1. Introduction
Viruses, particularly respiratory viruses, such as
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), pose
immense challenges to public health as they easily transmit through
aerosols and droplets, as well as through direct contact with
contaminated surfaces. The oral cavity provides a prime location
for viral replication, thus becoming a reservoir for viral
particles; saliva also plays a vital role in this regard.
Accordingly, reducing viral loads in oral fluids has come to occupy
a place of prominence in efforts toward halting the spread of
viruses, particularly in high-risk environments such as clinics and
hospitals (1,2). Antiviral mouthwashes are easily
applied, are inexpensive and are an accessible means of infection
control. Traditionally used mouthwashes aid in the maintenance of
oral hygiene as they are bactericidal or fungicidal agents;
however, there is currently an increasing interest in the
evaluation of their virucidal potentials. Studies have primarily
focused on povidone-iodine (PVP-I), chlorhexidine gluconate (CHX),
cetylpyridinium chloride (CPC), essential oils and newer compounds
phthalocyanine derivatives. These agents function through the
disruption of the viral envelopes or interference with replication
processes, thus leading to a loss of viral infectivity and shedding
(3). Schürmann et al
(2) focused on CPC-based
formulations as they could break viral lipid membranes, which
prevented coronaviruses and other related pathogens from spreading.
Rodríguez-Casanovas et al (3) and Carrouel et al (4) in their study demonstrated that few
mouthwashes can effectively eliminate viruses.
Mouthwashes with essential oils have
anti-inflammatory properties that have helped individuals deal with
the systemic effects of viral infections and have also made
antiviral treatment more flexible. The efficiency of such
mouthwashes in decreasing viral shedding among patients having mild
or asymptomatic COVID-19 has been proven through clinical studies
performed by Takeda et al (5) and Vilhena et al (6). Martínez Lamas et al (7) in their study has confirmed the
virucidal properties of PVP-I, suggesting its clinical use,
particularly in dentistry. The antiseptic CHX, frequently used in
dentistry, has also demonstrated short-term virucidal activity.
Jain et al (8) found that a
0.2% CHX solution inactivated >99.9% of SARS-CoV-2 within 30
sec, demonstrating its rapid action compared to PVP-I. Evidence
from the literature has indicated that CHX only reduces the viral
load for a short period of time and is therefore less effective as
a standalone treatment. Its combination with other agents, such as
PVP-I needs to be evaluated in a clinical setting (9). CHX is a rapid, yet temporary addition
to more long-lasting agents, such as PVP-I, and it can greatly
reduce oral viral loads when used in conjunction with
phthalocyanine derivatives and CPC (10). Perussolo et al (10) conducted a study where they tested
three types of mouthwash randomly. They discovered that these
mouthwashes were able to decrease the load of SARS-CoV-2 among
hospitalized patients. This supports the concept that mouthwashes
may limit the spread of the virus. Mouthwashes containing
phthalocyanine derivatives have also shown promising efficacy in
reducing hospitalization periods for patients with COVID-19, and
CPC-containing mouthwashes have also shown promising in
vitro activity against enveloped viruses (11,12).
Both CPC and phthalocyanine derivatives possess notable antiviral
activity. CPC functions by disrupting the lipid envelope of
viruses, which leads to rapid virucidal effects (5), while phthalocyanine derivatives
inhibit viral replication and reduce inflammation, which renders
them very useful in high-risk and hospitalized patients (6).
The majority of the formulations integrate
antimicrobial and anti-inflammatory properties, thereby enhancing
their efficacy in reducing viral loads and improving clinical
outcomes (13). Although various
mouthrinses that act against viruses, as studied in previous
literature (14-17),
have been proven to be useful and affordable, their functional
properties against these viruses need to be fully investigated.
The present review provides an overview of the
effectiveness of antiviral mouthwashes, particularly based on
scientific studies, in an aim to assess the role of these
mouthwashes in reducing the spread of viruses. For this purpose,
the PubMed and Google Scholar databases were searched to provide
related data for the present review, which concentrated on
systematic reviews, as well as in vitro, in vivo,
clinical and observational studies evaluating the virucidal
activity, real-world efficacy and preventive potential of antiviral
mouthwashes that were published between 2020 and 2025. The present
review discusses the mechanisms and relative efficacy, clinical
effectiveness and preventive potential of several mouthwash
formulations.
2. Antiviral mouthrinses
Types of antiviral mouthwashes
Antiviral mouthwashes are formulated to degrade the
structures of viruses, inhibit multiplication and decrease
infectivity in the oral environment. There is a plethora of types,
each with its mode of action:
PVP-I. PVP-I is an iodine-based antiseptic
solution. This disrupts the lipid envelope and then causes
oxidation of the viral proteins. As a result, the viruses becomes
inactivated. This product has wide applicability over viruses, such
as SARS-CoV-2 and is hence used quite commonly for preprocedural
rinsing and infection control in health care settings (1,18).
CPC. This compound is a quaternary ammonium
agent that functions at the lipid envelope level leading to
membrane disintegration and the release of viral genetic material,
ultimately resulting in viral inactivation. It also has some
anti-inflammatory effects and can effectively kill viruses, such as
influenza A and respiratory syncytial virus (RSV). It is very
commonly used for everyday oral hygiene, although it can also be
used in managing symptomatic viral infections (5,13).
CPC is a broad-spectrum antiseptic agent often mixed with zinc or
other additives to enhance its performance, according to
Garcia-Sanchez et al (19).
Hydrogen peroxide (H2O2).
H2O2 produces reactive oxygen species (ROS),
which oxidizes viral proteins, rendering viruses, including
SARS-CoV-2, vulnerable to oxidative stress. It is commonly applied
in general disinfection as well as a mouthrinse; however, it is not
effective compared to PVP (20).
According to the systematic review performed by Ortega et al
(16), there is no scientific
evidence supporting the use of hydrogen peroxide mouthwash for
reducing the viral load of SARS-CoV-2 in saliva.
Phthalocyanine-based mouthwashes.
Phthalocyanine derivatives present in mouthwashes interfere with
nucleic acid synthesis and thus inhibit viral replication. They are
particularly effective for use in high-risk environments and for
use preventively in immunocompromised individuals (21).
Alcohol-based mouthwashes. These mouthwashes
contain 20-30% of ethanol, they interfere with the lipid envelope
of enveloped viruses and can denature proteins, leading to viral
inactivation. There are only a limited number of clinical studies
evaluating the antiviral potential of mouthrinses (22). Although mouthwashes are used for
routine oral hygiene maintenance, they have limited direct efficacy
against SARS-CoV-2 since more ethanolic concentration is required
for virucidal property. However, their broad-spectrum antiseptic
properties render them a valuable component of infection control
protocols (18,23). Listerine contains essential oils
and alcohol, which are effective against enveloped viruses, such as
herpes simplex virus and influenza A. However, its efficacy against
SARS-CoV-2 is uncertain (24,25).
CHX. CHX has been extensively studied for its
broad-spectrum antimicrobial activity, even against viruses. It
disrupts cell membranes and consequently does not allow viral
replication, in addition to inactivating the viruses within the
oral cavity. CHX has generated interest as it can significantly
reduce the viral load of SARS-CoV-2. The study by Huang and Huang
(26) revealed a marked decrease
in the levels of SARS-CoV-2 RNA in oropharyngeal samples following
treatment with CHX mouthwash, thus supporting its use in infection
control protocols. CHX also plays a role in reducing the risk of
viral transmission in healthcare settings, particularly when used
preprocedurally. Other studies have suggested that CHX can be
incorporated into regular oral hygiene to assist in lowering oral
viral loads, even in patients with symptomatic viral infections
(27,28).
Other solutions. Other active ingredients,
such as zinc-based solutions and enzyme-protein mixtures are
virucidal. Zinc-based solutions help to prevent viral
multiplication in the nasopharyngeal region (29). In addition, the enzyme-protein
mixtures have been found to be capable of inhibiting surrogate
viruses, such as feline coronavirus, thereby rendering them useful
in extended antiviral applications (14).
Antiviral mouthwashes: Mechanisms and
efficacy
The efficacy of antiviral mouthwashes depends on
their active ingredients, concentration and mechanisms of action. A
standardized comparison of these formulations, incorporating virus
strain, test type, effectiveness and real-world applications is
provided in Table I. Various
mechanisms of viral disruption include the following:
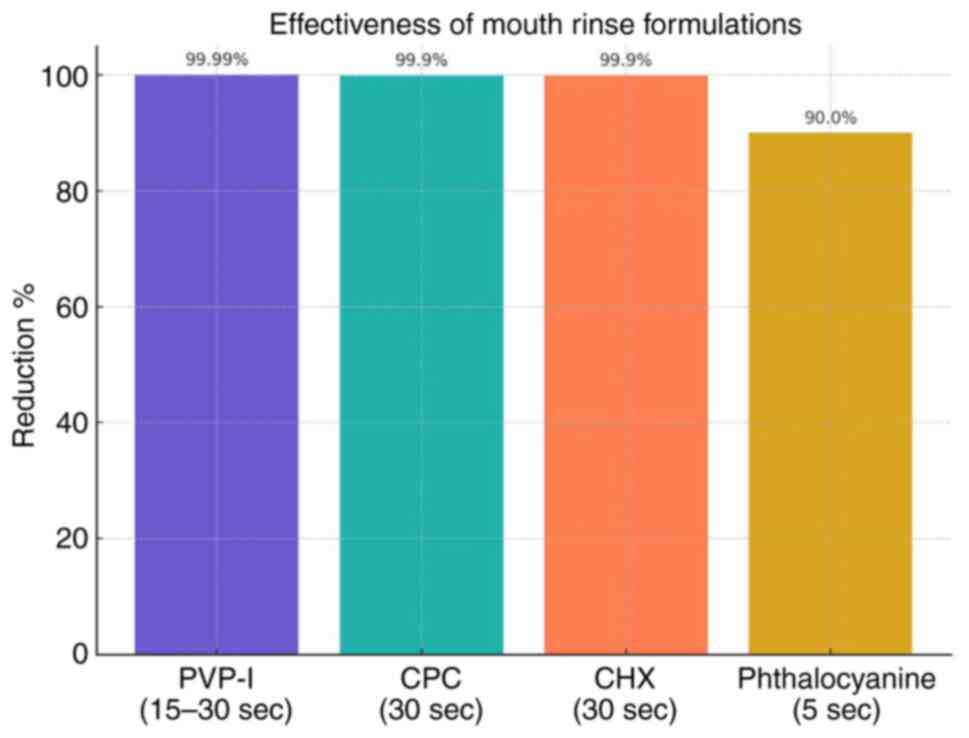
 | Table IComparison of mouthrinse
formulations. |
Table I
Comparison of mouthrinse
formulations.
| Mouthwash
formulation | Mechanism of
action | Virus efficacy | Types of
studies | Concentration | Reduction % | Contact time | (Refs.) |
|---|
| Povidone-iodine
(PVP-I) | Lipid envelope
disruption; protein oxidation | SARS-CoV-2 | In
vitro | 1% | 99.99% | 15-30 sec | (1) |
| Cetylpyridinium
chloride (CPC) | Lipid envelope
disruption; anti-inflammatory properties | SARS-CoV-2,
influenza A, RSV | In vitro and
clinical | 0.005% | 99.9% | 30 sec | (5) |
| Chlorhexidine
(CHX) | Lipid envelope
disruption; protein oxidation | SARS-CoV-2 | Clinical (RCT) | 0.12-0.2% | 99.9% | 30 sec | (8) |
|
Phthalocyanine-based mouthwash | Inhibition of viral
replication; anti-inflammatory effects | SARS-CoV-2 | In
vitro | 0.02-0.05% | 90% | 30 sec, 1 min and 5
min | (21) |
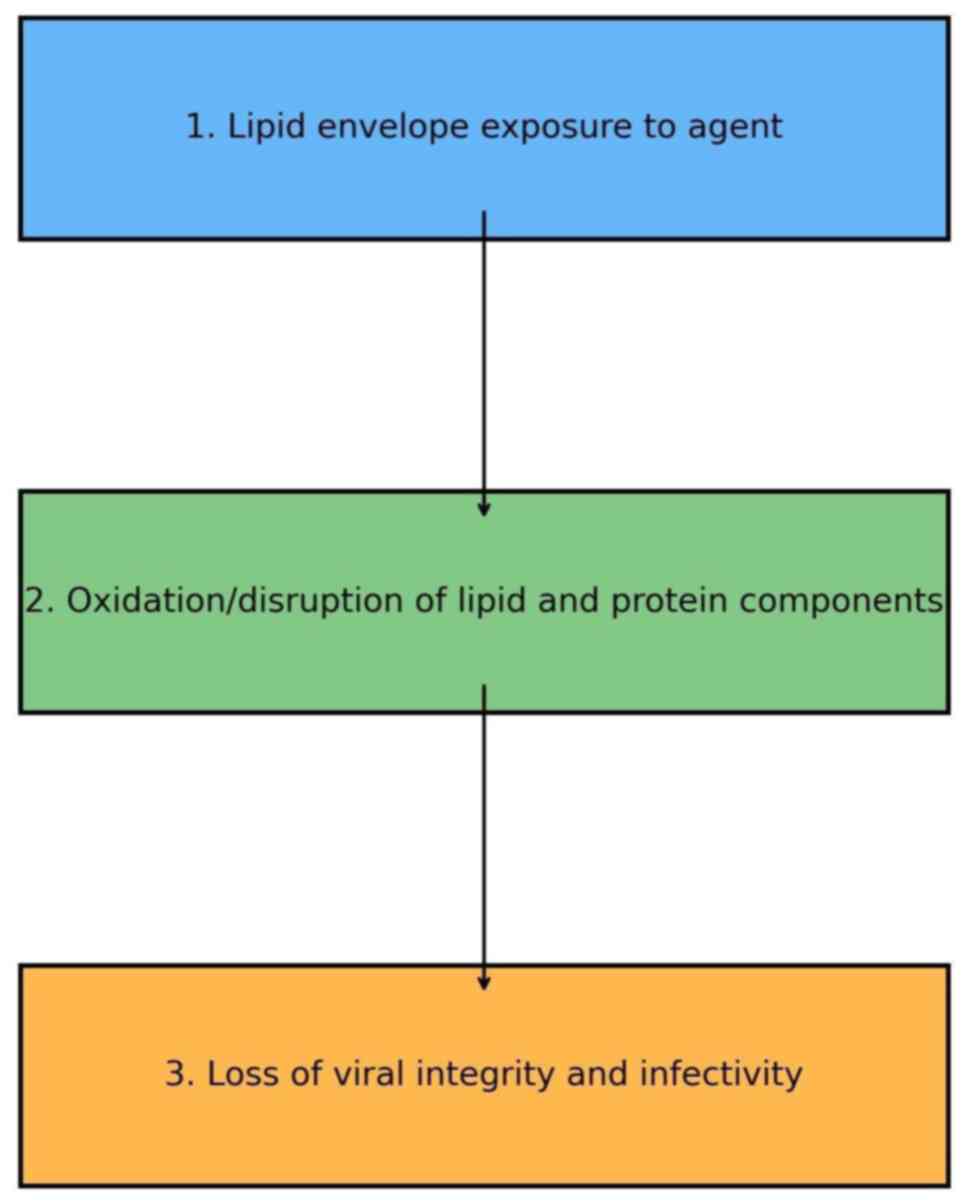
Lipid envelope disruption. The lipid envelope
is essential for the infectivity of enveloped viruses, such as
SARS-CoV-2. If this structure is disrupted, the virus is no longer
infectious. Some of the common agents that function by lipid
envelope disruption mechanism are as follows:
i) PVP-I. PVP-I is an oxidizing agent; it
destroys the lipid and protein moieties of the viral envelope
causing a structural collapse that leads to the inactivation of the
virus. The recent study by Hassandarvish et al (1) indicated that concentrations of PVP-I
from 0.23-1% provide >99.99% inactivation of SARS-CoV-2 in only
15 sec after contact. The study by Barrueco et al (28) also highlighted the efficacy of
PVP-I in reducing viral infectivity in clinical and public health
settings. Therefore, PVP-I has proven to be very useful in the
preparation of pre-procedural oral rinses for use in the healthcare
environment as it can markedly reduce viral loads in aerosols
(Fig. 1).
ii) CPC. CPC disrupts the lipid envelope due
to its capability to penetrate it and leak out the viral content.
Rius-Salvador et al (30)
reported a reduction in the viral titers of influenza A and RSV by
>99.9% with an oral care concentration within 30 sec (30). Thus, CPC is very safe and effective
for day-to-day oral hygiene and can be used effectively in
non-clinical settings to regulate enveloped viruses.
iii) CHX. CHX disrupts the lipid envelope of
SARS-CoV-2, similar to PVP-I and CPC. Its positive charge
destabilizes and inactivates the viral membrane by binding to its
negatively charged components. CHX rapidly inactivates viruses at
0.1-0.2%, rendering it a useful pre-procedural rinse in dentistry
and healthcare (8).
Alcohol-based solutions
These ethanol solutions achieve the disruption of
the lipid envelope bilayer around the virus; furthermore, ethanol
can facilitate protein denaturation inside the virus. SARS-CoV-2
and various other enveloped viruses can become deactivated with
solutions containing >60% alcohol for less than half a second or
instant (18,23). Such fluids are frequently
implemented for hand sanitizer disinfections; these same solutions
have a low utilization in mucous membranes.
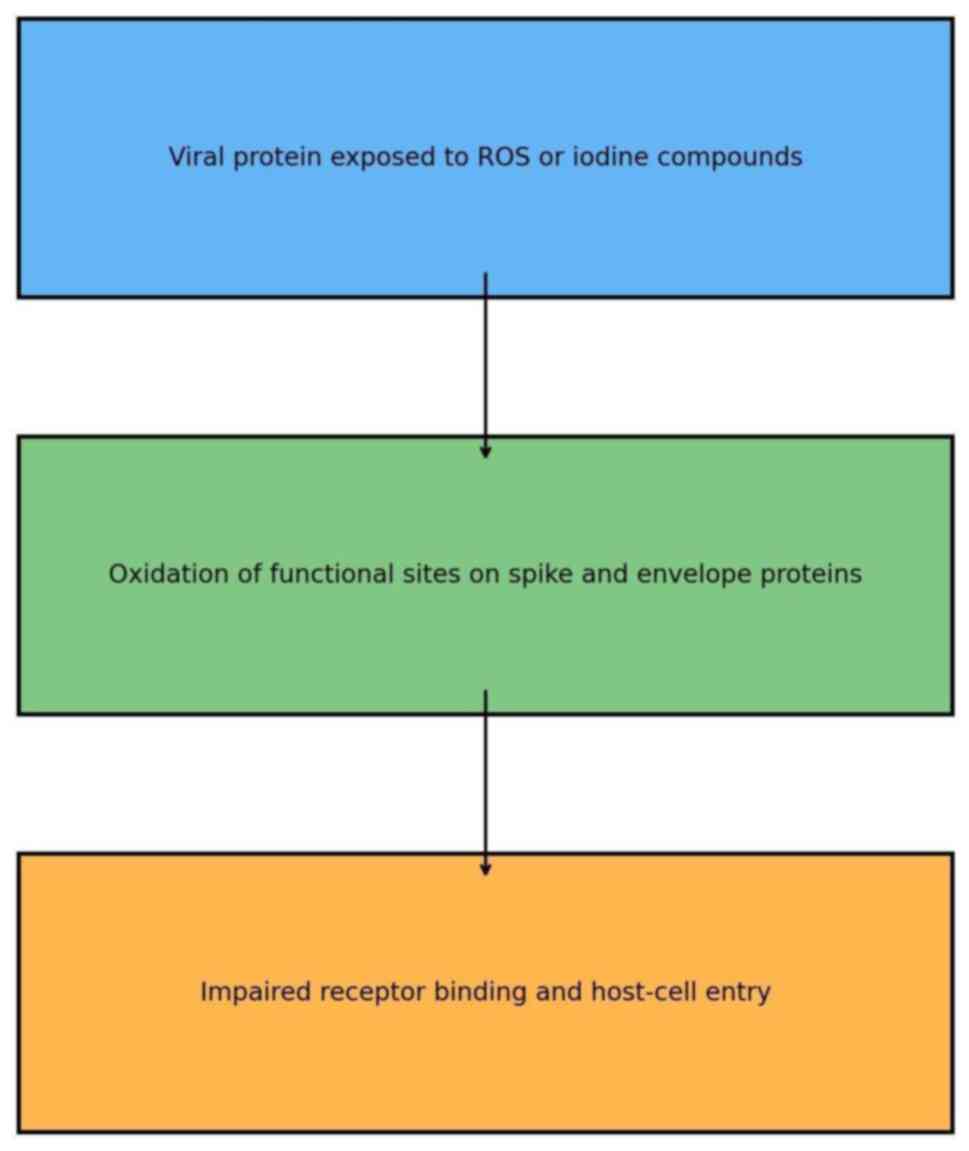
Protein oxidation
Surface proteins, such as the SARS-CoV-2 spike
protein mediate host-cell entry; the oxidation of these proteins is
now known to block viral infection (Fig. 2).
PVP-I. PVP-I rapidly oxidizes viral surface
proteins, thereby neutralizing them and preventing viral attachment
to host cells. Hassandarvish et al (1) demonstrated that PVP-I reaches a 4-log
reduction in infectivity within 15 sec of exposure. Thus, this
speed renders PVP-I suitable for general oral hygiene use,
particularly in risk settings where the speed of viral inactivation
is necessary.
H2O2. H2O2 produces
volatile ROS that oxidizes viral proteins, thus hindering virus
penetration into host cells. In their systematic review, Ortega
et al (16) suggested that
1.5-3% H2O2 reduces the infectivity of
viruses by as much as 80% within a period of 30-60 sec.
CHX. By rupturing the viral envelope, CHX
helps to inactivate viral proteins even though it does not directly
oxidize them. The viral surface proteins, including the spike
protein, are disrupted; thus, the virus cannot bind to host cells.
This action accentuates its general antiviral action (9).
Membrane disruption
Of note, apart from protein oxidation, some agents
such as PVP-I and CPC can affect the lipid envelope of viruses
causing structural instability that enhances viral inactivation.
Such disruption can supplement protein oxidation, leading to a
greater extent of reduction in infectivity of the virus (31).
Viral load reduction
Antiviral mouthwashes are critical in reducing viral
loads in the oral cavity, minimizing the risk of transmission,
particularly in high-risk environments such as clinical and dental
settings.
PVP-I and CPC. Both are effective in
achieving rapid and significant reductions in viral loads. PVP-I,
as a pre-procedural rinse can reduce the salivary viral loads by
>99%; effects can be observed up to 30 min post-rinse (1,18).
Similarly, CPC provides comparable effectiveness and confers
anti-inflammatory benefits; thus, it can be considered as a good
option for oral hygiene in clinical as well as nonclinical
environments (31).
H2O2 and alcohol-based mouthwashes.
H2O2 must remain in contact for 1-2 min in
order to be able to reduce viral loads by 80%. Higher
concentrations will achieve this in a more rapid manner; however,
the residual activity would not last as long (16,22).
Alcohol-based mouthwashes compromise viral lipid envelopes, but
lack prolonged virucidal activity, requiring repeated use for
sustained viral reduction (23).
CHX. CHX has been shown to rapidly reduce
viral load in the oral cavity. In a systematic review, Rahman et
al suggested that 0.12% CHX solution decreased SARS-CoV-2 viral
load by 99.9% within 30 sec (32).
This renders CHX an excellent choice for pre-procedural rinses
(Fig. 3), which help to reduce
viral transmission in dental and healthcare settings (32).
Anti-inflammatory effects
Antiviral mouthwashes are indispensable in lowering
viral loads within the oral cavity, thus markedly reducing the
inflammatory mediators by indirectly decreasing the stimulus to the
inflammatory cells (2).
CPC. CPC suppresses pro-inflammatory
cytokines, such as IL-6 and TNF-α by reducing the viral load. This
will thereby reduce both oral cavity site-specific inflammation, as
well as the associated systemic inflammation. Onozuka et al
(33) reported that the regular
use of CPC enhances promotes the recovery of patients from mild and
moderate COVID-19 symptoms (5,33).
Phthalocyanine derivatives. These derivatives
scavenge ROS, thus averting morbidity through the inhibition of
pro-inflammatory cytokines production. Vilhena et al
(6) demonstrated its efficacy in
reducing oral inflammation in patients with COVID-19, pointing
towards increasing their role in high-risk hospital setups. The key
feature is its antiviral action that also has role in reducing the
inflammatory burden, which provides great value to patients that
have immunocompromised states (6).
CHX. CHX reduces oral inflammation. It can
improve oral health in patients with symptomatic COVID-19 by
reducing gingival inflammation and bacterial load. This helps
patients with systemic diseases, such as diabetes, where
inflammation can accelerate disease progression (9,10,26).
The anti-inflammatory effects of CPC, CHX and phthalocyanine
derivatives have effects beyond oral health due to reduction in
viral load, contributing to the systemic benefits of reduced
complications, such as cytokine storms and secondary infections in
patients with viral infections, such as COVID-19.
Applications during the COVID-19
pandemic
Among the various mouthwashes, PVP-I at
concentrations between 0.2 to 2.5% has been observed to
significantly decrease up to 99.99% of SARS-CoV-2 particles with an
incubation period of <15 sec following its application (1,18).
Due to its potent virucidal effect, PVP-I is the preferred
pre-procedural oral rinse for several types of healthcare settings,
particularly in dental practices where aerosol-generating
procedures are inevitable, resulting in a higher risk of airborne
transmission of viruses (18).
Furthermore, the study conducted by Choudhury et al
(11) also established that PVP-I
mouthwashes decrease nasopharyngeal viral loads and help decrease
secondary transmission in patients, particularly those who are mild
or asymptomatic carriers. Additionally, the adaptability of PVP-I,
which also has wide-range effectiveness against other viruses
including Enteroviruses and Coxsackieviruses, has been further
justified as a management agent for the future viral pandemic
outbreak (34). Other
formulations, including CPC and chlorhexidine, were also found to
be helpful during the pandemic and found a niche in medical and
dental care, where CPC was shown to be particularly useful. CPC
mouthwashes have been shown to decrease viral titers and also
effective for aerosol-generating procedures (5). CHS is another antiviral agent that
has been strongly indicated as effective in combating the pandemic.
Jain et al (8) reported
that CHX mouth rinse may significantly reduce the levels of
SARS-CoV-2 RNA in the oral cavity. CHX mouthwashes were widely used
in aerosol-generating procedures in dental and healthcare settings,
primarily to decrease viral loads (8). In addition, the use of CHX as a
topical antimicrobial agent, as reported by Huang and Huang
(26), led to the reduction in
inflammatory burden caused by viral infections, thus contributing
to a more rapid recovery in patients with COVID-19(26). The pandemic also witnessed the
emergence of novel antiviral agents, including phthalocyanine-based
mouthwashes, which have been demonstrated to significantly reduce
viral loads and inflammation (6).
These formulations, which are used in critical care settings, were
incorporated into infection control plans with other antiviral
treatments to maximize protection (6,20).
Zinc-based and chlorhexidine solutions, which have broad-spectrum
antimicrobial activity, were applied to further limit the spread of
viruses, particularly in nasopharyngeal areas, by inhibiting viral
replication (28,35).
3. Challenges, limitations and future
directions
Although antiviral mouthwashes have demonstrated
their utility, they are not free of challenges as regards their
widespread use. A major hindrance is that the formulations vary,
and so do the designs of studies conducted, which makes it
challenging to standardize procedures for testing their efficacy.
For example, some compositions are deactivated by saliva or organic
matter when applied in practice, which could lower their
effectiveness during real-time application (3,36).
Another limitation is the long-term safety profile of oral
antiseptics. CPC-containing mouthwashes alter the oral microbiome,
leading to a possible risk of dysbiosis and poor oral health
(13). Likewise, CHX formulations
may cause taste impairment and tooth discoloration. According to
Jain et al (8), future
formulations require a balance of efficacy and minimal oral
disruption friendly to the microbiome. Long-term clinical trials
are required to evaluate the systemic impact of these formulations
(37). One other less commonly
used mouthwash is Chlorine dioxide, that has been proven to be
effective in reducing viral load (38). User compliance is also an issue.
Taste, cost and accessibility are the factors that prevent regular
use, particularly outside clinical settings. Educational programs
emphasizing the role of mouthwashes in infection control, along
with palatable formulations, will enhance adoption. Public
education programs, particularly for preprocedural mouth rinsing in
high-risk settings such as dental offices, will lead to further
increased use (34). Mouthrinses
have short-term advantages; however, their place in more
comprehensive infection control practices will be better understood
with further research. Researchers are investigating newer
formulations as possible remedies for these issues; these
formulations have improved efficacy, longer duration of action and
fewer side-effects.
Enzyme-based mouthwashes
These mouthwashes use lysozyme and proteases to
break down viral structures. The ability to neutralize feline
coronaviruses, a model for SARS-CoV-2, was shown by Buonavoglia
et al (14).
Nanoparticle-infused mouthwashes
Silver and zinc oxide nanoparticles, for example,
are used in mouthwashes to provide broad-spectrum antiviral action.
Zinc oxide may protect existing formulations by interfering with
viral replication (14).
Biofilm-targeting mouthwashes
Overcoming a significant limitation of conventional
mouthwashes, biofilm-targeting mouthwashes, such as solutions
infused with lactoferrin, aid in preventing viral adhesion to oral
tissues.
Smart pH-responsive formulations
These change their acidity when viruses are
detected, helping them function for longer periods of time to kill
viruses. The long-term virucidal activity is sustained by
pH-responsive CPC and CHX solutions, according to Rius-Salvador
et al (30). Future
research is required to focus on maintaining antiviral potency in
the presence of organic matter, possibly through zinc-based
antivirals combined with phthalocyanine derivatives. Further
optimization of efficacy while minimizing adverse effects could be
achieved by pairing CHX with anti-inflammatory agents and
optimizing CHX concentration (8).
To assess efficacy across various populations and healthcare
environments, further varied clinical trials are required. As these
advanced formulations develop, their integration into routine
infection control policies will necessitate additional validation.
Antiviral mouthwashes may reinforce masks, physical distance and
ventilation. Their widespread implementation in preprocedural
medical settings has the potential to significantly improve patient
safety and contribute to the long-term prevention of infectious
diseases.
4. Conclusion
Antiviral mouthwashes are an integral, yet
developing tool for preventing infections, particularly in dental
and medical settings. While existing formulations successfully
reduce viral loads, issues such as temporary efficacy, microbiome
impact and compliance barriers must be addressed. Improving the
stability of the formulation and creating designs that are easy for
patients to use are crucial steps towards making them really
useful.
In the future, standard clinical testing and
innovative formulation approaches will be integral to incorporating
mouthwashes in routine infection control practices. Given the
progress to date and potential for continued improvements, these
could become an increasingly accepted preventive tool, both to
augment existing prevention strategies in daily practice and
prepare for future pandemics.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
OT wrote the original draft of the manuscript, and
conducted the literature search. GK conceptualized the study. SB
performed the literature review. PLR supervised the study and
edited and revised the manuscript. All authors have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hassandarvish P, Tiong V, Mohamed NA,
Arumugam H, Ananthanarayanan A, Qasuri M, Hadjiat Y and Abubakar S:
In vitro virucidal activity of povidone iodine gargle and mouthwash
against SARS-CoV-2: Implications for dental practice. Br Dent J:
Dec 10, 2020 (Epub ahead of print).
|
|
2
|
Schürmann M, Aljubeh M, Tiemann C and
Sudhoff H: Mouthrinses against SARS-CoV-2: Anti-inflammatory
effectivity and a clinical pilot study. Eur Arch Otorhinolaryngol.
278:5059–5067. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rodríguez-Casanovas HJ, De la Rosa M,
Bello-Lemus Y, Rasperini G and Acosta-Hoyos AJ: Virucidal activity
of different mouthwashes using a novel biochemical assay.
Healthcare (Basel). 10(63)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carrouel F, Valette M, Gadea E, Esparcieux
A, Illes G, Langlois ME, Perrier H, Dussart C, Tramini P, Ribaud M,
et al: Use of an antiviral mouthwash as a barrier measure in the
SARS-CoV-2 transmission in adults with asymptomatic to mild
COVID-19: A multicentre, randomized, double-blind controlled trial.
Clin Microbiol Infect. 27:1494–501. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Takeda R, Sawa H, Sasaki M, Orba Y, Maishi
N, Tsumita T, Ushijima N, Hida Y, Sano H, Kitagawa Y and Hida K:
Antiviral effect of cetylpyridinium chloride in mouthwash on
SARS-CoV-2. Sci Rep. 12(14050)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vilhena FV, Brito Reia VC, da Fonseca
Orcina B, Santos CA, Zangrando M, Cardoso de Oliveira R and da
Silva Santos PS: The use of antiviral phthalocyanine mouthwash as a
preventive measure against COVID-19. GMS Hyg Infect Control.
16(Doc24)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martínez Lamas L, Diz Dios P, Pérez
Rodríguez MT, Del Campo Pérez V, Cabrera Alvargonzalez JJ, López
Domínguez AM, Fernandez Feijoo J, Diniz Freitas M and Limeres Posse
J: Is povidone iodine mouthwash effective against SARS-CoV-2? First
in vivo tests. Oral Dis. 28 (Suppl 1):S908–S911. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jain A, Grover V, Singh C, Sharma A, Das
DK, Singh P, Thakur KG and Ringe RP: Chlorhexidine: An effective
anticovid mouth rinse. J Indian Soc Periodontol. 25:86–88.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fernandez MDS, Guedes MIF, Langa GPJ,
Rösing CK, Cavagni J and Muniz FWMG: Virucidal efficacy of
chlorhexidine: A systematic review. Odontology. 110:376–392.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Perussolo J, Teh MT, Gkranias N, Tiberi S,
Petrie A, Cutino-Moguel MT and Donos N: Efficacy of three
antimicrobial mouthwashes in reducing SARS-CoV-2 viral load in the
saliva of hospitalized patients: A randomized controlled pilot
study. Sci Rep. 13(12647)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Choudhury MIM, Shabnam N, Ahsan T, Kabir
MS, Khan RM and Ahsan SMA: Effect of 1% povidone iodine
mouthwash/gargle, nasal and eye drop in COVID-19 patient. Biores
Commun. 7:919–23. 2021.
|
|
12
|
Arefin MK, Rumi SKNF, Uddin AKMN, Banu SS,
Khan M, Kaiser A, Chowdhury JA, Khan MAS and Hasan MJ: Virucidal
effect of povidone iodine on COVID-19 in the nasopharynx: An
open-label randomized clinical trial. Indian J Otolaryngol Head
Neck Surg. 74 (Suppl 2):S2963–S2967. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weber J, Bonn EL, Auer DL, Kirschneck C,
Buchalla W, Scholz KJ and Cieplik F: Preprocedural mouthwashes for
infection control in dentistry-an update. Clin Oral Investig. 27
(Suppl 1):S33–S44. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Buonavoglia A, Camero M, Lanave G, Catella
C, Trombetta CM, Gandolfi MG, Palazzo G, Martella V and Prati C:
Virucidal activity in vitro of mouthwashes against a feline
coronavirus type II. Oral Dis. 28 (Suppl 2):S2492–S2499.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Elzein R, Abdel-Sater F, Fakhreddine S,
Abi Hanna P, Feghali R, Hamad H and Ayoub F: In vivo evaluation of
the virucidal efficacy of chlorhexidine and povidone-iodine
mouthwashes against salivary SARS-CoV-2. A randomized-controlled
clinical trial. J Evid Based Dent Pract. 21(101584)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ortega KL, Rech BD, El Haje GL, Gallo CD,
Pérez-Sayáns M and Braz-Silva PH: Do hydrogen peroxide mouthwashes
have a virucidal effect? A systematic review. J Hosp Infect.
106:657–662. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen MH and Chang PC: The effectiveness of
mouthwash against SARS-CoV-2 infection: A review of scientific and
clinical evidence. J Formos Med Assoc. 121:879–885. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Imran E, Khurshid Z, M Al Qadhi AA, A
Al-Quraini AA and Tariq K: Preprocedural use of povidone-iodine
mouthwash during dental procedures in the COVID-19 pandemic. Eur J
Dent. 14:S182–S184. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Garcia-Sanchez A, Peña-Cardelles JF,
Salgado-Peralvo AO, Robles F, Ordonez-Fernandez E, Ruiz S and Végh
D: Virucidal activity of different mouthwashes against the salivary
load of SARS-CoV-2: A narrative review. Healthcare (Basel).
10(469)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kramer A, Eggers M, Hübner NO, Walger P,
Steinmann E and Exner M: Virucidal gargling and virucidal nasal
spray. GMS Hyg Infect Control. 16(Doc02)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Santos C, da Fonseca Orcina B, Brito Reia
VC, Ribeiro LG, Grotto RMT, Prudenciatti A, de Moraes LN,
Ragghianti Zangrando M, Vilhena FV and da Silva Santos PS:
Virucidal activity of the antiseptic mouthwash and dental gel
containing anionic phthalocyanine derivative: In vitro study. Clin
Cosmet Investig Dent. 13:269–274. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moosavi MS, Aminishakib P and Ansari M:
Antiviral mouthwashes: Possible benefit for COVID-19 with
evidence-based approach. J Oral Microbiol.
12(1794363)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mezarina Mendoza JPI, Trelles Ubillús BP,
Salcedo Bolívar GT, Castañeda Palacios RDP, Herrera Lopez PSG,
Padilla Rodríguez DA and Uchima Koecklin KH: Antiviral effect of
mouthwashes against SARS-COV-2: A systematic review. Saudi Dent J.
34:167–193. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu C, Wang A, Hoskin ER, Cugini C,
Markowitz K, Chang TL and Fine DH: Differential effects of
antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro.
Pathogens. 10(272)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang M, Meng N, Duo H, Yang Y, Dong Q and
Gu J: Efficacy of mouthwash on reducing salivary SARS-CoV-2 viral
load and clinical symptoms: A systematic review and meta-analysis.
BMC Infect Dis. 23(678)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang YH and Huang JT: Use of
chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19
patients. J Med Virol. 93:4370–4373. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kuznetsova MV, Nesterova LY, Mihailovskaya
VS, Selivanova PA, Kochergina DA, Karipova MO, Valtsifer IV,
Averkina AS and Starčič Erjavec M: Nosocomial Escherichia coli,
klebsiella pneumoniae, pseudomonas aeruginosa, and staphylococcus
aureus: Sensitivity to chlorhexidine-based biocides and prevalence
of efflux pump genes. Int J Mol Sci. 26(355)2025.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sánchez Barrueco Á, Mateos-Moreno MV,
Martínez-Beneyto Y, García-Vázquez E, Campos González A, Zapardiel
Ferrero J, Bogoya Castaño A, Alcalá Rueda I, Villacampa Aubá JM,
Cenjor Español C, et al: Effect of oral antiseptics in reducing
SARS-CoV-2 infectivity: Evidence from a randomized double-blind
clinical trial. Emerg Microbes Infect. 11:1833–1842.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Green A, Roberts G, Tobery T, Vincent C,
Barili M and Jones C: In vitro assessment of the virucidal activity
of four mouthwashes containing Cetylpyridinium Chloride, ethanol,
zinc and a mix of enzyme and proteins against a human coronavirus.
BioRxiv: 2020-10, 2020.
|
|
30
|
Rius-Salvador M, García-Múrria MJ, Rusu L,
Bañó-Polo M, León R, Geller R, Mingarro I and Martinez-Gil L:
Cetylpyridinium chloride and chlorhexidine show antiviral activity
against Influenza A virus and Respiratory Syncytial virus in vitro.
PLoS One. 19(e0297291)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Saud Z, Tyrrell VJ, Zaragkoulias A, Protty
MB, Statkute E, Rubina A, Bentley K, White DA, Rodrigues PDS,
Murphy RC, et al: The SARS-CoV2 envelope differs from host cells,
exposes procoagulant lipids, and is disrupted in vivo by oral
rinses. J Lipid Res. 63(100208)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rahman GS, Alshetan AAN, Alotaibi SSO,
Alaskar BMI and Baseer MA: Is chlorhexidine mouthwash effective in
lowering COVID-19 viral load? A systematic review. Eur Rev Med
Pharmacol Sci. 27:366–377. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Onozuka D, Takatera S, Matsuo H, Yoshida
H, Hamaguchi S, Yamamoto S, Sada RM, Suzuki K, Konishi K and
Kutsuna S: Oral mouthwashes for asymptomatic to mildly symptomatic
adults with COVID-19 and salivary viral load: A randomized,
placebo-controlled, open-label clinical trial. BMC Oral Health.
24(491)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ang WX, Tan SH, Wong KT, Perera D,
Kuppusamy UR and Ong KC: Antiviral activity of povidone-iodine
gargle and mouthwash solution against Enterovirus A71,
Coxsackieviruses A16, A10 and A6. Trop Biomed. 41:241–250.
2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vergara-Buenaventura A and Castro-Ruiz C:
Use of mouthwashes against COVID-19 in dentistry. Br J Oral
Maxillofac Surg. 58:924–927. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Verma SK, Dev Kumar B, Chaurasia A and
Dubey D: Effectiveness of mouthwash against viruses: 2020
Perspective. A systematic review. Minerva Dent Oral Sci.
70:206–213. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jaiyesimi R, Oyeniyi S and Bosun-Arije F:
The potential benefit of mouthwashes in reducing COVID-19 viral
load: A mini review. Proc Niger Acad Sci 13: ISSN 0794-7976,
2020.
|
|
38
|
Avhad SK, Bhanushali M, Sachdev SS, Save
SS, Kalra D and DN K: Comparison of effectiveness of chlorine
dioxide mouthwash and chlorhexidine gluconate mouthwash in
reduction of oral viral load in patients with COVID-19. Indian J
Public Health Res Deve. 11:27–32. 2020.
|