Introduction
Nipple adenoma is a rare, benign tumor of the breast
that occurs between the fourth and fifth decades of life,
predominantly affecting females, and very rarely encountered in
accessory breasts (1). The breast
(mammary gland) normally develops in the early embryonic period as
two ectodermal thickenings known as mammary milk lines, which form
along the sides of the embryo during the sixth week of development
and extend from the axillary region to the groin. During normal
development, the majority of the embryonic mammary ridges along the
milk line undergo involution except for two segments in the
pectoral area that eventually become breasts. When one of these
ridges fails to involute, supernumerary breasts form. This
condition, variably known as supernumerary breast, ectopic breast,
or polymastia (2). In 2022, benign
breast tumors were extensively studied with 839 publications.
Common types include fibroadenoma, fat necrosis, papilloma, cyst,
mastitis, hyperplasia, adenoma and granular cell tumors (3). Nipple adenoma, also termed nipple
duct adenoma, papillary adenoma, erosive adenomatosis, florid
papillomatosis, papillomatosis of the nipple and subareolar duct
papillomatosis, represents a subtype of intraductal papilloma
involving the terminal portion of the galactophorous ducts.
Clinically, nipple adenoma can be mistaken for Paget's disease,
while pathologically, it could be misclassified as a tubular
carcinoma. Although axillary tumors have a variety of differential
diagnoses ranging from benign to malignant, nipple neoplasms
arising from axillary accessory breasts are rarely mentioned in the
English literature (4).
The present study reports the case of a patient with
adenoma arising from an accessory breast nipple, and also provides
a brief literature review. The references have been inspected for
reliability (5).
Case report
Patient information
A 23-year-old female patient presented to Smart
Health Tower (Sulaymaniyah, Iraq) with a 4-year history of right
axillary discomfort, pain and swelling. The patient, a non-smoker
with no history of diabetes, had one child and breastfed for ~17
months, with the last lactation occurring ~2 years prior to the
current presentation. An investigation into the surgical history of
the patient revealed a cesarean section, while an analysis of her
family history did not reveal any notable findings.
Clinical finding
Upon a clinical examination, a tender, firm lump was
detected in the right axillary region. The lump did not display
signs of inflammation, such as redness and warmth.
Diagnostic approach
A breast ultrasound (data not shown) revealed a
homogeneous background echotexture with a fibroglandular pattern.
Bilateral accessory axillary breasts were observed, with the right
side being larger, displaying normal morphology without any solid
masses or distortions in either breast. Both primary and accessory
nipples were normal. The breasts exhibited normal skin thickness
and contours, and the axillary lymph nodes were unremarkable.
Unfortunately, the ultrasound images were not captured due to
limited resources in the present country, but the ultrasonography
report was available. Additionally, the complete blood count
results were within the normal range.
Therapeutic intervention
The mass was excised under local anesthesia, with
hemostasis achieved without complications, and the wound was closed
using absorbable sutures. Upon a gross examination, the skin
excisional biopsy measured 1.2x0.8 cm with a thickness of 0.3 cm,
revealing a 0.5-cm skin-covered, exophytic nodule, away from the
peripheral and deep margins.
The histopathological examination was performed as
follows: The specimen was fixed in 10% neutral-buffered formalin at
room temperature for a period of 1 to 3 days. It was then processed
using the DiaPath Donatello automated processor, progressing
through the designated stages: Samples were held in 10%
neutral-buffered formalin for an average duration of 20 min, then
placed in deionized water for 10 min. Dehydration was conducted
using alcohol, beginning with a concentration of 70% for 1 h,
followed by 95% for 1 h, 99% for 1 h and 30 min, and another
station of 99% for 1 h and 30 min. Clearing was performed through
three stations of xylene, each lasting 1 h for a total of 3 h.
Finally, infiltration with paraffin wax was carried out in three
stations, each lasting 1 h for a total of 3 h. The blocks were
subsequently embedded in paraffin wax using the Sakura Tissue-Tek
embedding center (Sakura Finetek USA). The blocks were then trimmed
and sectioned with the Sakura Accu-Cut SRM microtome (Sakura
Finetek USA) to a thickness of 5 µm. The sections were floated in a
Sakura 1451 water bath, maintained at 40-50˚C, and then mounted
onto standard glass slides. These slides were then placed in an
oven set at 60-70˚C and left overnight. The following day, the
slides were stained with hematoxylin and eosin using the DiaPath
Giotto autostainer, following these stages: First, the slides were
immersed in xylene for three intervals of 7, 7 and 5 min. They were
then treated with 100% alcohol in three stages lasting 7, 6 and 5
min, followed by 90% alcohol for 4 min, 70% alcohol for 3 min, and
then rinsed with tap water for 2 min. They were then stained with
Hematoxylin Gill II for 8 min, prepared from Sigma-Aldrich
Hematoxylin Natural Black 1. This was followed by rinsing in tap
water for 4 min, a brief dip in ammonia water for 1 min, another
tap water rinse for 1 min, and then 70% alcohol for 2 min.
Subsequently, the slides were stained with Eosin for 5 min,
prepared from Sigma-Aldrich Eosin Y disodium salt, followed by a
1-min rinse in tap water. The dehydration process involved 70%
alcohol for 15 sec, 90% alcohol for 2 min, and 100% alcohol in
three stages of 3, 3 and 4 min. The slides were then cleared in
xylene in three stages of 3, 5 and 4 min. After drying for 5 min,
the slides were covered with SurgiPath Sub-X mounting medium and a
coverslip. The microscope used for examination was an Olympus BX-51
microscope with a camera adaptor (Olympus U-TV0.5XC-3) (Olympus
Corporation) for obtaining images.
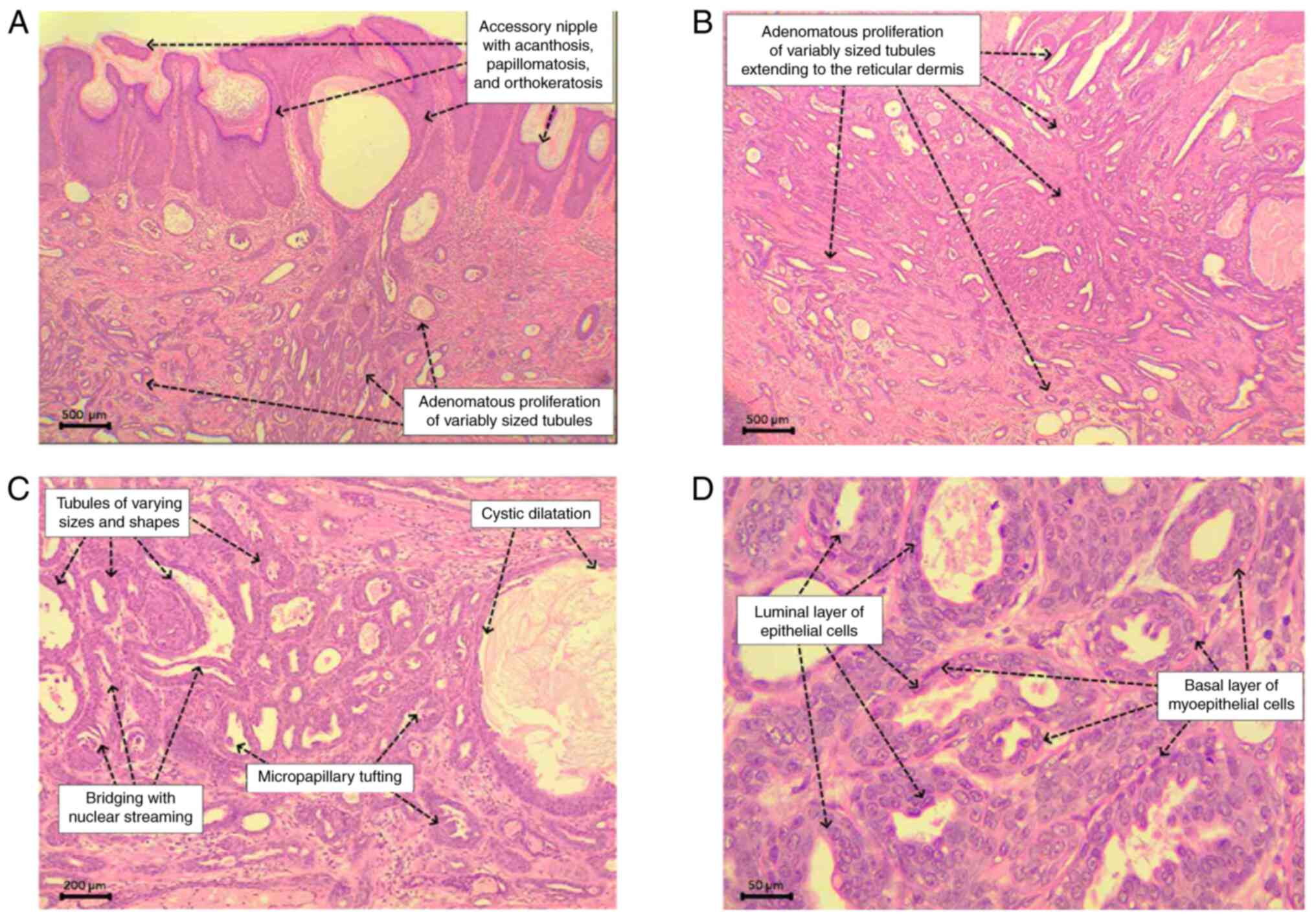
The histopathological examination revealed a
relatively ill-defined proliferation of variably sized ducts within
the dermis. These ducts comprised a basal layer of flat to cuboidal
myoepithelial cells with oval nuclei and fine chromatin, and a
luminal epithelial layer of cuboidal to low columnar cells with
oval nuclei exhibiting more open chromatin and conspicuous
nucleoli, devoid of significant cytologic atypia and atypical
mitotic figures. Some ducts displayed intraluminal micropapillary
proliferation and bridging with streaming nuclei. The surrounding
dermis exhibited fibrosis with patchy and mild mixed inflammatory
cells but lacked a desmoplastic reaction. The overlying epidermis
showed acanthosis, papillomatosis, and orthokeratosis. These
findings were consistent with an adenoma arising within an
accessory nipple (Fig. 1).
Follow-up and outcome
The patient was discharged in good health on the
first post-operative day. A 4-month follow-up revealed no clinical
signs of recurrence.
Discussion
Accessory breast tissue is a relatively uncommon
occurrence ranging from 0.4 to 0.6%, typically originating
alongside the embryogenic mammary ridge, extending from the axilla
to the groin, and is commonly found in the axilla (2). Neoplasms in the axillary nipple can
manifest as either benign or malignant lesions. Nipple adenoma, a
rare benign tumor, develops within the superficial part of the
nipple (6). Paget's disease of the
nipple is the primary differential diagnosis (1). According to the study by Shinn et
al (1), until 2015, only four
published reports of nipple adenoma in an accessory nipple had been
identified. A clinical examination often reveals a palpable lump
and an eroded, ulcerated, or crusted nipple as typical findings
(7). Previous case reports have
documented varying clinical presentations of nipple adenoma in
accessory breasts. For example, Shioi et al (4) described the case of an 82-year-old
female patient with a painful right axillary tumor, whereas,
Malagimani et al (6)
reported the case of a 24-year-old female patient experiencing
continuous pain and bleeding from her right accessory nipple, with
examination revealed no neoplastic lesions or palpable axillary
lymph nodes. In the case in the present study, the patient
presented with a right-sided axillary swelling and a tender firm
lump upon examination.
Nipple adenoma microscopically presents with the
proliferation of a small tubular structures exhibiting a
double-layered appearance, although it can display a wide range of
histopathological features (4).
Rosen and Caicco divided nipple adenomas into four morphological
patterns: i) Sclerosing papillomatosis, which can resemble
sclerosing papilloma; ii) papillomatosis, characterized by florid
papillary hyperplasia of ductal epithelium; iii) adenosis,
featuring evident myoepithelial hyperplasia; and iv) a combination
of these proliferative forms (8).
However, there is no apparent indication of prognostic relevance
associated with these patterns, and there is also a lack of
evidence suggesting variance in their underlying pathogenesis
(9). The case described herein
exhibited a predominant pattern of adenosis admixed with focal
micropapillary formations.
Radiographically, an ultrasound, mammography and
magnetic resonance imaging (MRI) are commonly utilized modalities
for identifying and characterizing nipple adenomas. Among these, an
ultrasound is considered the most clinically useful, as it can
delineate soft tissue nodularity, assess internal vascularity and
facilitate image-guided fine-needle aspiration. The lesion
typically appears well-defined and hypoechoic on the ultrasound
scan, and may exhibit posterior echo enhancement. The MRI
enhancement curve of nipple adenomas may mimic that of malignant
lesions, displaying rapid uptake, peaking at 60-90 sec followed by
washout, which can lead to diagnostic confusion. The results of
mammography may vary, ranging from benign calcification to dense
lesions with well-defined or irregular edges. It should be noted
that no single imaging modality should be relied upon in isolation
for diagnosing nipple adenoma (10). In the present study, a breast
ultrasound unexpectedly revealed normal accessory breasts and
nipples without evidence of a mass-forming lesion in either the
accessory breasts or the normal breast tissue. In terms of
management, the treatment of choice for an adenoma of the accessory
nipple is the surgical excision of the tumor. This approach ensures
complete removal of the tumor, thus alleviating any associated
symptoms. The prognosis following surgical intervention is
generally favorable, with a low risk of recurrence (10). While the link between breast
adenomas and carcinomas remains incompletely understood, breast
adenomas are generally considered benign tumors. Complete excision
and a histopathological examination are crucial for an accurate
diagnosis and for the prevention of the misdiagnosis of cancer,
thereby avoiding unnecessary and costly surgery (4). In the case presented herein, the mass
was successfully excised under local anesthesia with no
complications, and the subsequent histopathological examination
confirmed the benign nature of the tumor. It is worth highlighting
that the efficient management of the case in the present study
aligns with previous cases reported by in the studies by Shinn
et al (1), Shioi et
al (4) and Malagimani et
al (6).
Nipple adenoma, despite its benign nature, has been
found to be associated with breast cancer in clinical studies, with
some cases revealing concurrent active breast cancer or
clinically-occult invasive disease. Although establishing a direct
causal link remains challenging due to the rarity of malignant
transformation, heightened suspicion for co-existing breast cancer
is warranted in cases of nipple adenoma (8,11,12).
Additionally, the expanding growth pattern and potential for local
recurrence of nipple adenoma can significantly affect the quality
of life of patients, particularly in women of childbearing age
reliant on intact nipple function for breastfeeding. Thus, a
comprehensive clinical evaluation and screening for co-existing
breast cancer are imperative in the management of nipple adenoma
(13).
While specific risk factors for its occurrence in
younger patients have not been extensively studied, several
potential contributors have been suggested. Hormonal fluctuations
due to puberty, menstrual cycles, or hormonal medications may
stimulate breast tissue growth. Genetic predisposition,
particularly with a family history of breast conditions, could
elevate the risk. Reproductive factors, such as early menarche or
nulliparity may also play a role, along with breast trauma or
injury. Lifestyle factors such as diet and alcohol consumption may
contribute as well. However, the exact association between these
factors and nipple adenoma in younger patients requires further
investigation due to the limited evidence available since a
significant portion of the literature concerning nipple adenoma
comprises only individual case reports and series of cases
(14).
In conclusion, adenoma of accessory nipples is a
rare condition that necessitates careful clinical evaluation and
diagnostic workup to ensure proper management. Surgical excision
remains the standard approach for addressing this benign tumor,
with favorable long-term outcomes for affected individuals.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RMA and AMS were major contributors to the
conception of the study, as well as in the literature search for
related studies. AAQ and FHK were involved in the literature
review, the design of the study and in the writing of the
manuscript. AMA was the pathologist who performed the
histopathological diagnosis. LRAP, HOB, HOA, HMD and ROM were
involved in the literature review, the design of the study, in the
critical revision of the manuscript, and in the processing of the
figure. FHK and RMA confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
In our locality, ethical approval is not required
for case studies involving fewer than three cases. Written informed
consent was obtained from the patient for her participation in the
present study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present study and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shinn L, Woodward C, Boddu S, Jha P,
Fouroutan H and Péley G: Nipple adenoma arising in a supernumerary
mammary gland: A case report. Tumori. 97:812–814. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yefter ET and Shibiru YA: Fibroadenoma in
axillary accessory breast tissue: A case report. J Med Case Rep.
16:1–6. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Salih A, Ahmed A, Mohammed S, Qadir A,
Bapir N and Fatah G: Benign tumor publication in one year (2022): A
cross-sectional study. Barw Med J. 1:20–25. 2023.
|
|
4
|
Shioi Y, Nakamura SI, Kawamura S and
Kasami M: Nipple adenoma arising from axillary accessory breast: A
case report. Diagn Pathol. 7(162)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Muhialdeen AS, Ahmed JO, Baba HO, Abdullah
IY, Hassan HA, Najar KA, Mikael TM, Mustafa MQ, Mohammed DA, Omer
DA, et al: Kscien's list; A new strategy to discourage predatory
journals and publishers (Second Version). Barw Med J. 1:30–32.
2023.
|
|
6
|
Malagimani SC, Jashvanth CT,
Kasasomasekhar K and Paga U: Case report: A rare case of accessory
nipple (Polythelia) presenting as papillary adenoma. J Evolution
Med Dent Sci. 3:2005–2008. 2014.
|
|
7
|
Healy CE, Dijkstra B, Walsh M, Hill AD and
Murphy J: Nipple adenoma: A differential diagnosis for Paget's
disease. Breast J. 9:325–326. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rosen PP and Caicco JA: Florid
papillomatosis of the nipple: A study of 51 patients, including
nine with mammary carcinoma. Am J Surg Pathol. 10:87–101.
1986.PubMed/NCBI
|
|
9
|
Mills SE, Carter D, Greenson JK, Reuter VE
and Stoler MH (eds): Sternberg's diagnostic surgical pathology. 5th
edition. Lippincott Williams & Wilkins, Philadelphia, PA,
2012.
|
|
10
|
Tatterton MR and Fiddes R: Nipple adenoma:
A review of the literature. Ann Breast Surg. 3(e8586)2019.
|
|
11
|
Jones MW and Tavassoli FA: Coexistence of
nipple duct adenoma and breast carcinoma: A clinicopathologic study
of five cases and review of the literature. Mod Pathol. 8:633–636.
1995.PubMed/NCBI
|
|
12
|
Gudjónsdóttir A, Hägerstrand I and OStberg
G: Adenoma of the nipple with carcinomatous development. Acta
Pathol Microbiol Scand A. 79:676–680. 1971.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Combi F, Palma E, Montorsi G, Gambini A,
Segattini S, Papi S, Andreotti A and Tazzioli G: Management of
nipple adenomas during pregnancy: A case report. Int Breastfeed J.
18(19)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Berger O, Gersh G and Talisman R: Nipple
adenoma: Systematic review of literature. Plast Reconstr Surg Glob
Open. 12(e5827)2024.PubMed/NCBI View Article : Google Scholar
|