Introduction
The majority of types of cancer, including breast
cancer, result from genetic and epigenetic alterations that disrupt
normal cellular signaling, leading to uncontrolled proliferation
and survival (1-3).
Key pathways involved in breast cancer progression include the
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian
target of rapamycin (mTOR) and mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase (ERK) signaling
cascades, which not only drive tumorigenesis, but also contribute
to chemotherapeutic resistance (4,5).
These pathways regulate cell cycle progression by stimulating
cyclin D1, a critical protein that promotes cell division (6). The cell cycle, which consists of four
phases (G0/G1, S, G2 and M) (7),
is regulated by cyclins and cyclin-dependent kinases (CDKs) that
form cyclin/CDK complexes (8).
This process is negatively regulated by CDK inhibitors, such as
p21, which suppress cyclin/CDK activity (9). The dysregulation of p21 or the
overexpression of cyclins and CDKs can lead to uncontrolled cell
proliferation, a hallmark of cancer (8). Notably, cyclin D1 and CDK4/6 are
overexpressed in breast cancer, along with elevated levels of the
proliferation marker, Ki-67(10).
Ki-67 levels rapidly decrease during cancer cell apoptosis
following treatment with chemotherapy (11). However, the adverse effects of
chemotherapy on normal cells often limit its therapeutic success
(12).
Natural products are increasingly being explored for
their anticancer potential due to their fewer adverse effects.
Sulfated galactan (SG), a polysaccharide isolated from the red
seaweed, Gracilaria fisheri (G. fisheri), has been
shown to induce cell cycle arrest in cholangiocarcinoma (CCA) by
downregulating cyclin and CDK expression, while upregulating tumor
suppressors, such as p21 and p53(13). It has also been reported to
suppress CCA cell invasion and migration (14). Additionally, chemical
modifications, such as reducing molecular weight and substituting
functional groups, have been found to enhance the anticancer
properties of polysaccharides (15). In a recent study, modified SG from
G. fisheri with low molecular weight (LSG), supplemented
with an octanoyl ester (LSGO), was found to exhibit an enhanced
wound healing activity (16).
However, the effects of SG and its derivatives, including LSG and
LSGO, on breast cancer remain unexplored. Therefore, the present
study aimed to evaluate the anticancer activities of SG and its
derivatives in the MCF-7 breast cancer cell line. The mechanisms by
which SG and its derivatives affect MCF-7 cell proliferation, cell
cycle regulation, protein expression and mRNA expression were also
investigated.
Materials and methods
Cell lines and cell culture
Human mammary gland epithelial adenocarcinoma
(MCF-7; cat. no. HTB-22) and normal fibroblast (L929; cat. no.
CCL-1) cell lines were purchased from the American Type Culture
Collection (ATCC). MCF-7 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco™, Thermo Fisher Scientific, Inc.)
containing L-glutamine, pyridoxine hydrochloride and sodium
bicarbonate (NaHCO3), supplemented with 10% fetal bovine
serum (FBS; Invitrogen, Thermo Fisher Scientific, Inc.) and 1%
antibiotic-antimycotic (penicillin/streptomycin; Invitrogen, Thermo
Fisher Scientific, Inc.). L929 cells were cultured in minimum
essential medium (MEM; Gibco™, Thermo Fisher Scientific, Inc.),
which contains L-glutamine and phenol red, supplemented with 10%
FBS and 1% antibiotic-antimycotic. Both MCF-7 and L929 cells were
maintained under standard conditions at 37˚C in a humidified
atmosphere of 5% CO2.
Evaluation of the cytotoxicity of SG
and its derivatives in MCF-7 and L929 cells
SG, LSG and LSGO were prepared as previously
described by Rudtanatip et al (16). MTT assay was used to assess the
cytotoxicity of SG, LSG and LSGO in MCF-7 and L929 cell cultures.
MCF-7 and L929 cells were seeded into 96-well plates at a density
of 2x104 cells/well and allowed to attach for 24 h under
standard incubator conditions. The cells were then treated with
various concentrations of SG, LSG and LSGO (0, 125, 250, 500 and
1,000 µg/ml), as well as paclitaxel (PTX; Intaxel® 6
mg/ml paclitaxel injection, Fresenius Kabi) and gemcitabine (GEM;
gemcitabine hydrochloride, Supelco, MilliporeSigma) (0, 0.2, 0.4,
0.6, 0.8 and 1 µg/ml) for 48 h. PTX and GEM served as the positive
controls, while untreated cells were used as the normal control.
Following the treatment period, 20 µl MTT solution (5 mg/ml;
MilliporeSigma) were added to each well and incubated for 3 h at
37˚C. The medium was then removed, and 100 µl dimethyl sulfoxide
(DMSO; RCI Labscan™ Limited) were added to dissolve the purple
formazan crystals. The plate was shaken on a microplate shaker for
20 min, and the absorbance was measured at 540 nm using a
microplate reader (Varioskan® LUX, cat. no. N16044;
Thermo Fisher Scientific, Inc.). The results were reported as
IC50 values, as previously described (13). The concentrations of SG and its
derivatives, as well as those of PTX and GEM used in the present
study were based on a previous study by the authors and other
relevant studies (16-18).
Evaluation of cell morphology
The morphology of the MCF-7 and L929 cells treated
with PTX, GEM, SG, LSG and LSGO was also examined. The MCF-7 and
L929 cells (6x104 cells/well) were cultured in a 24-well
plate for 24 h, and the cells were divided into six groups as
follows: i) The normal control (NC), no treatment; ii) PTX, cells
were treated with 0.5 µg/ml PTX; iii) GEM, cells were treated with
0.5 µg/ml GEM; iv) SG, cells were treated with 500 µg/ml SG; v)
LSG, cells were treated with 500 µg/ml LSG; and vi) LSGO, cells
were treated with 500 µg/ml LSGO. The cells were treated with 2 ml
of the respective solution and incubated for 48 h at 37˚C before
observing cell morphology under a Nikon ECLIPSE TS100 inverted
microscope (Nikon Corporation). Cell counts were analyzed in three
randomly selected fields at x200 magnification using ImageJ
software version 1.54g (National Institutes of Health).
A concentration of 500 µg/ml was selected for use in
subsequent assays, as both 500 and 1,000 µg/ml exerted comparable
moderate effects on cell viability without reaching the
IC50 value. The results of MTT assay confirmed that 500
µg/ml produced similar outcomes to 1,000 µg/ml, supporting its use
in further experiments.
Evaluation of the anti-proliferative
effect of SG and its derivatives on MCF-7 cells
The results of cytotoxicity assay indicated that
although SG and its derivatives were not markedly cytotoxic to the
MCF-7 cells, they reduced cell numbers by 20% compared to the
control. This suggests that SG and its derivatives may inhibit
MCF-7 cell proliferation, as previously reported (13). The anti-proliferative effects of SG
and its derivatives were further investigated.
Experimental design
The MCF-7 cells were cultured and divided into six
groups as follows: i) NC, no treatment; ii) PTX, cells were treated
with 0.5 µg/ml paclitaxel; iii) GEM, cells were treated with 0.5
µg/ml GEM; iv) SG, cells were treated with 500 µg/ml SG; v) LSG,
cells were treated with 500 µg/ml LSG; and vi) LSGO, cells were
treated with 500 µg/ml LSGO.
Hoechst/propidium iodide (PI) dual
staining
The cytotoxic effects of SG and its derivatives were
further confirmed using Hoechst/PI dual staining. The MCF-7 cells
were plated on round coverslips in a 24-well plate at a density of
6x104 cells/well in DMEM and incubated for 24 h at 37˚C.
Following incubation, the cells were treated with the respective
solution for 48 h. The cells were then washed with PBS and stained
with Hoechst (Merck KGaA) and PI (BioChemica, PanReac AppliChem) at
a 1:1 ratio for 30 min at room temperature. After staining, the
cells were washed three times with PBS in the dark. The coverslips
containing the stained cells were then mounted on glass slides with
a droplet of anti-fade solution. Finally, the cells were observed
under a confocal microscope (ZEISS LSM 800, Carl Zeiss AG), as
previously described (16). The
laser intensity wavelengths were 405 nm with a pinhole size of 1.18
AU/45 µm for Hoechst staining and 561 nm with a pinhole size of
0.90 AU/47 µm for PI staining. PI fluorescent intensity was
measured in three randomly selected fields at a x200 total
magnification using ImageJ software version 1.54g (National
Institutes of Health).
Immunofluorescence staining of cell
proliferation using the Ki-67 biomarker
The MCF-7 cells seeded on round coverslips in a
24-well plate were treated with the respective solution for 48 h.
Following treatment, the cells were fixed with 4% paraformaldehyde
in 0.1% Triton X-100 at room temperature for 15 min and were then
washed three times with PBS. Subsequently, the cells were blocked
with 5% bovine serum albumin (BSA; Capricorn Scientific GmbH) for
30 min at room temperature. After blocking, the cells were washed
with PBS and incubated overnight at 4˚C with a primary antibody
specific to mouse anti-Ki-67 (dilution 1:500; cat no. 14569982;
Invitrogen, Thermo Fisher Scientific, Inc.). The following day, the
cells were incubated with a fluorescent-labeled secondary antibody
(goat anti-mouse FITC-conjugated; dilution 1:500; cat no. AP308F;
Merck Millipore) for 1 h, at room temperature in the dark.
Following incubation, the cells were washed with PBS in the dark
and counterstained with Cell Mask™ Deep Red plasma membrane stain
(dilution 1:500; cat no. C10066; Invitrogen, Thermo Fisher
Scientific, Inc.) for 20 min at room temperature. A total of 5 µl
of antifade solution conjugated with DAPI dye (ProLong™ Diamond
Antifade Mountant with DAPI, Invitrogen, Thermo Fisher Scientific,
Inc.) was then added to a glass slide. The round coverslips
containing the cells were then placed on the glass slide with a
droplet of antifade solution. Finally, the fluorescence intensity
of Ki-67 was observed using a confocal microscope (ZEISS LSM 800,
Carl Zeiss AG), as previously described (19).
Evaluation of cell cycle arrest using
flow cytometry
A flow cytometry cell cycle arrest assay was used to
evaluate the mechanisms of action of SG and its derivatives. The
MCF-7 cells were seeded in a six-well plate at a density of
1.5x105 cells/well and allowed to adhere overnight. The
cells were then treated with PTX, GEM, SG, LSG and LSGO solutions
for 48 h. Following treatment, all adherent cells were harvested by
trypsinization, transferred to sterile Eppendorf tubes, and
centrifuged at 2,795 x g at 4˚C for 5 min to pellet the cells. The
cell pellets were washed three times with cold PBS and centrifuged
at 2,795 x g at 4˚C for 5 min. The supernatant was discarded, and
ice-cold 70% ethanol was added to fix the cells. The pellets were
then resuspended to obtain a single-cell suspension and incubated
at -20˚C for 1 week. Following incubation, the cell pellets were
washed three times with PBS, and the supernatant was removed.
Nuclei were stained with PI/RNase staining solution (FxCycle™
PI/RNase, Thermo Fisher Scientific, Inc.) and incubated for 40 min
in the dark at room temperature. Flow cytometric analysis was
performed using a FACSCanto II flow cytometer (Model FACSCanto II,
BD Biosciences). The percentage of cells in each phase of the cell
cycle was then analyzed (20).
Analysis of mRNA expression using
reverse transcription-quantitative PCR (RT-qPCR)
TRIzol reagent (200 µl; MilliporeSigma) was used for
RNA extraction. RNA purity and concentration were determined by
measuring the absorbance ratio at 260/280 nm using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). Complementary
DNA (cDNA) was synthesized from 1 µg RNA using the RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) through
incubation at 42˚C for 60 min, followed by heating at 70˚C for 5
min. Subsequently, mRNA expression was analyzed using qPCR with the
synthesized cDNA and PCR Master Mix (Molecular Biology, Thermo
Fisher Scientific, Inc.), and forward and reverse primers. The
cycling conditions were as follows: 50˚C for 2 min, 95˚C for 10
min, followed by 40 cycles of 95˚C for 15 sec, 60˚C for 30 sec and
72˚C for 30 sec. Data quantification calculated using the
2-ΔΔCq method (21) was
performed using the Bio-Rad CFX Maestro software (Bio-Rad
Laboratories, Inc.). The primers used in the present study included
Ki-67, PI3K, AKT, mTOR, cyclin D1, CDK4, cyclin A, CDK2, p21, ERK
1/2 and GAPDH (Bionics). Forward and reverse primer sequences were
designed using NCBI/Primer-Blast, and their sequences are provided
in Table I.
 | Table INucleotide sequences of specific
primers used for mRNA expression. |
Table I
Nucleotide sequences of specific
primers used for mRNA expression.
| Gene names | Primers | Nucleotide
sequences (5' to 3') |
|---|
| Ki-67 | Forward |
GAAAGAGTGGCAACCTGCCTTC |
| | Reverse |
GCACCAAGTTTTACTACATCTGCC |
| PI3K | Forward |
AACACAGAAGACCAATACTC |
| | Reverse |
TTCGCCATCTACCACTAC |
| AKT | Forward |
TCTATGGCGCTGAGATTGTG |
| | Reverse |
CTTAATGTGCCCGTCCTTGT |
| mTOR | Forward |
GCTTGATTTGGTTCCCAGGAC |
| | Reverse |
GTGCTGAGTTTGCTGTACCCA |
| Cyclin D1 | Forward |
GCATGTTCGTGGCCTCTAAG |
| | Reverse |
CGTGTTTGCGGATGATCTGT |
| CDK4 | Forward |
TGAGGGTCTCCCTTGATCTGAG |
| | Reverse |
AGGGATACATCTCGAGGCCA |
| p21 | Reverse |
GCTTCATGCCAGCTACTTCC |
| | Forward |
CCCTTCAAAGTGCCATCTGT |
| Cyclin A | Forward |
GTCAGAGAGGGGATGGCAT |
| | Reverse |
CCAGTCCACCAGAATCGTG |
| CDK2 | Forward |
GAATCTCCAGGGAATAGGGC |
| | Reverse |
CTGAAATCCTCCTGGGCTG |
| ERK1/2 | Forward |
TGGCAAGCACTACCTGGATCAG |
| | Reverse |
GCAGAGACTGTAGGTAGTTTCGG |
| GAPDH | Reverse |
GGTGAAGGTCGGTGTGAACG |
| | Forward |
CTCGCTCCTGGAAGATGGTG |
Analysis of protein expression
The expression of proteins involved in cell
proliferation was determined after the MCF-7 cells were treated for
48 h using western blot analysis. Cells were harvested for protein
extraction. The protein concentration was quantified using a
NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.),
and 50 µg protein samples were separated by electrophoresis using
12% sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE). The separated proteins were then transferred onto a
nitrocellulose membrane (Amersham™ Protran™ Premium 0.45 µm NC
nitrocellulose western blotting membranes), which was blocked with
4% BSA for 1 h at room temperature. Each membrane was probed with
primary antibodies (dilution 1:1,000) against mouse anti-Ki-67
(cat. no. 14569982; Invitrogen, Thermo Fisher Scientific, Inc.),
rabbit anti-PI3K (cat. no. 4255S; Cell Signaling Technology, Inc.),
rabbit anti-phosphorylated (p)-AKT (cat. no. sc-135651; Santa Cruz
Biotechnology, Inc.), rabbit anti-AKT (cat. no. 8596S; Cell
Signaling Technology, Inc.), rabbit anti-p-mTOR (cat. no. 5536S;
Cell Signaling Technology Inc.), rabbit anti-mTOR (cat. no. 2972S;
Cell Signaling Technology Inc.), mouse anti-cyclin D1 (cat. no.
AHF0082; Invitrogen, Thermo Fisher Scientific, Inc.), mouse
anti-CDK4 (cat. no. AHZ0202; Invitrogen, Thermo Fisher Scientific,
Inc.), rabbit anti-p21 (cat. no. E2R7A; Cell Signaling Technology
Inc.), rabbit anti-cyclin A (cat. no. AF0142; Affinity
Biosciences), rabbit anti-CDK2 (cat. no. MA5-17052; Invitrogen,
Thermo Fisher Scientific, Inc.), mouse anti-ERK1/2 (cat. no. 9102S;
Cell Signaling Technology Inc.) and rabbit anti-β-actin (cat. no.
AF7018; Affinity Biosciences) and incubated overnight at 4˚C. The
membranes were then incubated with a secondary antibody conjugated
with horseradish peroxidase (dilution 1:2,000; cat. no. 31460 for
anti-rabbit antibody and cat. no. 626520 for anti-mouse antibody;
Invitrogen, Thermo Fisher Scientific, Inc.) at room temperature for
1 h. Following incubation, the membranes were washed with
Tris-buffered saline-Tween 20 (TBS-T) solution, and
immunoreactivity was enhanced using a chemiluminescence ECL-Western
blotting substrate kit (Clarity™ Western ECL substrate, Bio-Rad,
USA). Finally, protein bands were detected using the ChemiDoc Touch
imaging system (Amersham™ ImageQuant™ 800 biomolecular imager,
Cytiva). Relative protein expression was quantified using ImageJ
software version 1.32j (National Institutes of Health) with band
intensities standardized to β-actin (22).
Statistical analysis
All experiments were performed in triplicate, and
data are presented as the mean ± SEM. Statistical analysis was
conducted using one-way ANOVA followed by Tukey's multiple
comparisons test using GraphPad Prism software version 5
(Dotmatics).
Results
Effects of SG and its derivatives on
the viability of MCF-7 and L929 cells
The cytotoxic effects of SG and its derivatives on
MCF-7 breast cancer and L929 normal fibroblast cells were evaluated
and expressed as a percentage of the control following 48 h of
exposure at concentrations of 125, 250, 500 and 1,000 µg/ml. The
results revealed that at 500 and 1,000 µg/ml, MCF-7 cell viability
significantly decreased from 100 to 80% (Fig. 1A), while L929 cell viability
exhibited no significant differences between the groups (Fig. 1B). However, the IC50
values of SG, LSG and LSGO in the MCF-7 cells were calculated to be
3,027, 2,416 and 1,757 µg/ml, respectively. Additionally, the
anticancer drugs, PTX and GEM, were used as positive controls to
compare the cytotoxic effects. PTX and GEM significantly reduced
the viabilities of both the MCF-7 and L929 cells, with low
IC50 values of 0.54 and 0.57 µg/ml, respectively
(Fig. 1C and D). Cell morphology observed under a phase
contrast microscope revealed that the MCF-7 and L929 cells treated
with PTX and GEM (Fig. 1E and
F) exhibited characteristic
cellular changes, including shrinkage, membrane bleb formation and
the loss of normal shape. Notably, the L929 cells treated with SG
and its derivatives did not exhibit any noticeable changes compared
to the control. Moreover, the quantitative cell counts of MCF-7 and
L929 cells following treatment, shown in Fig. 1G and H, were consistent with the results of MTT
assay. These findings suggest a selective effect of the compounds
on cancer cells without affecting normal cells.
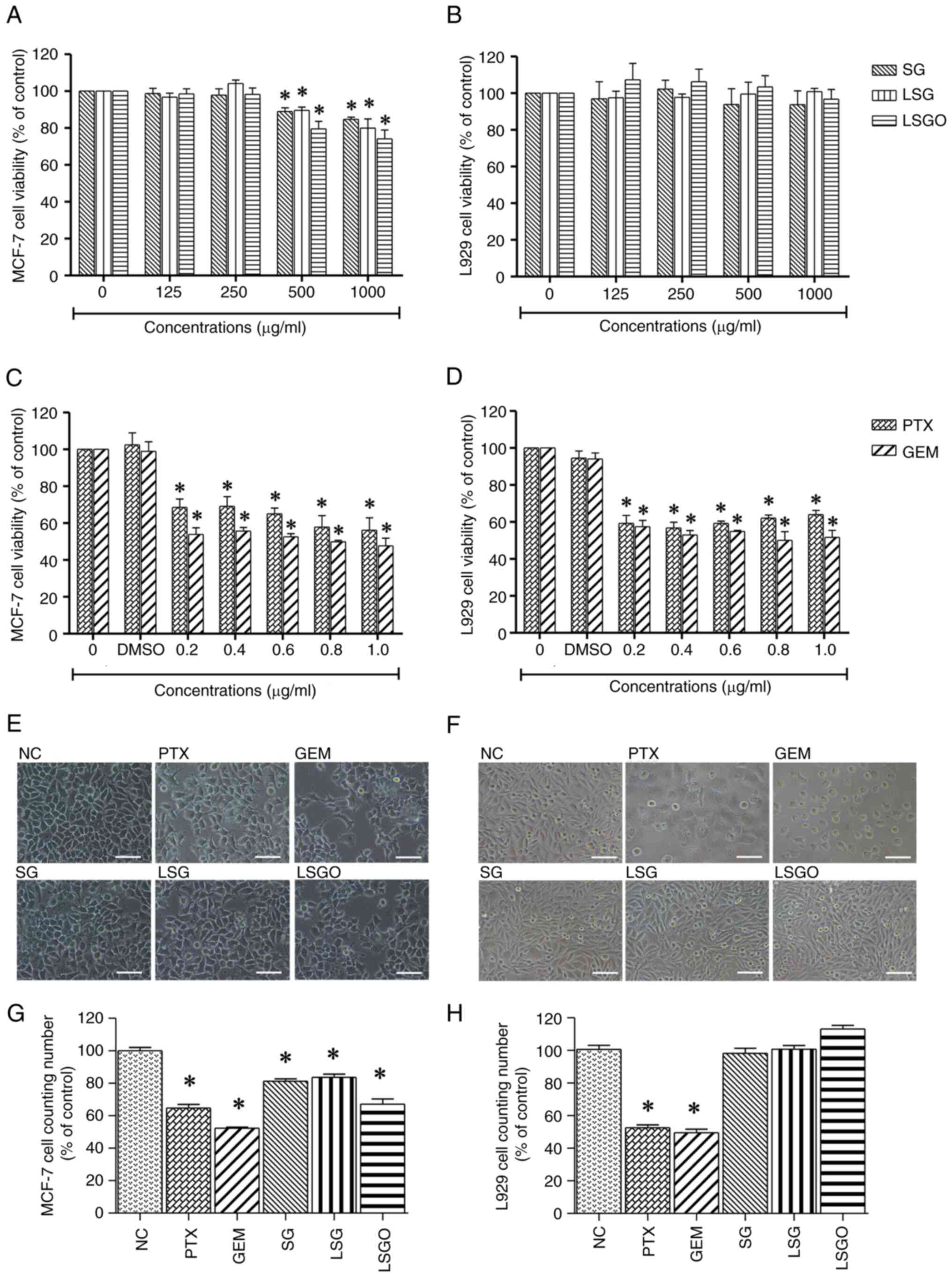 | Figure 1Effects of PTX and GEM (at
concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 µg/ml) and SG, LSG and
LSGO (at concentrations of 125, 250 500, and 1,000 µg/ml) on the
viability of MCF-7 breast cancer cells and L929 normal fibroblast
cells, as assessed using MTT assay. (A) Viability of MCF-7 cells
treated with SG, LSG and LSGO. (B) Viability of L929 cells treated
with SG, LSG and LSGO. (C) Viability of MCF-7 cells treated with
PTX and GEM. (D) Viability of L929 cells treated with PTX and GEM.
(E) Phase-contrast images illustrating morphological changes in
MCF-7 cells following treatment with PTX, GEM, SG, LSG and LSGO.
(F) Phase-contrast images illustrating morphological changes in
L929 cells following treatment with PTX, GEM, SG, LSG and LSGO.
Scale bars, 100 µm. (G) Quantitative cell counts of MCF-7 cells
treated with PTX, GEM, SG, LSG and LSGO. (H) Quantitative cell
counts of L929 cells treated with PTX, GEM, SG, LSG and LSGO. The
results are presented as the mean±SEM (n=3) from three independent
experiments. *P<0.05, statistically significant
difference compared to the normal control at a 95% confidence
level. PTX, paclitaxel; GEM, gemcitabine; SG, sulfated galactan;
LSG low molecular weight SG; and LSGO, octanoyl ester-supplemented
SG. |
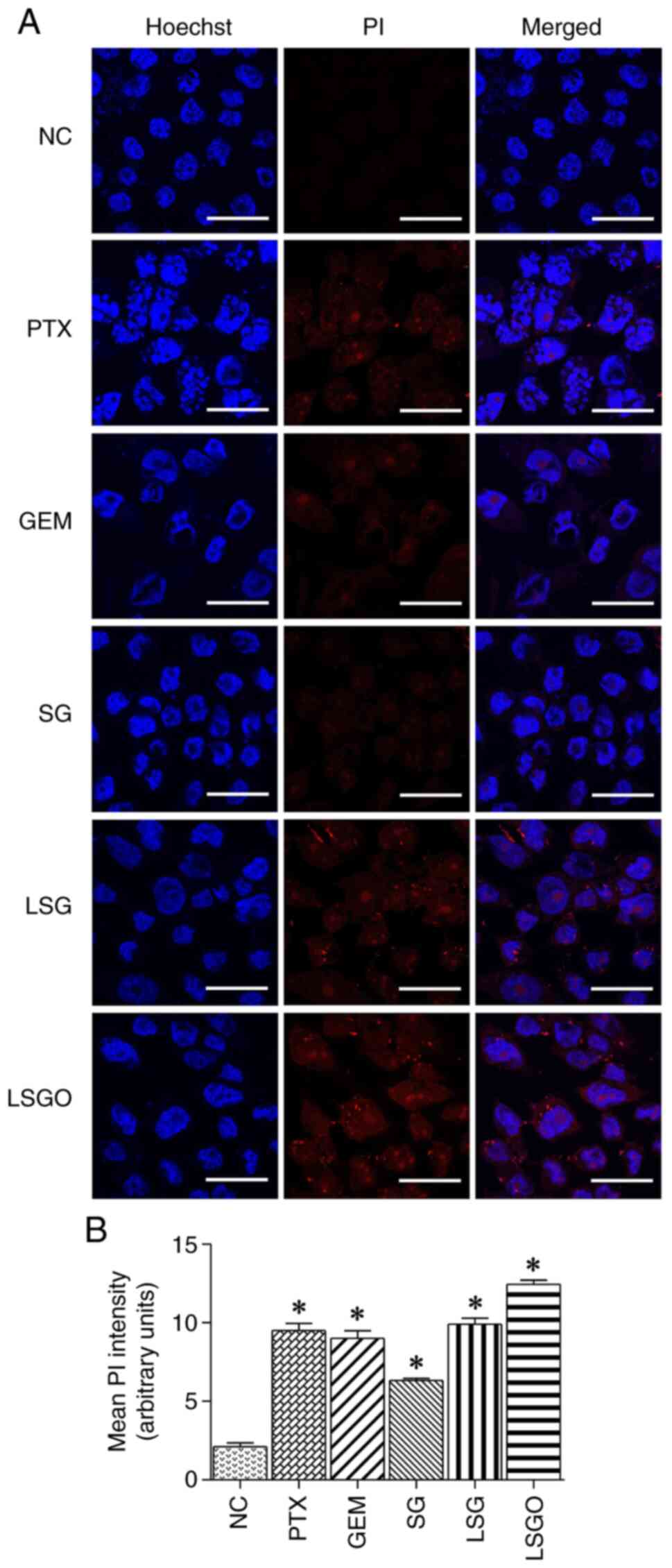
Determination of the death and
proliferation of MCF-7 cells caused by SG and its derivatives
The decreased viability of the MCF-7 breast cancer
cells following treatment with SG and its derivatives may be
associated with cell death and/or inhibited cell proliferation. To
investigate this, immunofluorescence staining was performed using
Hoechst/PI dual staining and the Ki-67 biomarker. The results
revealed that the cells treated with 500 µg/ml SG and its
derivatives exhibited a slight increase in fluorescent PI intensity
compared to the normal control, consistent with the effects
observed in the PTX- and GEM-treated cells (Fig. 2). By contrast, treatment with 500
µg/ml SG and its derivatives resulted in a significant reduction in
the fluorescent Ki-67 intensity compared to the normal control,
aligning with the results observed in the PTX- and GEM-treated
cells (Fig. 3A). Additionally, the
mRNA and protein expression levels of Ki-67 were significantly
decreased in the MCF-7 cells treated with SG derivatives,
particularly LSGO (Fig. 3B and
C), corresponding to the effects
observed with PTX and GEM. These findings suggest that SG and its
derivatives exert anti-proliferative effects on MCF-7 cells.
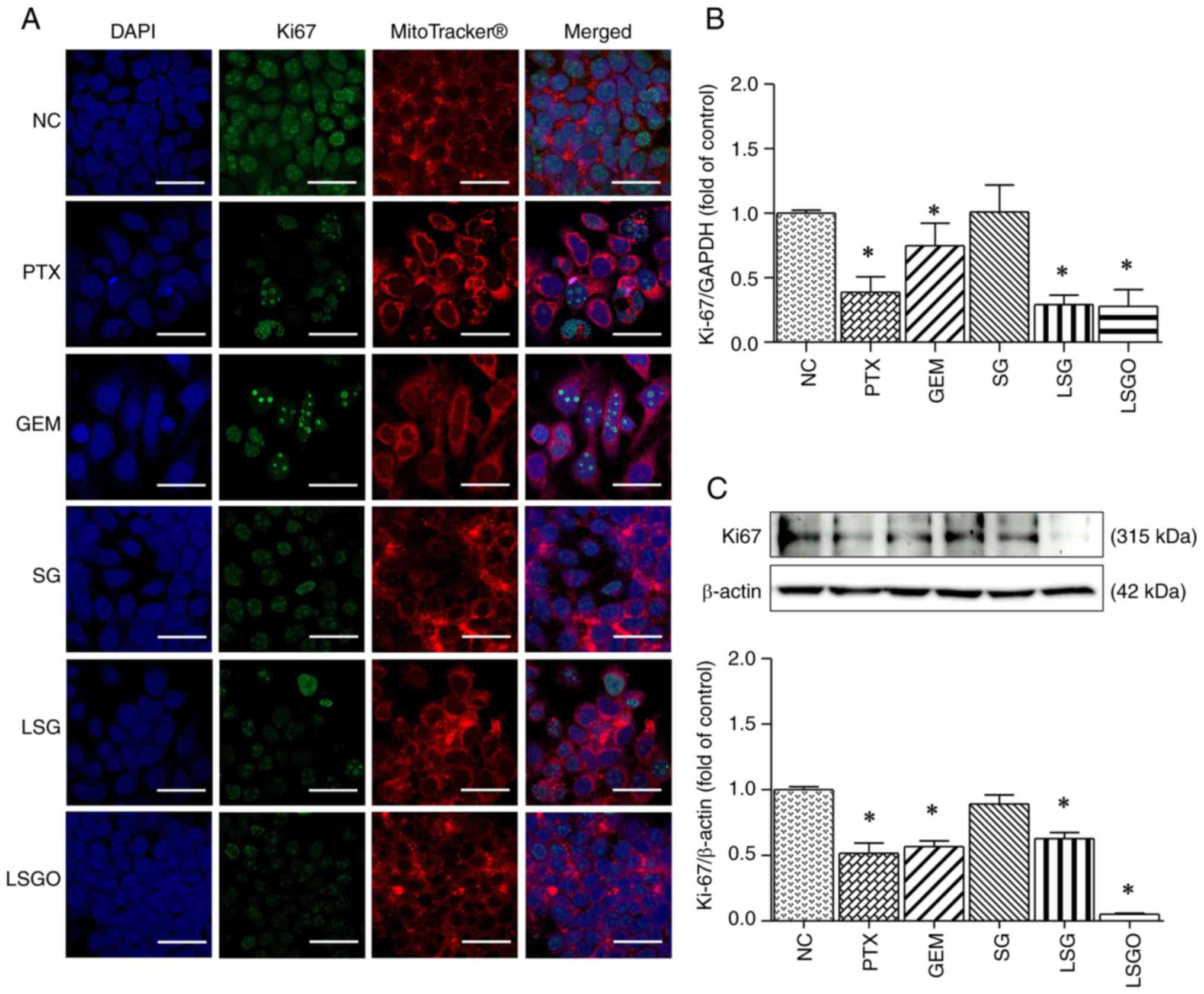 | Figure 3Expression levels of Ki-67 in MCF-7
cells following treatment with PTX, GEM, SG, LSG and LSGO. (A)
Immunofluorescence confocal micrographs illustrating Ki-67
expression in MCF-7 cells. Ki-67 protein is shown in green, nuclei
were stained with DAPI (blue), and the cell plasma membrane was
stained with MitoTracker® Deep Red (red). Scale bars, 20
µm. (B) mRNA expression levels of Ki-67 in MCF-7 cells, assessed
using reverse transcription-quantitative PCR. (C) Protein
expression levels of Ki-67 in MCF-7 cells, assessed using western
blot analysis. The results are presented as the mean ± SEM (n=3)
from three independent experiments. *P<0.05,
statistically significant difference compared to the normal control
at a 95% confidence level. PTX, paclitaxel; GEM, gemcitabine; SG,
sulfated galactan; LSG low molecular weight SG; and LSGO, octanoyl
ester-supplemented SG. |
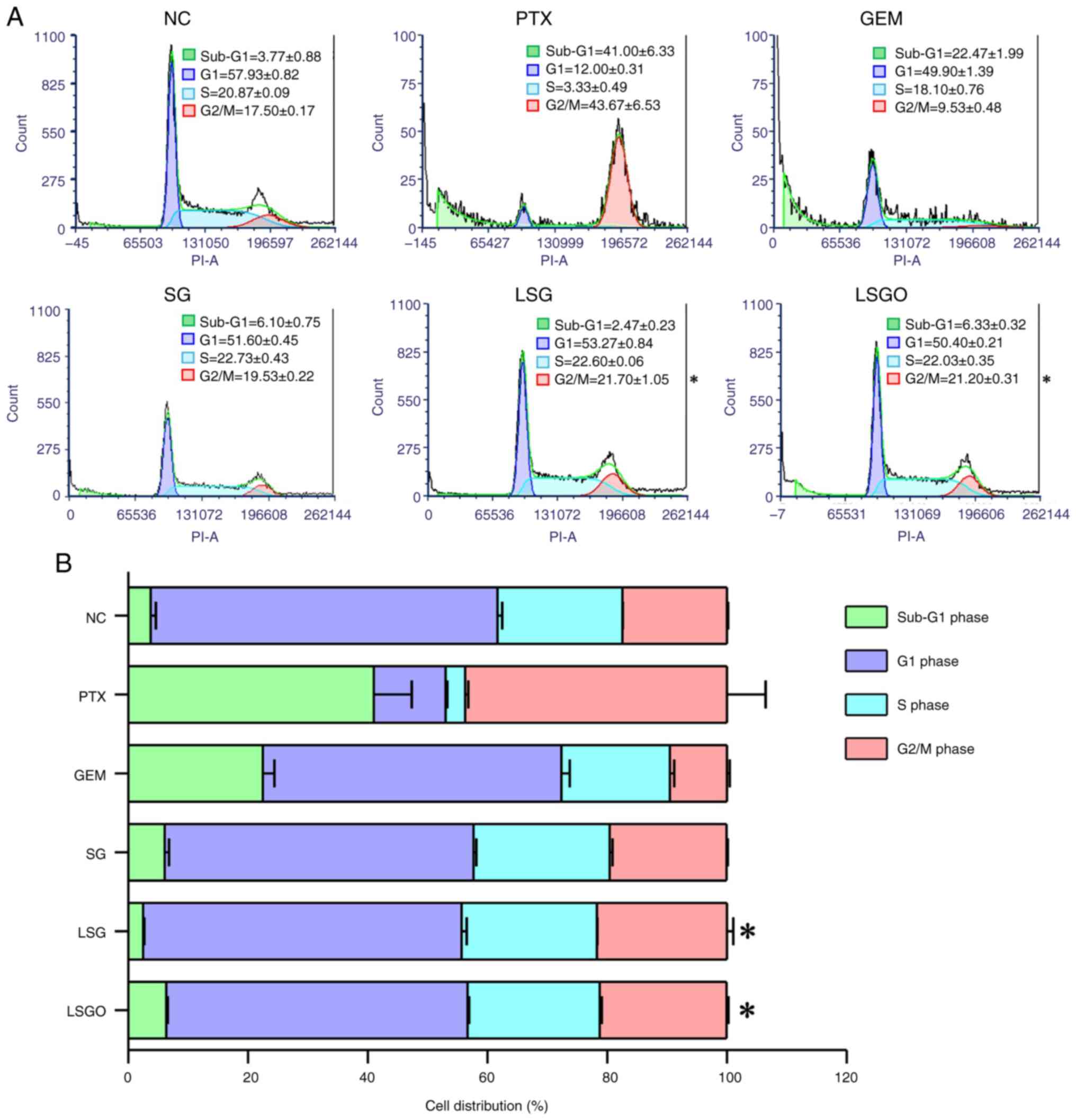
SG derivatives promote MCF-7 cell
cycle arrest
The present study further investigated the
anti-proliferative effects of SG and its derivatives on MCF-7 cells
by analyzing the DNA content across different cell cycle phases
using PI staining. Flow cytometry profiles of the nuclear DNA
content revealed alterations in cell population distribution across
phases following treatment with SG and its derivatives compared to
the normal control (untreated cells), as shown in Fig. 4. In the normal control, the cells
in the sub-G1/G1 phase accounted for 61.70%, the S phase for 20.87%
and the G2/M phase for 17.50%. However, the cells treated with SG
and its derivatives exhibited a decrease in the number of cells in
the sub-G1/G1 phase, a slight increase in the number of cells in
the S phase, and a significant increase in the number of cells in
the G2/M phase compared to the normal control. Specifically, in the
SG group, the number of cells in the sub-G1/G1, S and G2/M phases
were 57.70, 22.73 and 19.53%, respectively. In the LSG group, the
number of cells in the sub-G1/G1, S and G2/M phases were 55.74,
22.6 and 21.70%, respectively, while in the LSGO group, the number
of cells in the sub-G1/G1, S and G2/M phases were 56.73, 22.03 and
21.20%, respectively. This increase in G2/M phase arrest was
consistent with the results observed in the PTX-treated group.
Additionally, the analysis of GEM-treated cells revealed a higher
percentage of cells in the sub-G1/G1 phase compared to the other
phases. These findings suggest that SG derivatives may effectively
inhibit MCF-7 cell proliferation by inducing cell cycle arrest at
the G2/M phase.
Decreased expression of mRNA and
proteins involved in MCF-7 cell cycle arrest caused by SG
derivatives
The expression of key mRNA and proteins involved in
tumor cell proliferation in MCF-7 cells, including those in the
PI3K/Akt/mTOR, MAPK and CDK pathways, was assessed following
treatment with SG and its derivatives using RT-qPCR and western
blot analysis. Compared to the controls, the expression levels of
PI3K/Akt/mTOR, MAPK and CDKs were altered in the cells treated with
SG and its derivatives, in accordance with the effects observed in
the PTX- and GEM-treated groups. The results of RT-qPCR revealed
that the mRNA expression levels of PI3K, AKT, mTOR, cyclin D1,
CDK4, cyclin A, CDK2 and ERK were significantly decreased in the
cells treated with LSGO compared to the normal control (Fig. 5). Similarly, as demonstrated in
Fig. 6, the protein expression
levels of PI3K, p-AKT/AKT, p-mTOR/mTOR, cyclin D1, CDK4, cyclin A,
CDK2 and ERK were significantly decreased in the cells treated with
LSGO compared to the NC. However, the expression levels of some
proteins (p-mTOR/mTOR, CDK2 and ERK) were unaltered in the cell
treated with SG and LSG. Of note, p21 expression remained unaltered
at both the mRNA and protein levels in the cells treated with SG
derivatives. Notably, treatment with LSGO exerted a significantly
greater reduction in both mRNA and protein expression levels,
suggesting a superior anti-proliferative effect of LSGO on MCF-7
cells.
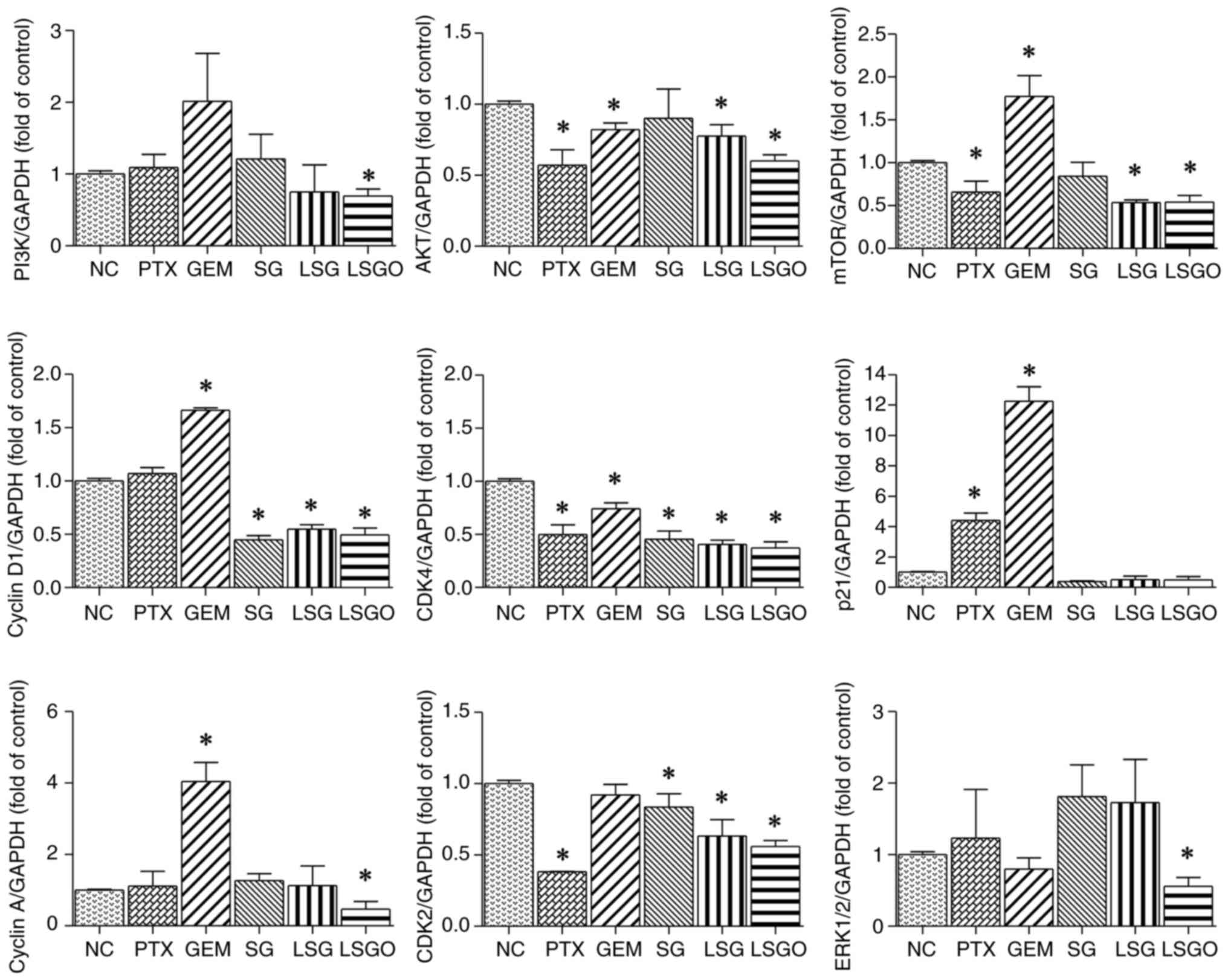 | Figure 5mRNA expression levels of PI3K, AKT,
mTOR, cyclin D1, CDK4, p21, cyclin A, CDK2 and ERK 1/2 in MCF-7
cells treated with PTX, GEM, SG, LSG and LSGO, assessed relative to
GAPDH using reverse transcription-quantitative PCR. The results are
presented as the mean±SEM (n=3) from three independent experiments.
*P<0.05, statistically significant difference
compared to the normal control at a 95% confidence level. PTX,
paclitaxel; GEM, gemcitabine; SG, sulfated galactan; LSG low
molecular weight SG; and LSGO, octanoyl ester-supplemented SG. |
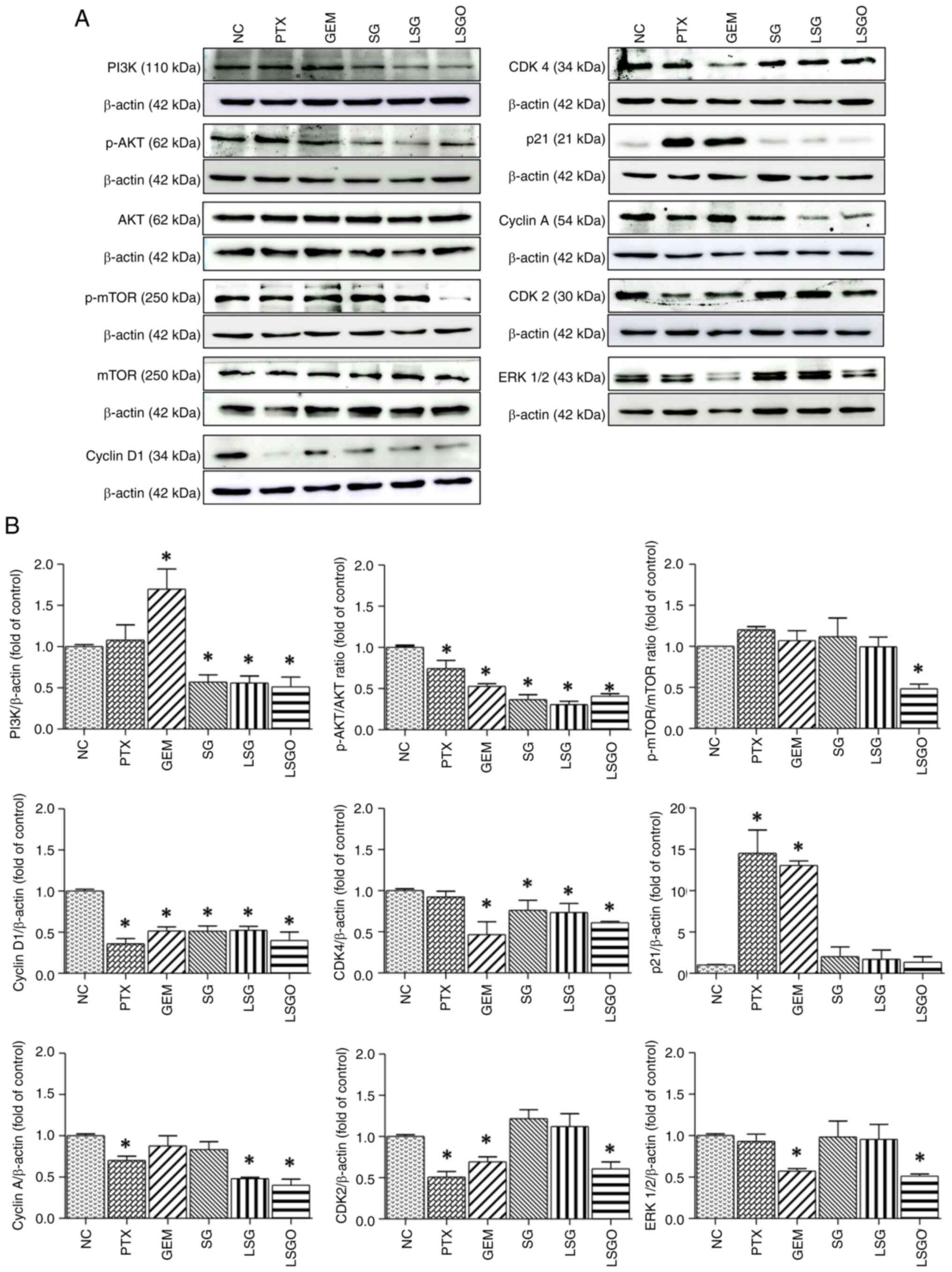 | Figure 6Effects of PTX, GEM, SG, LSG and LSGO
on protein expression levels in MCF-7 cells, assessed using western
blot analysis. (A) Protein expression levels of PI3K, p-AKT, AKT,
p-mTOR, mTOR, cyclin D1, CDK4, p21, cyclin A, CDK2 and ERK 1/2 in
MCF-7 cells treated with PTX, GEM, SG, LSG and LSGO, assessed using
western blot analysis. (B) Quantitative analysis of PI3K,
p-AKT/AKT, p-mTOR/mTOR, cyclin D1, CDK4, p21, cyclin A, CDK2 and
ERK 1/2 expression, normalized to β-actin. The results are
presented as the mean±SEM (n=3) from three independent experiments.
*P<0.05, statistically significant difference
compared to the normal control at a 95% confidence level. PTX,
paclitaxel; GEM, gemcitabine; SG, sulfated galactan; LSG low
molecular weight SG; and LSGO, octanoyl ester-supplemented SG. |
Discussion
Breast cancer remains one of the most prevalent
malignancies worldwide (23).
Despite advancements being made in treatment strategies, managing
the disease remains challenging, with chemotherapeutic resistance
and associated side-effects often leading to treatment failure
(24). In response, researchers
are exploring the potential of natural compounds in breast cancer
therapy, particularly for mitigating chemotherapy-induced
side-effects. Previous studies have demonstrated that
polysaccharides inhibit cancer cell proliferation and survival by
inducing cell cycle arrest and apoptosis. For instance, serum
collected from fucoidan-treated rats was shown to inhibit the
proliferation and increase the apoptosis of MCF-7 cells (25). Fucoidan from the brown algae,
Fucus vesiculosus, at concentrations ranging from 100 to 300
µg/ml, has also been reported to induce apoptosis and promote cell
cycle arrest in CL-6 CCA cells by increasing the levels of
apoptotic protein markers and decreasing the expression of cyclin
and CDK molecules (26). SG,
extracted from the red algae, G. fisheri, is a sulfated
polysaccharide (SP) with a galactose backbone and a high percentage
of sulfate groups (27). It has
been shown to inhibit cell proliferation and induce cell cycle
arrest in HuCCA-1 cells by downregulating cyclin/CDK expression
following treatment at concentrations of 10 and 50 µg/ml (13). Thus, the anticancer activity of
polysaccharides may be influenced by several factors, including
their molecular structure, dosage and the type of cancer cells
involved (28). Additionally,
reducing molecular weight (15,29)
and supplementing SP with an octanoyl ester (16) have been found to enhance their
biological effectiveness. In the present study, the anticancer
activity of SG from G. fisheri, along with its structurally
modified derivatives, LSG and LSGO, was investigated in MCF-7
breast cancer cells and L929 normal lung fibroblasts. The effects
were compared with those of unmodified SG and the anticancer drugs,
PTX and GEM. The concentrations used for SG and its derivatives
(125-1,000 µg/ml) in the present study reflect typical dosing
ranges for marine polysaccharides (17), which generally exhibit lower
cytotoxicity per unit mass compared to small-molecule drugs such as
PTX and GEM (0.2-1 µg/ml) (18).
This difference in dosage is necessary due to variations in
molecular size, bioavailability and mechanisms of action.
The results of the present study demonstrated that
SG and its derivatives decreased cell viability and suppressed
MCF-7 cell proliferation, in accordance with the reduced Ki-67
expression and the induction of cell cycle arrest, particularly in
the cells treated with LSGO, similar to the effects observed with
PTX and GEM. Treatment with LSGO in MCF-7 cells exerted a greater
anti-proliferative effect, indicating that its molecular structure
is positively associated with its biological activity (30). Furthermore, the enhanced
anti-proliferative activity of LSGO suggests that it may improve
cellular internalization, thereby increasing its overall efficacy
(31). A high Ki-67 expression has
been shown to be associated with cancer proliferation and it is a
well-established indicator of prognosis and clinical outcomes
(32). Since Ki-67 remains active
during the G1, S, G2 and M phases of the cell cycle, it serves as a
reliable marker of cell proliferation and a recognized hallmark of
oncogenesis (33). Notably, the
decreased Ki-67 expression through antisense oligonucleotides has
been shown to inhibit cancer cell proliferation and tumor growth
(34). Herein, the reduction of
Ki-67 expression in MCF-7 cells following SG and its derivatives
treatment suggests anti-proliferative activity, consistent with
previous findings on polysaccharides extracted from
Crataegus (Hawthorn), which inhibited human colon cancer
HCT116 cell proliferation by reducing Ki-67 protein expression and
inducing cell cycle arrest (35).
However, these effects were not observed in L929 normal
fibroblasts, indicating a selective effect on breast cancer cells
(36). This selectivity may be
attributed to differences in cellular metabolism, surface receptor
expression, and intracellular signaling between normal and cancer
cells (37).
In the present study, these effects were achieved
through the downregulation of upstream signaling molecules of
PI3K/Akt/mTOR, ERK, and CDKs at both the gene and protein levels.
This downregulation may be attributed to the structural similarity
of SG derivatives to heparan sulfate proteoglycans (HSPGs), which
can bind to growth factor receptors and/or ligands, thereby
inhibiting receptor-ligand complex formation (13). It has been demonstrated that
natural SPs share structural similarities with HSPGs and can mimic
their functions. These SPs can interfere with the binding of growth
factors to their receptors or co-receptors, leading to the
inhibition of downstream signaling pathway activation in cancer
cells (38). However, previous
research indicates that SG derived from G. fisheri does not
directly interact with EGF, but instead interacts with the EGFR,
resulting in the downregulation of EGFR activity in CCA cells
(14). The competitive binding of
SG may disrupt receptor dimerization, leading to a reduction in
p-EGFR and p-ERK levels (14).
Therefore, by inhibiting growth factor receptors such as EGFR, SG
derivatives may indirectly regulate PI3K/Akt/mTOR and ERK signaling
molecules.
In MCF-7 cells treated with LSGO, the decreased
expression of PI3K, AKT, mTOR and ERK at both the mRNA and protein
levels resulted in a reduction of cyclin D1 and CDK4 expression,
the first protein complex to become active in the G1 phase
(39). The downregulation of
cyclin D1 and CDK4 leads to the decreased transcription of key
target genes, including cyclin E, cyclin B, CDK2 and CDK1, thereby
disrupting cell cycle progression from the G1 to S phase (40). Furthermore, the downregulation of
genes involved in the G1 and S phases also led to reduced
expression of cyclin A and CDK2 at both the gene and protein
levels. Given their critical roles in DNA synthesis, proper S phase
progression, and the transition from S to G2 phase, this reduction
may significantly affect cell cycle regulation (41). The findings of the present study
indicate that SG derivatives, particularly LSGO induced cell cycle
arrest at the G2/M phase in MCF-7 cells, potentially through the
downregulation of cyclin/CDK complexes, particularly cyclin A and
CDK2. These results are in contrast to those of previous studies
reporting that SG derived from G. fisheri induces cell cycle
arrest at the G1 phase in CCA cells by downregulating cyclin D,
cyclin E, CDK4 and CDK2. This reduction in cyclin/CDK expression is
associated with the upregulation of p53 and p21, a key regulator of
cyclin/CDK inhibition (13). By
contrast, these findings suggest that the anti-proliferative effect
of SG derivatives in MCF-7 cells is not mediated by the increased
expression of p21.
Another possible explanation for why SG derivatives
inhibit MCF-7 cell proliferation at the G2/M phase, including the
reduction of cyclin A and CDK2, may be related to the
structure-activity association of SG derivatives and cancer cell
type specificity (26).
Polysaccharides from different sources have been reported to induce
cell cycle arrest at various phases in different cancer cell lines
(35). For example, SP from
Laetiporus sulphureus fruiting bodies has been shown to
induce cell cycle arrest at the G0/G1 phase in triple-negative
breast cancer MDA-MB-231 cells (42). Similarly, a homogeneous
polysaccharide from Crataegus (Hawthorn) inhibited the
proliferation of human colon cancer HCT116 cells by inducing cell
cycle arrest at the S/G2 phase (35). Additionally, the combination of
fucoidan with natural/organic ingredients, including vegetable
juice, mulberry and wheatgrass, has been reported to increase the
proportion of cells arrested in the G2/M phase in oral squamous
cell carcinoma (43). The
implications of the findings of the present study extend beyond
breast cancer treatment, as the cell cycle regulatory proteins
targeted by SG derivatives are involved in various cancer types.
While previous studies have reported individual chemical
modifications of SG, such as molecular weight reduction and
esterification, the present study is the first to combine both
modifications in a single structure. This combination resulted in
the disruption of breast cancer cells and the inhibition of key
signaling pathways in MCF-7 cells. These findings support the
potential use of LSGO as an optimized SG derivative with enhanced
anti-proliferative activity compared to either unmodified SG or SG
with reduced molecular weight alone. Although the present study
demonstrates the modulation of the PI3K/AKT and ERK signaling
pathways following treatment with SG and its derivatives, further
studies incorporating pathway-specific inhibitors or gene silencing
approaches are warranted to validate the causal involvement of
these pathways in mediating the observed anti-proliferative and
cytotoxic effects. Additionally, future research is required to
include Annexin V/PI staining to clarify the apoptosis-related
mechanisms of SG and its derivatives, investigate their molecular
interactions with regulatory proteins, evaluate their efficacy in
in vivo models, and explore their potential synergy with
existing chemotherapeutic agents to reduce side-effects in with
breast cancer.
In conclusion, the results of the present study
indicate that SG derivatives from G. fisheri exhibit mild,
yet effective anti-proliferative activity in MCF-7 cells by
inhibiting cell proliferation and inducing cell cycle arrest at the
G2/M phase. This effect is mediated through the inhibition of
upstream signaling molecules, cyclins and CDKs, which regulate
MCF-7 cell proliferation. These findings highlight the potential
use of SG derivatives, particularly LSGO, as natural compounds for
breast cancer treatment and warrant further investigation into
their clinical applications.
Acknowledgements
The authors would like to thank Dr Dylan Southard
(the KKU Publication Clinic, Khon Kaen University, Khon Kaen,
Thailand) for editing the manuscript.
Funding
Funding: The present study was supported by a Postgraduate Study
Support Grant of the Faculty of Medicine, Khon Kaen University and
the Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
(Grant no. IN66081).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JP and TR conceived and designed the study. JP, SS
and TR performed the experiments. JP, SS, WS, TS, JK, KW and TR
were responsible for data analysis. JP, SS and TR participated in
the drafting of the manuscript. JK, KW and TR edited and finalized
the manuscript. JK, KW and TR supervised the study. JP and TR
provided funding and managed the project. JP, SS and TR confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ilango S, Paital B, Jayachandran P, Padma
PR and Nirmaladevi R: Epigenetic alterations in cancer. Front
Biosci (Landmark Ed). 25:1058–1109. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Damiescu R, Efferth T and Dawood M:
Dysregulation of different modes of programmed cell death by
epigenetic modifications and their role in cancer. Cancer Lett.
584(216623)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer
development and progression: Risk factors, cancer stem cells,
signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 5:77–106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee S, Rauch J and Kolch W: Targeting MAPK
signaling in cancer: Mechanisms of drug resistance and sensitivity.
Int J Mol Sci. 21(1102)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong C, Wu J, Chen Y, Nie J and Chen C:
Activation of PI3K/AKT/mTOR pathway causes drug resistance in
breast cancer. Front Pharmacol. 12(628690)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mangé A, Coyaud E, Desmetz C, Laurent E,
Béganton B, Coopman P, Raught B and Solassol J: FKBP4 connects
mTORC2 and PI3K to activate the PDK1/Akt-dependent cell
proliferation signaling in breast cancer. Theranostics.
9:7003–7015. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Palmer N and Kaldis P: Less-well known
functions of cyclin/CDK complexes. Semin Cell Dev Biol. 107:54–62.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao
H, Yu M, Lin J and Cui Q: The roles of cyclin-dependent kinases in
cell-cycle progression and therapeutic strategies in human breast
cancer. Int J Mol Sci. 21(1960)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al Bitar S and Gali-Muhtasib H: The role
of the cyclin dependent kinase inhibitor p21cip1/waf1 in
targeting cancer: Molecular mechanisms and novel therapeutics.
Cancers (Basel). 11(1475)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Menon SS, Guruvayoorappan C, Sakthivel KM
and Rasmi RR: Ki-67 protein as a tumour proliferation marker. Clin
Chim Acta. 491:39–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Burcombe R, Wilson GD, Dowsett M, Khan I,
Richman PI, Daley F, Detre S and Makris A: Evaluation of Ki-67
proliferation and apoptotic index before, during and after
neoadjuvant chemotherapy for primary breast cancer. Breast Cancer
Res. 8(R31)2006.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Liyanage UE, Law MH, Han X, An J, Ong JS,
Gharahkhani P, Gordon S, Neale RE and Olsen CM: 23andMe Research
Team et al. Combined analysis of keratinocyte cancers
identifies novel genome-wide loci. Hum Mol Genet. 28:3148–3160.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sae-Lao T, Tohtong R, Bates DO and
Wongprasert K: Sulfated galactans from red seaweed Gracilaria
fisheri target EGFR and inhibit cholangiocarcinoma cell
proliferation. Am J Chin Med. 45:615–633. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sae-Lao T, Luplertlop N, Janvilisri T,
Tohtong R, Bates DO and Wongprasert K: Sulfated galactans from the
red seaweed Gracilaria fisheri exerts anti-migration effect on
cholangiocarcinoma cells. Phytomedicine. 36:59–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fan J, Zhu J, Zhu H, Zhang Y and Xu H:
Potential therapeutic target for polysaccharide inhibition of colon
cancer progression. Front Med (Lausanne).
10(1325491)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rudtanatip T, Somintara S, Sakaew W,
El-Abid J, Cano ME, Jongsomchai K, Wongprasert K and Kovensky J:
Sulfated galactans from Gracilaria fisheri with supplementation of
octanoyl promote wound healing activity in vitro and in vivo.
Macromol Biosci. 22(e2200172)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Srimongkol P, Songserm P, Kuptawach K,
Puthong S, Sangtanoo P, Thitiprasert S, Thongchul N, Phunpruch S
and Karnchanatat A: Sulfated polysaccharides derived from marine
microalgae, Synechococcus sp. VDW, inhibit the human colon cancer
cell line Caco-2 by promoting cell apoptosis via the JNK and p38
MAPK signaling pathway. Algal Res. 69(102919)2023.
|
|
18
|
Aslama A, Bergerb MR, Ullaha I, Hameeda A
and Masoodc F: Preparation and evaluation of cytotoxic potential of
paclitaxel containing poly-3-hydroxybutyrate-co-3-hydroxyvalarate
(PTX/PHBV) nanoparticles. Braz J Biol. 83(e275688)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dehghani N, Tafvizi F and Jafari P: Cell
cycle arrest and anti-cancer potential of probiotic
Lactobacillus rhamnosus against HT-29 cancer cells.
Bioimpacts. 11:245–252. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Intuyod K, Hahnvajanawong C, Pinlaor P and
Pinlaor S: Anti-parasitic drug ivermectin exhibits potent
anticancer activity against gemcitabine-resistant
cholangiocarcinoma in vitro. Anticancer Res. 39:4837–4843.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shendge AK, Basu T and Mandal N:
Evaluation of anticancer activity of Clerodendrum viscosum
leaves against breast carcinoma. Indian J Pharmacol. 53:377–383.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Marquette C and Nabell L:
Chemotherapy-resistant metastatic breast cancer. Curr Treat Options
Oncol. 13:263–275. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He X, Xue M, Jiang S, Li W, Yu J and Xiang
S: Fucoidan promotes apoptosis and inhibits EMT of breast cancer
cells. Biol Phram Bull. 42:442–447. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chantree P, Na-Bangchang K and Martviset
P: Anticancer activity of fucoidan via apoptosis and cell cycle
arrest on cholangiocarcinoma cell. Asian Pac J Cancer Prev.
22:209–217. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wongprasert K, Rudtanatip T and Praiboon
J: Immunostimulatory activity of sulfated galactans isolated from
the red seaweed Gracilaria fisheri and development of resistance
against white spot syndrome virus (WSSV) in shrimp. Fish Shellfish
Immunol. 36:52–60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li N, Wang C, Georgiev MI, Bajpai VK,
Tundis R, Simal-Gandara J, Lu X, Xiao J, Tang X and Qiao X:
Advances in dietary polysaccharides as anticancer agents:
Structure-activity relationship. Trends Food Sci Technol.
111:360–377. 2021.
|
|
29
|
Lu J, Shi KK, Chen S, Wang J, Hassouna A,
White LN, Merien F, Xie M, Kong Q, Li J, et al: Fucoidan extracted
from the New Zealand Undaria pinnatifida-physicochemical
comparison against five other fucoidans: Unique low molecular
weight fraction bioactivity in breast cancer cell lines. Mar Drugs.
16(461)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Y, Xing M, Cao Q, Ji A, Liang H and
Song S: Biological activities of fucoidan and the factors mediating
its therapeutic effects: A review of recent studies. Mar Drugs.
17(183)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sakaew W, Somintara S, Jongsomchai K, El
Abid J, Wongprasert K, Kovensky J and Rudtanatip T: Octanoyl
esterification of low molecular weight sulfated galactan enhances
the cellular uptake and collagen expression in fibroblast cells.
Biomed Rep. 19(99)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Davey MG, Hynes SO, Kerin MJ, Miller N and
Lowery AJ: Ki-67 as a prognostic biomarker in invasive breast
cancer. Cancers (Basel). 13(4455)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang C, Zhang J, Ding M, Xu K, Li L, Mao L
and Zheng J: Ki67 targeted strategies for cancer therapy. Clin
Transl Oncol. 20:570–575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ma L, Xu GB, Tang X, Zhang C, Zhao W, Wang
J and Chen H: Anti-cancer potential of polysaccharide extracted
from hawthorn (Crataegus.) on human colon cancer cell line HCT116
via cell cycle arrest and apoptosis. J Funct Foods.
64(103677)2020.
|
|
36
|
Abdala-Díaz RT, Casas-Arrojo V,
Castro-Varela P, Riquelme C, Carrillo P, Medina MA, Cárdenas C,
Becerra J and Pérez Manríquez C: Immunomodulatory, antioxidant, and
potential anticancer activity of the polysaccharides of the fungus
Fomitiporia chilensis. Molecules. 29(3628)2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Akhtar MJ, Ahamed M, Alhadlaq HA,
Alrokayan SA and Kumar S: Targeted anticancer therapy:
Overexpressed receptors and nanotechnology. Clin Chim Acta.
436:78–92. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cheng JJ, Chang CC, Chao CH and Lu MK:
Characterization of fungal sulfated polysaccharides and their
synergistic anticancer effects with doxorubicin. Carbohydr Polym.
90:134–139. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Asghar U, Witkiewicz AK, Turner NC and
Knudsen ES: The history and future of targeting cyclin-dependent
kinases in cancer therapy. Nat Rev Drug Discov. 14:130–146.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Finn RS, Aleshin A and Slamon DJ:
Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen
receptor-positive breast cancers. Breast Cancer Res.
18(17)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gopinathan L, Tan SL, Padmakumar VC,
Coppola V, Tessarollo L and Kaldis P: Loss of Cdk2 and cyclin A2
impairs cell proliferation and tumorigenesis. Cancer Res.
74:3870–3879. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jen CI, Lu MK, Lai MN and Ng LT: Sulfated
polysaccharides of Laetiporus sulphureus fruiting bodies exhibit
anti-breast cancer activity through cell cycle arrest, apoptosis
induction, and inhibiting cell migration. J Ethnopharmacol.
321(117546)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen PH, Chiang PC, Lo WC, Su CW, Wu CY,
Chan CH, Wu YC, Cheng HC, Deng WP, Lin HK and Peng BY: A novel
fucoidan complex-based functional beverage attenuates oral cancer
through inducing apoptosis, G2/M cell cycle arrest and retarding
cell migration/invasion. J Funct Foods. 85(104665)2021.
|