1. Introduction
Breast cancer (BC) is the most prevalent type of
cancer among women worldwide, with 2.3 million new cases diagnosed
each year (1). The aetiology of BC
is multifactorial and involves genetic, hormonal, environmental and
lifestyle factors. While the precise mechanisms remain poorly
understood, research has identified various risk factors, including
non-modifiable factors such as age, genetic predisposition
(BRCA1/2 mutations) and hormone levels. Modifiable factors
include lifestyle choices, the use of oral contraceptives or
hormone replacement therapy (2)
and parity (3). In addition, a
high mammographic density has been found to be associated with an
increased risk of BC development (4).
At a cellular level, the majority of BC cases
originate in the epithelial lining of breast ducts and lobules,
termed ductal or lobular carcinoma in situ. Once malignant
cells spread to nearby structures or tissues, the cancer becomes
invasive carcinoma. BC can be classified into four main molecular
subtypes based on the hormone receptor (HR; i.e., oestrogen and
progesterone) and the human epidermal growth factor receptor 2
(HER2) status and include luminal A (HR+ and
HER2-), luminal B (HR+ and HER2+),
triple-negative (HR- and HER2-), and HER2-positive (5). The current standard of care depends
on curative intent or palliative approaches, and can include a
combination of chemotherapy, radiotherapy and surgery. Targeted
approaches and hormonal therapies are also available depending on
the subtype, apart from triple-negative BC (TNBC) which is
associated with the poorest prognosis (6). While epithelial cells predominantly
drive BC pathogenesis, its progression is widely accepted to be
influenced by components of the breast microenvironment, a rich
milieu, which includes fibroblasts, adipocytes, and immune cells
such as macrophages, mast cells and T-cells (7-10).
Endocrine-disrupting chemicals (EDCs) are
environmental substances known for interfering with the normal
functioning of the endocrine system (11). These chemicals can mimic, block, or
disrupt various processes related to the production, release,
transport, metabolism, binding, action, or elimination of natural
hormones in the body (12). Hence,
there is potential to impact cellular function and increase the
risk of carcinogenesis. Exposure to EDCs can occur through
ingestion, inhalation or dermal contact, for example with
contaminated air, water, food, consumer products, or contaminated
surfaces (11,13-15).
EDCs interfere with and modulate the endocrine system, and can lead
to various adverse effects, such as alterations of the
hypothalamic-pituitary-adrenal axis and urogenital tract
malformations, and may also contribute to the development of BC
(16). By disrupting the cellular
microenvironment, EDCs may affect cell signalling, communication
and the extracellular matrix, contributing to a pro-carcinogenic
environment.
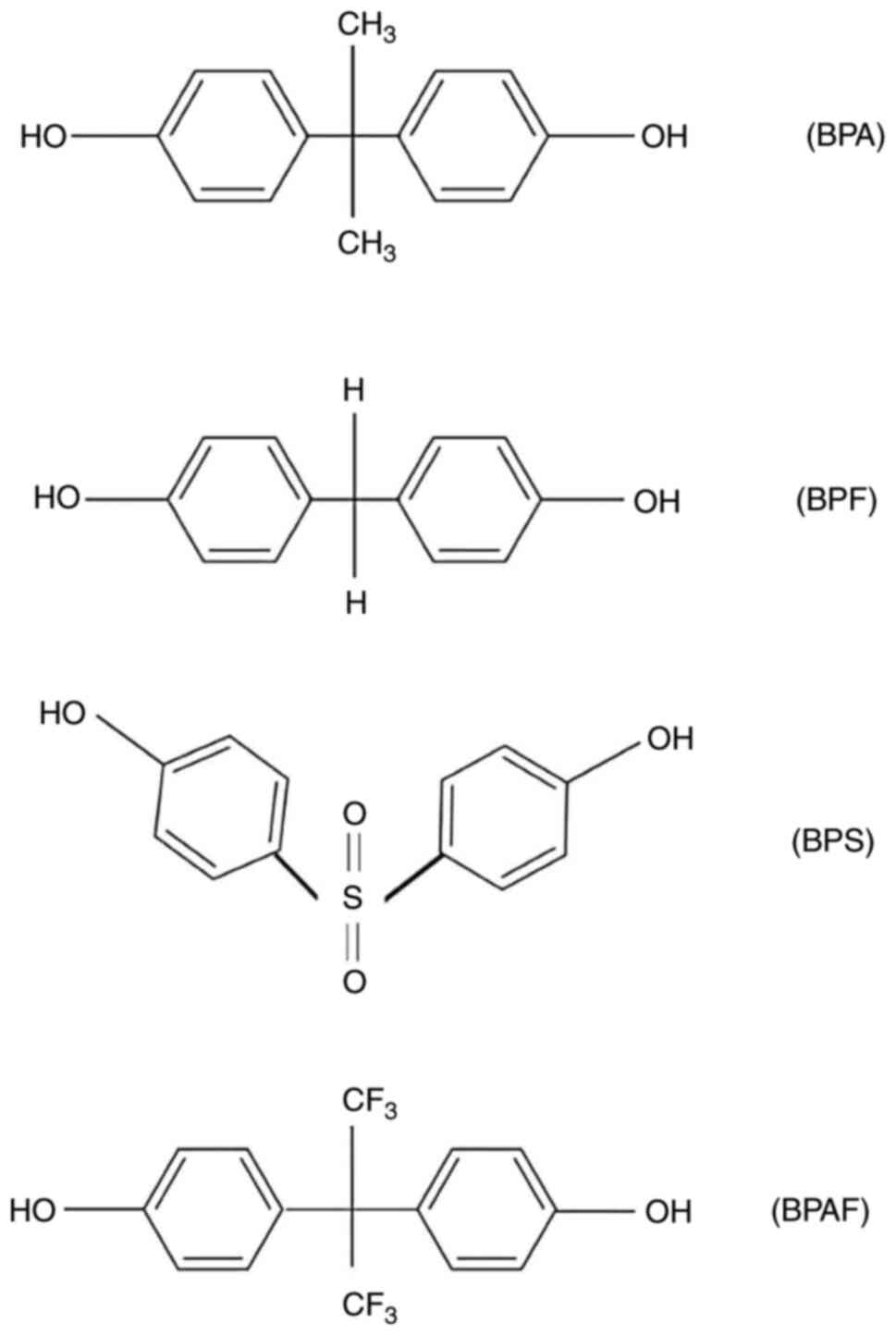
Bisphenols are some of the most commonly recognised
EDCs (11), with bisphenol A (BPA)
being the most well-known. Alternatives to BPA, include bisphenol
AF (BPAF), bisphenol S (BPS) and bisphenol F (BPF), which share
structural similarities with BPA (17). In response to regulatory measures,
use of BPA has been increasingly restricted (11) while alternatives, particularly BPS
and BPF, have gained favour as replacements (18). However, despite being postulated as
safer substitutes, BPAF, BPS and BPF exhibit comparable structural
characteristics and demonstrate similar oestrogenic, androgenic,
anti-oestrogenic and anti-androgenic activities both in vivo
and in vitro (19). These
properties suggest that these compounds may influence cellular
processes in ways that could elevate the risk of cancer
development. Therefore, a comprehensive evaluation of BPA and its
alternatives is essential to fully assess their potential
implications in BC development, and to determine whether these BPA
substitutes are safer alternatives.
The present narrative review aimed to provide a
comprehensive summary of the available literature to determine the
potential role of BPA and its chemical analogues (BPAF, S and F) on
the cell types that reside within the breast microenvironment
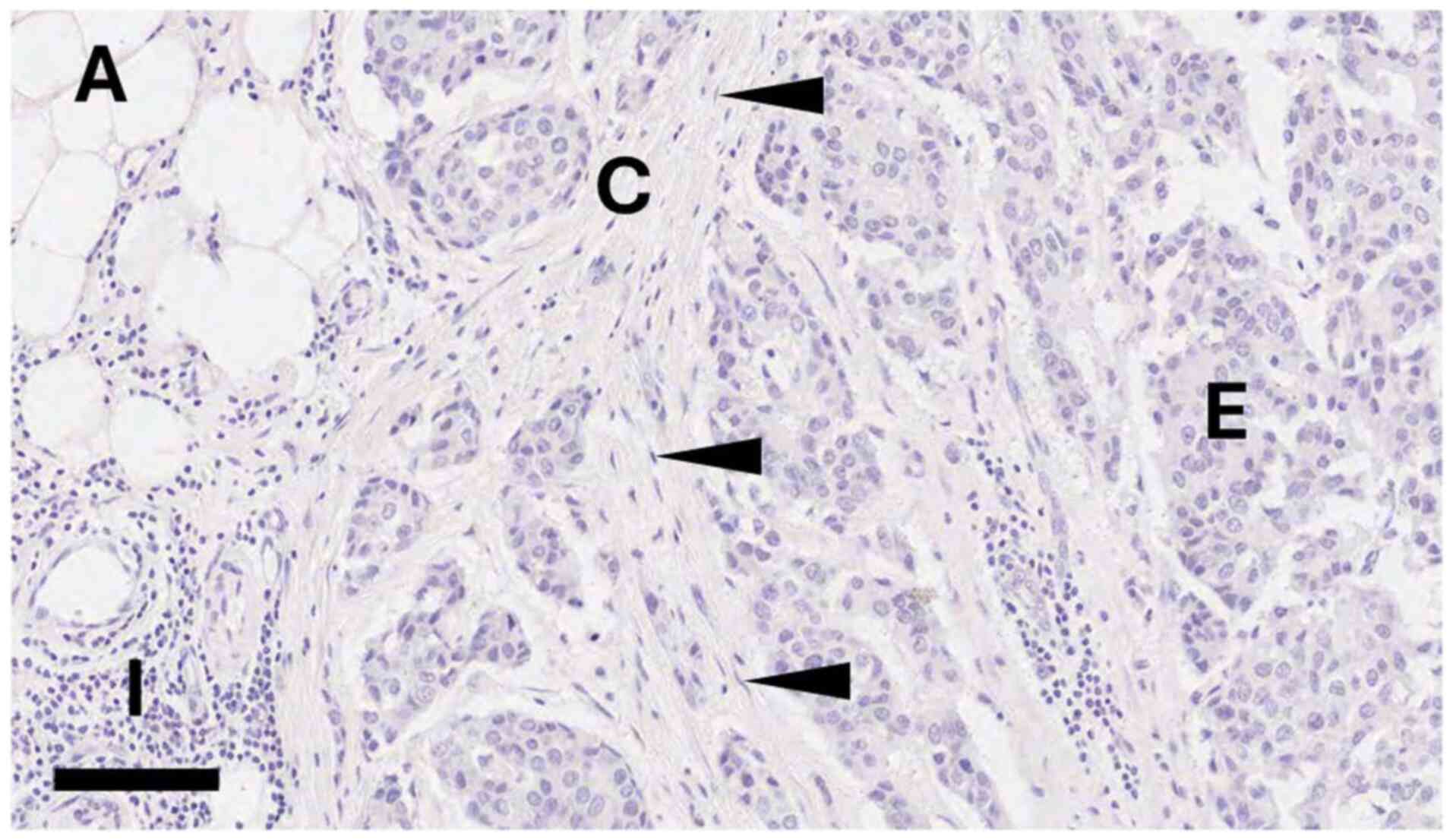
(Fig. 1) and to determine how this
may influence BC development.
2. Bisphenols
BPA. The structure of BPA is illustrated in
Fig. 2, a synthetic xenoestrogen
used in the manufacturing of polycarbonate plastics and epoxy
resins (20). BPA has been
extensively investigated for its effects on nuclear receptor
signalling pathways and has been found to be disruptive (21). For example, high-dose BPA
predominantly functions as an oestrogen receptor (ER) antagonist
through modulating genomic transcription (22). Conversely, at nanomolar doses, BPA
is considered to interfere with biological processes through
non-genomic mechanisms facilitated by membrane signalling (23). Materials containing BPA are various
and include plastic containers, tin cans, water bottles, toys,
healthcare equipment and up to 2020, thermal paper (including till
receipts) (24). Consumer exposure
primarily occurs through food coming into contact with
BPA-containing materials, such as polycarbonate baby bottles, food
containers, and epoxy resin-lined food and beverage cans. The
impact of BPA on human health has led to the implementation of
various regulatory measures aimed at limiting lifetime exposure.
For instance, the European Food Safety Authority (EFSA) has reduced
the recommended tolerable daily intake (TDI) of BPA from 4 µg/kg
bodyweight/day (set in 2015) to 0.2 ng/kg bodyweight/day (set in
2023) (25), a 20,000-fold
decrease. Additionally, the use of BPA in baby products was
completely banned in the EU from 2011 onwards (26). In 2024, the EFSA issued a statement
for phasing out BPA and its analogues in consumer products
(27). By contrast, in the USA,
the TDI has not been changed since it was first introduced in the
1980s and still stands at 50 µg/kg bodyweight/day (28). Furthermore, numerous petitions with
new evidence indicating the potential adverse effects of BPA have
failed to initiate a review or reduction of the current TDI
(29).
BPA chemical analogues. The growing public
awareness surrounding the potential adverse effects of BPA on human
health has placed manufacturers under significant pressure to
eliminate their use, resulting in the emergence of ‘BPA-free’
products. However, many of these contain BPA analogues, including
BPAF, BPS and BPF (Fig. 2), whose
use currently remains unregulated (30). Despite research efforts into the
potential oestrogenic effects of these alternatives, the available
literature remains limited. A single systematic review of 32
studies highlighted the significant knowledge gaps on their impact
(19). Nonetheless, findings from
preclinical studies have shown considerable endocrine-disrupting
effects of BPA, BPS and BPF at micromolar values (31,32).
Phenotypic and proteomic changes were observed within a
human-derived normal breast organoid model exposed to 15 nM BPA,
BPS and BPF. This included the disruption of tissue architecture
and abnormal branching (33).
Another study on human breast cancer cell lines demonstrated that
BPAF was a more potent activator of ERα than BPA (34). Moreover, animal studies have shown
that BPS and BPF have direct oestrogenic activity; for instance,
BPS and BPF can induce uterine growth in rodents (35,36)
and disrupt reproduction in zebrafish (37).
3. Bisphenols and the breast
microenvironment
Investigations into the potential oestrogenic
effects of BPA and its analogues have been extensive in normal and
cancerous breast epithelial cells, as reviewed extensively
elsewhere (38-40).
The present review provides insight into the potential effects of
BPA on other cells found within the breast microenvironment,
including fibroblasts, adipocytes and immune cells.
Fibroblasts. Fibroblasts are the primary
cellular constituents of the breast stroma and undergo activation
and proliferation in response to various stimuli, including
inflammation, wound repair and malignancy (41,42).
In addition, fibroblasts can regulate mammary epithelial cell
morphogenesis, including expansion, differentiation, ductal
elongation and invasion into adipose tissue (43). The development of fibrous
connective tissue, typically in response to injury or damage,
results in fibrosis, a process known to be associated with an
increased risk of malignancy (44). Fibroblasts and myofibroblasts
undergo genetic alterations, predisposing them to transform into
cancer-associated fibroblasts (CAFs), which play a pivotal role in
tumour development, stromal remodelling, and intercellular
communication pathways (45).
Collectively, these actions create a tumour-supportive
microenvironment and contribute to resistance to anticancer
therapies (46).
Previous studies on the immune regulation of mammary
fibroblasts have highlighted the role of immune cells in regulating
fibroblast function within the mammary gland, which impacts
mammographic density, a known risk factor for BC development
(47-50).
Mammographic density is dependent on the proportions of epithelial
and fibrous stromal tissues. Increased mammographic density is
analogous with a higher content of fibrous tissue within the stroma
and is associated with a 4-6-fold increased risk of developing BC
(50). Studies have shown that
exposure to BPA promotes collagen production by cardiac fibroblasts
via the ERK1/2 pathway (51), and
is associated with an elevated mammographic density (52) and increased mammary gland rigidity,
a property which is known to enhance proliferation and tumour
progression (53).
Fibroblast-driven paracrine signalling and extracellular matrix
remodelling, as mediated by the EGFR pathway; this further
influences epithelial morphogenesis and may play a role in tumour
initiation (54).
A previous study investigating the effects of BPA on
co-cultures of CAFs and breast cancer cells, demonstrated that BPA
mediated CAF proliferation and SkBr3 migration through the
activation of the G-protein coupled ER pathway (55). Primary mammary fibroblasts from
mouse mammary glands exposed to BPA exhibit the differential
expression of genes commonly associated with an increased risk of
developing BC (56).
BPA exposure significantly alters the gene
expression profiles of mesenchymal cells, including fibroblasts,
derived from murine mammary glands (57), disrupting their function and
behaviour by influencing pathways associated with extracellular
matrix production. These disruptions could potentially alter
fibroblast-epithelial interactions, affecting mammary gland
development and potentially predisposing the tissue to developing
malignancy.
There are a limited number of studies investigating
the effects of BPS and BPF on fibroblasts. Potential cytotoxic
effects of these compounds on mammalian fibroblasts, at 50-500 mM
doses has been reported; however, the use of such high
concentrations is questionable compared to real-world exposure
levels (58). In MRC5 human lung
fibroblasts exposed to up to 100 µM BPA, no effects were observed
on proliferation, cell cycle progression or apoptosis (59).
Adipocytes. Adipocytes are abundant in the
breast tumour microenvironment (TME). White adipocytes are a major
component and primarily store energy as triacylglycerol, releasing
this as fatty acids and glycerol under high metabolic demand
(60). Breast adipocytes are
increasingly associated with BC development and progression
(61). In a previous study using
mice exposed to oral BPA (5, 50, 500 and 5,000 µg/kg/day) alongside
normal chow, bodyweight and adiposity increased, indicating the
potential interaction of BPA with diet (62). Furthermore, BPA increased
circulating inflammatory factors leptin and tumour necrosis factor
(TNF)-α in lean female, but not male mice (62). At an endocrine level, obesity poses
a significant risk factor for ER+ BC due to the
aromatisation of androgens to oestrogens in adipocytes, which
elevates the overall oestrogen levels (63). This also extends to BC in males,
where research has demonstrated that the increasing number of males
receiving a BC diagnosis parallels with an increase in obesity
(64).
Some endocrine disruptors can modify inflammatory
responses in adipocytes (62).
Cancer-associated adipocytes (CAAs) are adipocytes located close to
the tumour leading edge and are characterised by loss of lipid
content, loss of mature adipocyte markers, such as peroxisome
proliferator-activated receptor γ (PPARγ), and an increase in
adipokines, primarily leptin and resistin, which can promote BC
development (65-67).
Additionally, CAAs display an increased secretion of inflammatory
mediators, including interleukin (IL)-1β, IL-6(68), vascular endothelial growth factor
(VEGF) and TNF-α, potentially in response to cancer cells (69). Moreover, CAAs release adipokines
that promote cancer cell migration, such as IL-6 and monocyte
chemoattractant protein-1 (MCP-1) (70), at higher levels compared to
non-cancer adipocytes (70). BPA
increases the expression of these adipokines to help facilitate
their role in breast carcinogenesis. For example, in a previous
study, when mature human adipocytes were treated with 0.1 nM BPA,
the G protein-coupled oestrogen receptor pathway was activated,
leading to the release of IL-6, IL-8 and MCP1α (23). Both IL-6 and MCP-1α can promote
cancer cell proliferation and migration, and IL-8 enhances the
invasiveness and angiogenesis of BC cells, particularly
ER+ subtypes (68,71).
The implication of these findings is that BPA may cause adipocytes
in the breast microenvironment to release pro-inflammatory
cytokines, potentially accelerating tumour development and
growth.
BPA has also been implicated in altering hormone
levels in adipose tissue by modulating the activity of key
regulators, such as lipoprotein lipase and aromatase (72,73).
Although limited, studies on the effects of BPA on breast
adipocytes have shown that nanomolar concentrations of BPA do not
stimulate the proliferation of adipocytes in human subcutaneous
adipose tissue (74). However,
several studies have demonstrated that BPA accelerates adipocyte
differentiation into mature cells in vitro (75-80).
For instance, murine 3T3-L1 cells and adipocyte stem cells undergo
accelerated differentiation into mature adipocytes upon exposure to
low concentrations of BPA (10-80 µM) (75-80).
Mature adipocytes are crucial for secreting adipokines, such as
adiponectin, which have anti-inflammatory and antioxidative
properties, and can inhibit cell proliferation and angiogenesis
(81). In addition, adipokines
promoted apoptosis in diabetes (82). If these effects are mirrored in BC,
this may provide potential protective effects. Conversely, exposure
to 0.1 and 1 nM of BPA has been shown to decrease adiponectin
release from human breast adipose tissue (83). In metabolic disorders, adiponectin
functions as a protective factor, promoting insulin sensitivity and
exhibiting anti-inflammatory and anti-tumorigenic properties
(84).
Both preadipocytes and mature adipocytes
significantly influence mammary gland development, with
preadipocytes promoting epithelial branching and elongation, as
demonstrated in mouse models (85). In vivo, murine studies have
indicated that prolonged exposure to both 1 and 10 µg/ml BPA,
classified as low and high concentrations, respectively, influences
obesity and hyperlipidaemia development during perinatal and
postnatal periods (86). In
another study, the in utero exposure of mice to BPA at 250
ng/kg/day increased the expression of PPARγ and other
adipogenesis-related genes (57).
In addition, BPA exposure has been shown to increase the
concentration of mature adipocytes, negatively influencing lumen
formation in the foetal mammary gland epithelium (57,87).
Additionally, oestradiol sensitivity is increased (88), potentially elevating risk of
carcinogenesis. Collectively, these results suggest that, at least
in mouse models, in utero BPA exposure may be a critical
window for abnormal changes in epithelial, stromal and adipose
cells, potentially enhancing carcinogenic potential in
adulthood.
BPA alternatives, BPAF, BPF and BPS have been shown
to alter leptin hormone levels and increase lipid accumulation in
the murine 3T3L1 cell line (89).
Leptin is secreted by adipocytes and plays a critical role in
energy balance and appetite regulation. It also exerts
pro-inflammatory effects and may affect cancer progression
(90). In a previous study, in
3T3-L1 cells, exposure to 32 µM BPS and BPF increased the number of
differentiated adipocytes and their ability to store more lipids
compared to BPA, indicating a higher obesogenic potential and
endocrine toxicity (91).
Furthermore, in adult mice, doses of both 10 nM and 1 µM BPS
resulted in the upregulation of the expression of PPARγ and
perilipin 1, both involved in preadipocyte differentiation, much
earlier in the differentiation process than BPA and BPF. Hence, BPS
exposure may contribute to increased adiposity and an increased
risk of developing BC. However, the effects of BPF on adiposity are
reduced (92). These contrasting
findings suggest uncertainty over the potential influence of BPS
and BPF on the risk of developing BC. On the other hand, a previous
study demonstrated that BPAF treatment (5 µM) increased lipid
accumulation and sensitivity to inflammatory cytokines, including
interferon-γ, in differentiating adipocytes (93). This increased sensitivity resulted
in a reduction in mitochondrial and cellular respiratory capacity
in adipocytes through the suppression of UCP1 by interferon-γ,
which may also influence BC progression (93). Collectively, these findings
demonstrate the potential impact of BPA and its analogues on
adipocyte function and the putative link of bisphenols in
increasing breast carcinogenesis.
Immune cells. Different immune cell
populations often infiltrate breast cancers. This varies between
different BC subtypes. For example, TNBC often has high numbers of
tumour-infiltrating lymphocytes, which is associated with an
improved survival (94). Several
studies have identified the importance of lymphoid and myeloid
cells, which are primarily located within the epithelium of breast
lobules, in breast carcinogenesis, progression and treatment
(95-97).
Lymphoid cells. Lymphoid cells, including
lymphocytes, such as T-cells, B-cells and natural killer (NK) cells
are key components of the immune system. In a previous study, human
T-cells exposed to nanomolar concentrations of BPA exhibited a
reduced telomerase activity in CD8+ but not
CD4+ T-cells, accompanied by telomere shortening and
human telomerase reverse transcriptase suppression, suggesting
adverse effects of BPA on T-cell responses (98). In another study, a 30-day chronic
exposure of the human BC cell lines, MCF-7, SkBr3 and MDA-MB-231 to
physiologically relevant BPA concentrations (10 nM) upregulated the
expression of genes associated with NK cell and T-cell activation,
suggesting the potential of BPA to modulate immune function
(99). Notably, each cell line
exhibited distinct gene expression changes related to immune
responses. In MCF-7 cells, IL-19 expression was upregulated,
while in SkBr3 cells, the upregulation of CXCL5 was linked
to BC progression (99). Further
research has highlighted an abundance of NK cells in
HER2+ and TNBC subtypes, with their presence being
associated with increased lymphocyte infiltration and a higher
grade (100), suggesting that NK
cell activation is associated with more advanced BC. These findings
underscore the complex immune-related gene expression alterations
induced by BPA, implicating its potential role in BC progression
through immune modulation.
Myeloid cells. Myeloid cells, including
monocytes, macrophages and granulocytes contribute to TMEs by
modulating immune responses, promoting inflammation, and supporting
tumour growth and metastasis. The immune subtyping of myeloid cells
in murine breast cancer models and in clinical datasets has
identified neutrophil- and macrophage-enriched subtypes (101).
Neutrophils circulate in the bone marrow and
peripheral blood, recognising pathogens through host protein
interaction, aiding in phagocytosis, pathogen clearance and tissue
regeneration. To the best of our knowledge, no studies to date have
examined the effect of BPA on neutrophils in BC. However, an
examination of the effects of BPA on human T-cells in vitro
indicated increased reactive oxygen species (ROS) production, which
is associated with breast carcinogenesis (102).
Macrophages are classified into two main types: M1,
which is associated with anti-tumour immunity, and M2, which is
linked to pro-tumorigenic properties (103). Evidence from animal models
suggests that BPA may alter tumour-associated macrophage (TAM)
phenotypes (104). Another study
assessed the effects of BPA on macrophage polarisation,
demonstrating a significant increase in M2 markers (Arg-1 and
CD206) and a reduction in TAMs with M1 markers in ductal carcinomas
exposed to 10 nM BPA, which is typically considered an
environmentally relevant concentration (105).
BPA alternatives may also influence immune cell
activation and differentiation (106). While there are no BC studies, at
least to the best of our knowledge, studies using zebrafish and
carp have demonstrated that BPS and BPF exert immunotoxic effects
by modulating immunoregulatory genes in a concentration-dependent
manner during early development. Furthermore, exposure to BPF
increases the levels of immunomodulating chemokines and cytokines
and is associated with elevated ROS levels, oxidative stress and
the upregulation of inflammatory cytokine genes (107,108). The effects of BPA on human
myeloid cells in BC macrophages have yet to be determined.
4. Discussion and future directions
The present narrative review demonstrates that BPA
and its chemical analogues; BPAF, BPS and BPF, exert diverse
effects on the breast microenvironment. Some of these effects may
promote BC development, for example, through structural and
epigenetic changes to epithelial cells, the disruption of
fibroblast functions and stromal modifications, altered adipocyte
differentiation, and influences on lymphoid immune activity.
Previous studies on the effects of BPA on the female breast
microenvironment have mainly focused on BPA, overlooking its
analogues and the critical role of non-epithelial cells in the
breast milieu. To the best of our knowledge, the present review is
the first to systematically compare the differential effects of BPA
and its analogues on adipocytes, providing new insight into how
these compounds may alter breast tissue composition and function.
In addition, the present review also highlights safety concerns
regarding BPA analogues and the urgent need for regulatory
guidelines.
As demonstrated herein, one of the most critical
issues surrounding research on BPA is the reliance on animal models
and in vitro cell lines, which take a reductionist approach
and fail to fully capture the complexity of the breast
microenvironment. While these models have provided valuable
insight, their translational relevance remains limited. For
instance, current animal model limitations include murine mammary
tissue, which contains terminal end buds (TEBs) vs. terminal ductal
lobular units (TDLUs) found in humans. TDLUs are specialised
structures containing collagen, hyaluronan and other matrix
proteins, and give rise to primary sites where ductal carcinomas
can occur (109). By contrast,
mouse TEBs lack such specialised stroma, primarily comprising
adipose tissue with few fibroblasts (109). Furthermore, research has
highlighted that murine mammary cytokines may not functionally
match human receptor interactions, limiting the translational
relevance of these models (110).
To address these shortcomings, recent advancements have introduced
humanised models including organoid cultures, 3D co-culture systems
and clinical sample analyses, which better replicate the breast
microenvironment by preserving multicellular contacts and immune
interactions. Incorporating these models could improve the clinical
applicability of BPA research in future. Additionally, the majority
of available studies have examined single compounds and have
overlooked the combined effects of multiple chemicals or ‘cocktail
effects’, which may lead to underestimating the real-world risks.
Future research is thus warranted to incorporate experimental
designs that consider mixed bisphenol exposures to provide a closer
representation of the environmental risks. Moreover, the heavy
reliance on cell lines does not address the potential role of BPA
in initiating breast carcinogenesis.
To overcome these limitations, future studies are
required to focus on the effects of BPA and its analogues on all
cells within the breast microenvironment, particularly in the
context of cancer-stromal interactions, using multicellular 3D
models (111). A critical aspect
of BPA-induced breast carcinogenesis may lie in its ability to
remodel the TME. CAFs, key components of the TME, play an essential
role in metabolic symbiosis with tumour cells and contribute to
immune escape mechanisms by modulating cytokine signalling.
Bisphenols may enhance CAF activation, leading to extracellular
matrix remodelling, increased tumour invasiveness and altered
immune responses. Thus, further studies using tumour niche models
and stroma-epithelium interaction assays are required to elucidate
the mechanisms through which BPA and its analogues promote breast
cancer progression. Studies should also aim to use more reflective
models and novel technological advances to better understand BPA
and its alternatives, and their impact on the TME, as well as to
determine safe cut-off levels to guide regulatory recommendations
to prevent toxic harm. The use of organoids derived from cancerous
and non-cancerous human breast tissue is recommended (112), that can recapitulate their
primary tissue both phenotypically and molecularly (113). Although definitive evidence is
currently lacking, an observational study of male BC across various
regions of Scotland found higher incidence rates in areas with
significant agricultural activity, where the use of pesticides and
EDCs may be more prevalent (114). As such, tissue collection efforts
should include both males and females (114). Multi-cellular 3D models of cell
lines (115), co-culture
approaches (116) and the use of
novel bioprinting techniques (117), would enable a more insightful
representation of microenvironmental complexity. In addition, the
use of breast-on-a-chip models would enable microfluidic techniques
to create controlled, miniaturised environments for studying
dynamic tissue interactions with greater precision (118). To permit the comprehensive
mapping of cellular interactions and molecular changes within
breast tissue exposed to BPA and its analogues, the use of emerging
spatial multi-omics technologies should also be utilised (119). Drawing insights from the Human
Breast Cell Atlas (120), a
comprehensive map of cell types found within the normal human
breast, may inform these studies and enhance our understanding of
the tissue-specific impacts of bisphenols. Lastly, a thorough
investigation into both the short- and long-term effects of BPA on
various mammary cells, alongside sensitivity testing across low,
medium, and high BPA exposure levels, is crucial for making any
meaningful study comparisons and guiding regulatory decisions on
safe exposure limits.
Another observation was the wide variation in doses
of bisphenols used experimentally, ranging from 500 mM (58) to 10 nM (105). Optimum concentrations should be
defined to allow their biological effects to be defined more
rigorously. It is also recognised that these chemicals are
pervasive in the environment which may be confounding, although
this is difficult to control.
Finally, from a regulatory and public health
perspective, there is a lack of consensus regarding the threshold
for BPA exposure, making it challenging to define what constitutes
‘safe’ exposure levels. This inconsistency is particularly evident
when comparing international guidelines; for instance, the US TDI
currently stands at 50 µg/kg body weight/day, which is 250,000-fold
higher than the European TDI of 0.2 ng/kg bodyweight/day (28,121). In the UK, recent estimates
indicate that average daily exposure to BPA is ~2.5 ng/kg body
weight/day, depending on dietary habits and environmental factors
(122); however, precise numbers
are difficult to assess. Moreover, the term ‘chronic BPA exposure’
is inconsistently defined among studies, impeding cross-study
comparability. Consequently, the potential impact of
bioaccumulation on breast carcinogenesis remains largely
unexplored. It is also important to highlight that BPAF, BPS and
BPF currently lack regulatory oversight, a concern underscored by
the findings presented herein, as these unregulated compounds may
be driving the same pheno- and genotypic changes as found in
BPA.
Acknowledgements
Not applicable.
Funding
Funding: The present study received funding from Breast Cancer
UK (RG/2017-VS; 2122029), Animal Free Research UK and the Cyril
Margaret Gates Charitable Trust.
Availability of data and materials
Not applicable.
Authors' contributions
ST was involved in the writing, reviewing and
editing of the manuscript and in the writing of the original draft,
as well as in visualization (figure preparation) and project
administration. KK was involved in the writing, reviewing and
editing of the manuscript. KP and AD were involved in the writing,
reviewing and editing of the manuscript, as well as in data
curation (assessing all the relevant research articles for
inclusion in the review). DPMC was involved in the writing,
reviewing and editing of the manuscript, in visualization and study
supervision, as well as in project administration, methodology,
investigation, formal analysis, data curation and in the
conceptualisation of the study. VS was involved in the writing,
reviewing and editing of the manuscript, as well as in
visualization, study supervision and project administration,
resources (funding for the study and the tissue image), and in the
conceptualisation of the study.
Ethics approval and consent to
participate
The tissue image included in Fig. 1 was generated from an anonymised
breast tissue sample donated with ethical approval from the Leeds
Breast Tissue Bank (REC 15/YH/0025), whose samples now reside in
the Breast Cancer Now Biobank (REC 23/EE/0229). As the sample was
provided with full anonymisation, the donor cannot be identified
meaning that written informed patient consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lippman ME, Krueger KA, Eckert S, Sashegyi
A, Walls EL, Jamal S, Cauley JA and Cummings SR: Indicators of
lifetime estrogen exposure: effect on breast cancer incidence and
interaction with raloxifene therapy in the multiple outcomes of
raloxifene evaluation study participants. J Clin Oncol.
19:3111–3116. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nguyen B, Venet D, Lambertini M, Desmedt
C, Salgado R, Horlings HM, Rothé F and Sotiriou C: Imprint of
parity and age at first pregnancy on the genomic landscape of
subsequent breast cancer. Breast Cancer Res. 21(25)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper
JL, Yaffe MJ and Paterson AD: Mammographic breast density as an
intermediate phenotype for breast cancer. Lancet Oncol. 6:798–808.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Perou CM, Sørile T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Pareja F, Geyer FC, Marchiò C, Burke KA,
Weigelt B and Reis-Filho JS: Triple-negative breast cancer: The
importance of molecular and histologic subtyping, and recognition
of low-grade variants. NPJ Breast Cancer. 2(16036)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Soysal SD, Tzankov A and Muenst SE: Role
of the tumor microenvironment in breast cancer. Pathobiology.
82:142–152. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Terceiro LEL, Edechi CA, Ikeogu NM, Nickel
BE, Hombach-Klonisch S, Sharif T, Leygue E and Myal Y: The breast
tumor microenvironment: A key player in metastatic spread. Cancers
(Basel). 13(4798)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weigelt B and Bissell MJ: Unraveling the
microenvironmental influences on the normal mammary gland and
breast cancer. Semin Cancer Biol. 18:311–321. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mittal S, Brown NJ and Holen I: The breast
tumor microenvironment: Role in cancer development, progression and
response to therapy. Expert Rev Mol Diagn. 18:227–243.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Diamanti-Kandarakis E, Bourguignon JP,
Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT and Gore AC:
Endocrine-disrupting chemicals: An endocrine society scientific
statement. Endocr Rev. 30:293–342. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yilmaz B, Terekeci H, Sandal S and
Kelestimur F: Endocrine disrupting chemicals: Exposure, effects on
human health, mechanism of action, models for testing and
strategies for prevention. Rev Endocr Metab Disord. 21:127–147.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gore AC, Chappell VA, Fenton SE, Flaws JA,
Nadal A, Prins GS, Toppari J and Zoeller RT: Executive summary to
EDC-2: The endocrine society's second scientific statement on
endocrine-disrupting chemicals. Endocr Rev. 36:593–602.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zoeller RT, Brown TR, Doan LL, Gore AC,
Skakkebaek NE, Soto AM, Woodruff TJ and Vom Saal FS:
Endocrine-disrupting chemicals and public health protection: A
statement of principles from the endocrine society. Endocrinology.
153:4097–4110. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cwiek-Ludwicka K and Ludwicki JK:
Endocrine disruptors in food contact materials; is there a health
threat? Rocz Panstw Zakl Hig. 65:169–177. 2014.PubMed/NCBI
|
|
16
|
La Merrill MA, Vandenberg LN, Smith MT,
Goodson W, Browne P, Patisaul HB, Guyton KZ, Kortenkamp A, Cogliano
VJ, Woodruff TJ, et al: Consensus on the key characteristics of
endocrine-disrupting chemicals as a basis for hazard
identification. Nat Rev Endocrinol. 16:45–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brody JG and Rudel RA: Environmental
pollutants and breast cancer. Environ Health Perspect.
111:1007–1019. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Hawke E: Human biomonitoring results
reveal widespread exposure of general public to harmful chemicals.
https://chemtrust.org/hbm4eu_conference/. Accessed on
December 4, 2024.
|
|
19
|
Rochester JR and Bolden AL: Bisphenol S
and F: A systematic review and comparison of the hormonal activity
of bisphenol A substitutes. Environ Health Perspect. 123:643–650.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
European Food Safety Authority: Bisphenol
A | EFSA, 2023. https://www.efsa.europa.eu/en/topics/topic/bisphenol.
Accessed on December 11, 2024.
|
|
21
|
Liu X, Sakai H, Nishigori M, Suyama K,
Nawaji T, Ikeda S, Nishigouchi M, Okada H, Matsushima A, Nose T, et
al: Receptor-binding affinities of bisphenol A and its
next-generation analogs for human nuclear receptors. Toxicol Appl
Pharmacol. 377(114610)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nadal A, Fuentes E, Ripoll C, Villar-Pazos
S, Castellano-Muñoz M, Soriano S, Martinez-Pinna J, Quesada I and
Alonso-Magdalena P: Extranuclear-initiated estrogenic actions of
endocrine disrupting chemicals: Is there toxicology beyond
paracelsus? J Steroid Biochem Mol Biol. 176:16–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cimmino I, Oriente F, D'Esposito V,
Liguoro D, Liguoro P, Ambrosio MR, Cabaro S, D'Andrea F, Beguinot
F, Formisano P and Valentino R: Low-dose bisphenol-A regulates
inflammatory cytokines through GPR30 in mammary adipose cells. J
Mol Endocrinol. 63:273–283. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
European Union: Comission Regulation (EU)
2016/2235, 2016. https://eur-lex.europa.eu/eli/reg/2016/2235/oj/eng.
Accessed on December 13, 2024.
|
|
25
|
EFSA Panel on Food Contact Materials,
Enzymes and Processing Aids (CEP). Lambré C, Barat Baviera JM,
Bolognesi C, Chesson A, Cocconcelli PS, Crebelli R, Gott DM, Grob
K, Lampi E, et al: Re-evaluation of the risks to public health
related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J.
21(e06857)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
European Commision: Internal Market,
Industry, Entrepreneurship and Smes-Bisphenol A: EU ban on use in
baby bottles. https://ec.europa.eu/newsroom/growth/items/44933/en.
Accessed on December 4, 2024.
|
|
27
|
European Commission: European
Commission-Daily News, 2024. https://ec.europa.eu/commission/presscorner/api/files/document/print/en/mex_24_3243/MEX_24_3243_EN.pdf.
Accessed on December 11, 2024.
|
|
28
|
U.S. Environmental Protection Agency:
Bisphenol A. (CASRN 80-05-7) | IRIS | US EPA. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0356_summary.pdf.
Accessed on December 4, 2024.
|
|
29
|
U.S. Food and Drug Administration (FDA):
Bisphenol A (BPA) | FDA. FDA, Silver Spring, MD, 2023. https://www.fda.gov/food/food-packaging-other-substances-come-contact-food-information-consumers/bisphenol-bpa.
Accessed on December 4, 2024.
|
|
30
|
European Chemicals Agency (ECHA):
Assessment of regulatory needs. ECHA, Helsinki, 2021. https://echa.europa.eu/documents/10162/7de6871f-30db-9cdc-0a13-20942f511e00.
Accessed on December 11, 2024.
|
|
31
|
Atlas E and Dimitrova V: Bisphenol S and
Bisphenol A disrupt morphogenesis of MCF-12A human mammary
epithelial cells. Sci Rep. 9(16005)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kim JY, Choi HG, Lee HM, Lee GA, Hwang KA
and Choi KC: Effects of bisphenol compounds on the growth and
epithelial mesenchymal transition of MCF-7 CV human breast cancer
cells. J Biomed Res. 31(358)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Winkler J, Liu P, Phong K, Hinrichs JH,
Ataii N, Williams K, Hadler-Olsen E, Samson S, Gartner ZJ, Fisher S
and Werb Z: Bisphenol A replacement chemicals, BPF and BPS, induce
protumorigenic changes in human mammary gland organoid morphology
and proteome. Proc Natl Acad Sci USA.
119(e2115308119)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mesnage R, Phedonos A, Arno M, Balu S,
Corton JC and Antoniou MN: Editor's highlight: Transcriptome
profiling reveals bisphenol A alternatives activate estrogen
receptor alpha in human breast cancer cells. Toxicol Sci.
158:431–443. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yamasaki K, Noda S, Imatanaka N and Yakabe
Y: Comparative study of the uterotrophic potency of 14 chemicals in
a uterotrophic assay and their receptor-binding affinity. Toxicol
Lett. 146:111–120. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stroheker T, Chagnon MC, Pinnert MF,
Berges R and Canivenc-Lavier MC: Estrogenic effects of food wrap
packaging xenoestrogens and flavonoids in female Wistar rats: A
comparative study. Reprod Toxicol. 17:421–432. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ji K, Hong S, Kho Y and Choi K: Effects of
bisphenol S exposure on endocrine functions and reproduction of
zebrafish. Environ Sci Technol. 47:8793–8800. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Darbre PD: Endocrine disrupting chemicals
and breast cancer cells. Adv Pharmacol. 92:485–520. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wan MLY, Co VA and El-Nezami H: Endocrine
disrupting chemicals and breast cancer: A systematic review of
epidemiological studies. Crit Rev Food Sci Nutr. 62:6549–6576.
2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Vandenberg LN: Endocrine disrupting
chemicals and the mammary gland. Adv Pharmacol. 92:237–277.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Avagliano A, Granato G, Ruocco MR, Romano
V, Belviso I, Carfora A, Montagnani S and Arcucci A: Metabolic
reprogramming of cancer associated fibroblasts: The slavery of
stromal fibroblasts. Biomed Res Int. 2018(6075403)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Camps JL, Chang SM, Hsu TC, Freeman MR,
Hong SJ, Zhau HE, von Eschenbach AC and Chung LW:
Fibroblast-mediated acceleration of human epithelial tumor growth
in vivo. Proc Natl Acad Sci USA. 87:75–79. 1990.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bissell MJ, Rizki A and Mian IS: Tissue
architecture: The ultimate regulator of breast epithelial function.
Curr Opin Cell Biol. 15:753–762. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kuziel G, Moore BN and Arendt LM: Obesity
and fibrosis: Setting the stage for breast cancer. Cancers (Basel).
15(2929)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ye F, Liang Y, Wang Y, Le Yang R, Luo D,
Li Y, Jin Y, Han D, Chen B, Zhao W, et al: Cancer-associated
fibroblasts facilitate breast cancer progression through exosomal
circTBPL1-mediated intercellular communication. Cell Death Dis.
14(471)2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hu D, Li Z, Zheng B, Lin X, Pan Y, Gong P,
Zhuo W, Hu Y, Chen C, Chen L, et al: Cancer-associated fibroblasts
in breast cancer: Challenges and opportunities. Cancer Commun
(Lond). 42:401–434. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Archer M, Dasari P, Walsh D, Britt KL,
Evdokiou A and Ingman WV: Immune regulation of mammary fibroblasts
and the impact of mammographic density. J Clin Med.
11(799)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Unsworth A, Anderson R and Britt K:
Stromal fibroblasts and the immune microenvironment: Partners in
mammary gland biology and pathology? J Mammary Gland Biol
Neoplasia. 19:169–182. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Thurfjell E: Breast density and the risk
of breast cancer. N Engl J Med. 347(866)2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
McCormack VA and dos Santos Silva I:
Breast density and parenchymal patterns as markers of breast cancer
risk: A meta-analysis. Cancer Epidemiol Biomarkers Prev.
15:1159–1169. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hu Y, Zhang L, Wu X, Hou L, Li Z, Ju J, Li
Q, Qin W, Li J, Zhang Q, et al: Bisphenol A, an environmental
estrogen-like toxic chemical, induces cardiac fibrosis by
activating the ERK1/2 pathway. Toxicol Lett. 250-251:1–9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sprague BL, Trentham-Dietz A, Hedman CJ,
Wang J, Hemming JD, Hampton JM, Buist DS, Aiello Bowles EJ, Sisney
GS and Burnside ES: Circulating serum xenoestrogens and
mammographic breast density. Breast Cancer Res.
15(R45)2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Schedin P and Keely PJ: Mammary gland ECM
remodeling, stiffness, and mechanosignaling in normal development
and tumor progression. Cold Spring Harb Perspect Biol.
3(a003228)2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Koledova Z, Zhang X, Streuli C, Clarke RB,
Klein OD, Werb Z and Lu P: SPRY1 regulates mammary epithelial
morphogenesis by modulating EGFR-dependent stromal paracrine
signaling and ECM remodeling. Proc Natl Acad Sci USA.
113:E5731–E5740. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pupo M, Pisano A, Lappano R, Santolla MF,
De Francesco EM, Abonante S, Rosano C and Maggiolini M: Bisphenol A
induces gene expression changes and proliferative effects through
GPER in breast cancer cells and cancer-associated fibroblasts.
Environ Health Perspect. 120:1177–1182. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wormsbaecher C, Hindman AR, Avendano A,
Cortes-Medina M, Jones CE, Bushman A, Onua L, Kovalchin CE, Murphy
AR, Helber HL, et al: In utero estrogenic endocrine disruption
alters the stroma to increase extracellular matrix density and
mammary gland stiffness. Breast Cancer Res. 22(41)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wadia PR, Cabaton NJ, Borrero MD, Rubin
BS, Sonnenschein C, Shioda T and Soto AM: Low-dose BPA exposure
alters the mesenchymal and epithelial transcriptomes of the mouse
fetal mammary gland. PLoS One. 8(e63902)2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hyun M, Rathor L, Kim HJ, McElroy T, Hwang
KH, Wohlgemuth S, Curry S, Xiao R, Leeuwenburgh C, Heo JD and Han
SM: Comparative toxicities of BPA, BPS, BPF, and TMBPF in the
nematode Caenorhabditis elegans and mammalian fibroblast cells.
Toxicology. 461(152924)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kim JY, Shin GS, Kim CH, Kim MJ, An MJ,
Lee HM and Kim JW: The cytotoxic effects of bisphenol A
alternatives in human lung fibroblast MRC5 cells. Mol Cell Toxicol.
17:267–276. 2021.
|
|
60
|
Li Q and Spalding KL: The regulation of
adipocyte growth in white adipose tissue. Front Cell Dev Biol.
10(1003219)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Vaysse C, Lømo J, Garred Ø, Fjeldheim F,
Lofteroed T, Schlichting E, McTiernan A, Frydenberg H, Husøy A,
Lundgren S, et al: Inflammation of mammary adipose tissue occurs in
overweight and obese patients exhibiting early-stage breast cancer.
NPJ Breast Cancer. 3(19)2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yang M, Chen M, Wang J, Xu M, Sun J, Ding
L, Lv X, Ma Q, Bi Y, Liu R, et al: Bisphenol A promotes adiposity
and inflammation in a nonmonotonic dose-response way in 5-week-old
male and female C57BL/6J mice fed a low-calorie diet.
Endocrinology. 157:2333–2345. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chetrite GS, Cortes-Prieto J, Philippe JC,
Wright F and Pasqualini JR: Comparison of estrogen concentrations,
estrone sulfatase and aromatase activities in normal, and in
cancerous, human breast tissues. J Steroid Biochem Mol Biol.
72:23–27. 2000.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Humphries MP, Jordan VC and Speirs V:
Obesity and male breast cancer: Provocative parallels? BMC Med.
13(134)2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hu Y, Liu L, Chen Y, Zhang X, Zhou H, Hu
S, Li X, Li M, Li J, Cheng S, et al: Cancer-cell-secreted
miR-204-5p induces leptin signalling pathway in white adipose
tissue to promote cancer-associated cachexia. Nat Commun.
14(5179)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gao Y, Chen X, He Q, Gimple RC, Liao Y,
Wang L, Wu R, Xie Q, Rich JN, Shen K and Yuan Z: Adipocytes promote
breast tumorigenesis through TAZ-dependent secretion of Resistin.
Proc Natl Acad Sci USA. 117:33295–33304. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zhu Q, Zhu Y, Hepler C, Zhang Q, Park J,
Gliniak C, Henry GH, Crewe C, Bu D, Zhang Z, et al: Adipocyte
mesenchymal transition contributes to mammary tumor progression.
Cell Rep. 40(111362)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Dirat B, Bochet L, Dabek M, Daviaud D,
Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S,
et al: Cancer-associated adipocytes exhibit an activated phenotype
and contribute to breast cancer invasion. Cancer Res. 71:2455–2465.
2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
De Palma M, Biziato D and Petrova TV:
Microenvironmental regulation of tumour angiogenesis. Nat Rev
Cancer. 17:457–474. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Fujisaki K, Fujimoto H, Sangai T,
Nagashima T, Sakakibara M, Shiina N, Kuroda M, Aoyagi Y and
Miyazaki M: Cancer-mediated adipose reversion promotes cancer cell
migration via IL-6 and MCP-1. Breast Cancer Res Treat. 150:255–263.
2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Todorović-Raković N and Milovanović J:
Interleukin-8 in breast cancer progression. J Interferon Cytokine
Res. 33:563–570. 2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Williams GP and Darbre PD: Low-dose
environmental endocrine disruptors, increase aromatase activity,
estradiol biosynthesis and cell proliferation in human breast
cells. Mol Cell Endocrinol. 486:55–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Linehan C, Gupta S, Samali A and O'Connor
L: Bisphenol A-mediated suppression of LPL gene expression inhibits
triglyceride accumulation during adipogenic differentiation of
human adult stem cells. PLoS One. 7(e36109)2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ahmed F, Sarsenbayeva A, Katsogiannos P,
Aguer C and Pereira MJ: The effects of bisphenol A and bisphenol S
on adipokine expression and glucose metabolism in human adipose
tissue. Toxicology. 445(152600)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Masuno H, Iwanami J, Kidani T, Sakayama K
and Honda K: Bisphenol a accelerates terminal differentiation of
3T3-L1 cells into adipocytes through the phosphatidylinositol
3-kinase pathway. Toxicol Sci. 84:319–327. 2005.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Sargis RM, Johnson DN, Choudhury RA and
Brady MJ: Environmental endocrine disruptors promote adipogenesis
in the 3T3-L1 cell line through glucocorticoid receptor activation.
Obesity (Silver Spring). 18:1283–1288. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ohlstein JF, Strong AL, McLachlan JA,
Gimble JM, Burow ME and Bunnell BA: Bisphenol A enhances adipogenic
differentiation of human adipose stromal/stem cells. J Mol
Endocrinol. 53:345–353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ahmed S and Atlas E: Bisphenol S- and
bisphenol A-induced adipogenesis of murine preadipocytes occurs
through direct peroxisome proliferator-activated receptor gamma
activation. Int J Obes (Lond). 40:1566–1573. 2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Salehpour A, Shidfar F, Hedayati M,
Neshatbini Tehrani A, Farshad AA and Mohammadi S: Bisphenol A
enhances adipogenic signaling pathways in human mesenchymal stem
cells. Genes Environ. 42(13)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Cohen IC, Cohenour ER, Harnett KG and
Schuh SM: BPA, BPAF and TMBPF alter adipogenesis and fat
accumulation in human mesenchymal stem cells, with implications for
obesity. Int J Mol Sci. 22(5363)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Pham DV and Park PH: Adiponectin triggers
breast cancer cell death via fatty acid metabolic reprogramming. J
Exp Clin Cancer Res. 41(9)2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kim Y and Park CW: Mechanisms of
adiponectin action: Implication of adiponectin receptor agonism in
diabetic kidney disease. Int J Mol Sci. 20(1782)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Hugo ER, Brandebourg TD, Woo JG, Loftus J,
Alexander JW and Ben-Jonathan N: Bisphenol A at environmentally
relevant doses inhibits adiponectin release from human adipose
tissue explants and adipocytes. Environ Health Perspect.
116:1642–1647. 2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lim S, Bae JH, Chun EJ, Kim H, Kim SY, Kim
KM, Choi SH, Park KS, Florez JC and Jang HC: Differences in
pancreatic volume, fat content, and fat density measured by
multidetector-row computed tomography according to the duration of
diabetes. Acta Diabetol. 51:739–748. 2014.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kimata K, Sakakura T, Inaguma Y, Kato M
and Nishizuka Y: Participation of two different mesenchymes in the
developing mouse mammary gland: Synthesis of basement membrane
components by fat pad precursor cells. J Embryol Exp Morphol.
89:243–257. 1985.PubMed/NCBI
|
|
86
|
Miyawaki J, Sakayama K, Kato H, Yamamoto H
and Masuno H: Perinatal and postnatal exposure to bisphenol a
increases adipose tissue mass and serum cholesterol level in mice.
J Atheroscler Thromb. 14:245–252. 2007.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Vandenberg LN, Maffini MV, Wadia PR,
Sonnenschein C, Rubin BS and Soto AM: Exposure to environmentally
relevant doses of the xenoestrogen bisphenol-A alters development
of the fetal mouse mammary gland. Endocrinology. 148:116–127.
2007.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Munoz-de-Toro M, Markey C, Wadia PR, Luque
EH, Rubin BS, Sonnenschein C and Soto AM: Perinatal exposure to
bisphenol-A alters peripubertal mammary gland development in mice.
Endocrinology. 146:4138–4147. 2005.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Ramskov Tetzlaff CN, Svingen T, Vinggaard
AM, Rosenmai AK and Taxvig C: Bisphenols B, E, F, and S and
4-cumylphenol induce lipid accumulation in mouse adipocytes
similarly to bisphenol A. Environ Toxicol. 35:543–552.
2020.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Frühbeck G, Méndez-Giménez L,
Fernández-Formoso JA, Fernández S and Rodríguez A: Regulation of
adipocyte lipolysis. Nutr Res Rev. 27:63–93. 2014.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Martínez M, Blanco J, Rovira J, Kumar V,
Domingo JL and Schuhmacher M: Bisphenol A analogues (BPS and BPF)
present a greater obesogenic capacity in 3T3-L1 cell line. Food
Chem Toxicol. 140(111298)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Drobna Z, Talarovicova A, Schrader HE,
Fennell TR, Snyder RW and Rissman EF: Bisphenol F has different
effects on preadipocytes differentiation and weight gain in adult
mice as compared with Bisphenol A and S. Toxicology. 420:66–72.
2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Chernis N, Masschelin P, Cox AR and Hartig
SM: Bisphenol AF promotes inflammation in human white adipocytes.
Am J Physiol Cell Physiol. 318:C63–C72. 2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Leon-Ferre RA, Jonas SF, Salgado R, Loi S,
de Jong V, Carter JM, Nielsen TO, Leung S, Riaz N, Chia S, et al:
Tumor-infiltrating lymphocytes in triple-negative breast cancer.
JAMA. 331:1135–1144. 2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Valenza C, Taurelli Salimbeni B, Santoro
C, Trapani D, Antonarelli G and Curigliano G: Tumor infiltrating
lymphocytes across breast cancer subtypes: Current Issues for
biomarker assessment. Cancers (Basel). 15(767)2023.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Qiu SQ, Waaijer SJH, Zwager MC, de Vries
EGE, van der Vegt B and Schröder CP: Tumor-associated macrophages
in breast cancer: Innocent bystander or important player? Cancer
Treat Rev. 70:178–189. 2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
No authors listed. Estrogen
receptor-positive breast cancer subtypes show differential
macrophage functions. Nat Cancer. 4:450–451. 2023.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Tran HTT, Herz C and Lamy E: Long-term
exposure to ‘low-dose’ bisphenol A decreases mitochondrial DNA copy
number, and accelerates telomere shortening in human CD8 + T cells.
Sci Rep. 10(15786)2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kim H, Kim HS and Moon WK: Comparison of
transcriptome expression alterations by chronic exposure to
low-dose bisphenol A in different subtypes of breast cancer cells.
Toxicol Appl Pharmacol. 385(114814)2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Bouzidi L, Triki H, Charfi S, Kridis WB,
Derbel M, Ayadi L, Sellami-Boudawara T and Cherif B: Prognostic
value of natural killer cells besides tumor-infiltrating
lymphocytes in breast cancer tissues. Clin Breast Cancer.
21:e738–e747. 2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Kim IS, Gao Y, Welte T, Wang H, Liu J,
Janghorban M, Sheng K, Niu Y, Goldstein A, Zhao N, et al:
Immuno-subtyping of breast cancer reveals distinct myeloid cell
profiles and immunotherapy resistance mechanisms. Nat Cell Biol.
21:1113–1126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Balistrieri A, Hobohm L, Srivastava T,
Meier A and Corriden R: Alterations in human neutrophil function
caused by bisphenol A. Am J Physiol Cell Physiol. 315:C636–C642.
2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Palacios-Arreola MI, Nava-Castro KE,
Río-Araiza VHD, Pérez-Sánchez NY and Morales-Montor J: A single
neonatal administration of Bisphenol A induces higher tumour weight
associated to changes in tumour microenvironment in the adulthood.
Sci Rep. 7(10573)2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Kim H, Kim HS, Piao YJ and Moon WK:
Bisphenol A promotes the invasive and metastatic potential of
ductal carcinoma in situ and protumorigenic polarization of
macrophages. Toxicol Sci. 170:283–295. 2019.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Pahović PŠ, Iulini M, Maddalon A, Galbiati
V, Buoso E, Dolenc MS and Corsini E: In vitro effects of bisphenol
analogs on immune cells activation and Th differentiation. Endocr
Metab Immune Disord Drug Targets. 23:1750–1761. 2023.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Oshi M, Gandhi S, Yan L, Tokumaru Y, Wu R,
Yamada A, Matsuyama R, Endo I and Takabe K: Abundance of reactive
oxygen species (ROS) is associated with tumor aggressiveness,
immune response, and worse survival in breast cancer. Breast Cancer
Res Treat. 194:231–241. 2022.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Qiu W, Shao H, Lei P, Zheng C, Qiu C, Yang
M and Zheng Y: Immunotoxicity of bisphenol S and F are similar to
that of bisphenol A during zebrafish early development.
Chemosphere. 194:1–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Dontu G and Ince TA: Of mice and women: A
comparative tissue biology perspective of breast stem cells and
differentiation. J Mammary Gland Biol Neoplasia. 20:51–62.
2015.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Cardiff RD and Wellings SR: The
comparative pathology of human and mouse mammary glands. J Mammary
Gland Biol Neoplasia. 4:105–122. 1999.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Roberts GC, Morris PG, Moss MA, Maltby SL,
Palmer CA, Nash CE, Smart E, Holliday DL and Speirs V: An
evaluation of matrix-containing and humanised matrix-free
3-dimensional cell culture systems for studying breast cancer. PLoS
One. 11(e0157004)2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Russo J and Russo IH: Genotoxicity of
steroidal estrogens. Trends Endocrinol Metab. 15:211–214.
2004.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Sachs N and Clevers H: Organoid cultures
for the analysis of cancer phenotypes. Curr Opin Genet Dev.
24:68–73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Reddington R, Galer M, Hagedorn A, Liu P,
Barrack S, Husain E, Sharma R, Speirs V and Masannat Y: Incidence
of male breast cancer in Scotland over a twenty-five-year period
(1992-2017). Eur J Surg Oncol. 46:1546–1550. 2020.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Roberts S, Peyman S and Speirs V: Current
and emerging 3D models to study breast cancer. Adv Exp Med Biol.
1152:413–427. 2019.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Weigelt B, Ghajar CM and Bissell MJ: The
need for complex 3D culture models to unravel novel pathways and
identify accurate biomarkers in breast cancer. Adv Drug Deliv Rev.
69-70:42–51. 2014.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Tang M, Jiang S, Huang X, Ji C, Gu Y, Qi
Y, Xiang Y, Yao E, Zhang N, Berman E, et al: Integration of 3D
bioprinting and multi-algorithm machine learning identified glioma
susceptibilities and microenvironment characteristics. Cell Discov.
10(39)2024.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Huh D, Hamilton GA and Ingber DE: From 3D
cell culture to organs-on-chips. Trends Cell Biol. 21:745–754.
2011.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Ståhl PL, Salmén F, Vickovic S, Lundmark
A, Navarro JF, Magnusson J, Giacomello S, Asp M, Westholm JO, Huss
M, et al: Visualization and analysis of gene expression in tissue
sections by spatial transcriptomics. Science. 353:78–82.
2016.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Reed AD, Pensa S, Steif A, Stenning J,
Kunz DJ, Porter LJ, Hua K, He P, Twigger AJ, Siu AJQ, et al: A
single-cell atlas enables mapping of homeostatic cellular shifts in
the adult human breast. Nat Genet. 56:652–662. 2024.PubMed/NCBI View Article : Google Scholar
|
|
121
|
EFSA Panel on Food Contact Materials,
Enzymes Flavourings and Processing Aids (CEF). Scientific opinion
on the risks to public health related to the presence of bisphenol
A (BPA) in foodstuffs. EFSA J. 13(3978)2015.
|
|
122
|
Dueñas-Moreno J, Mora A, Kumar M, Meng XZ
and Mahlknecht J: Worldwide risk assessment of phthalates and
bisphenol A in humans: The need for updating guidelines. Environ
Int. 181(108294)2023.PubMed/NCBI View Article : Google Scholar
|