1. Introduction
The escalating issue of meat inflation, the
increasing global population and the need for a nutritionally
balanced diet, while minimizing greenhouse gas emissions have all
contributed to a surge in demand for protein from plant sources.
Since the primary protein sources are derived from animals and the
strain on current animal sources is deemed unsustainable, there is
an urgent need for novel, sustainable protein sources that are safe
and of high nutritional quality. Therefore, the food industry and
researchers are tasked with finding alternative protein sources and
production methods to satisfy consumer demand and anticipated
global protein needs.
While plant-based proteins are nutritionally
beneficial, they require land and water, which are becoming
increasingly scarce. Moreover, the conversion of plant proteins
into meat proteins is inefficient, requiring 6 kg of plant proteins
to produce 1 kg of meat proteins (1). Single-cell proteins, mainly composed
of dried cells (biomass) produced by algae, yeast, bacteria and
fungi, present a viable alternative to traditional protein sources
in this context (2). Microalgae,
unicellular photosynthetic organisms, are considered promising
alternative protein sources (3).
They can produce biomass and oxygen by harnessing sunlight as an
energy source, CO2 as a carbon source and inorganic
salts as a carbon supply (4).
Microalgae exist in freshwater, marine and edaphic
habitats, with some species also found in humid soils (5). Unlike conventional food crops,
microalgae can thrive in various conditions without requiring
arable land, thus not competing with crops, such as soybeans and
cereal grains (6).
The broad spectrum of microalgae includes
>100,000 species divided into four distinct groups:
Chlorophyceae (green algae), Bacillariophyceae (eukaryotic
diatoms), Chrysophyceae (golden algae) and Cyanophyceae (blue-green
algae) (7). Despite their vast
diversity, microalgae remain under-researched and poorly understood
(4). Researchers have found that
microalgae are an attractive source for developing beneficial
products with significant value in the cosmetics, food and
nutrition, medicinal and pollution-prevention sectors (8).
Chlorella spp. was the first algae discovered
and isolated in culture by Beijerinck (9). Of note >20 Chlorella
species have been identified, with >100 strains documented
(10). The exploration of the
dietary benefit of Chlorella on human health began in the
early 1950s when Chlorella was introduced as a food source
during a global food crisis (11).
It was first grown and consumed in Asia, mainly in Japan, before
gaining popularity as a nutritional supplement worldwide (12).
Chlorella spp. contains protein levels
similar to traditional protein sources such as meat, egg, soybean
and milk (3). Research has shown
that it contains 55-67% protein, 1-4% chlorophyll, 9-18% dietary
fiber, and various minerals and vitamins on a dry matter basis
(13). In particular, Chlorella
vulgaris is known to contain up to 58% protein on a dry weight
basis (14) and is considered safe
for human consumption by the Food and Drug Administration (FDA) in
the USA, along with other microalgae species, such as
Arthrospira platensis (Spirulina) due to their long history
of human consumption and nutrient profile (15). However, limited reports are
available on the protein content and quality of Chlorella
sorokiniana (C. sorokiniana) and its application in
food. Thus, the present review aimed to evaluate C.
sorokiniana biomass as an alternative ingredient for food. The
present review aimed to enhance the understanding of the biomass
characteristics, bioactive substances, functional components,
biomass-based food products, and health advantages linked to the
consumption of C. sorokiniana microalgae.
2. Review methodology and literature
selection
A systematic approach was utilized to select
pertinent literature from databases such as Scopus, Web of Science,
CrossRef, PubMed and Google Scholar to ensure a comprehensive
review. The search for articles included in the present review
involved utilizing specific key terms, such as ‘C.
sorokiniana Characteristics’, ‘Macronutrients in C.
sorokiniana’, ‘Protein Quality of C. sorokiniana’,
‘Micronutrients in C. sorokiniana’, ‘Bioactive compounds in
C. sorokiniana’, and ‘C. sorokiniana Food Products’.
Subsequently, the databases were used in conjunction with VOSviewer
software to conduct a bibliometric analysis (16). Articles were selected based on
various criteria, including their relevance to the topic and the
presence of substantial reviews or experimental data on C.
sorokiniana microalgae.
3. C. sorokiniana biomass
characteristics
C. sorokiniana, a freshwater green microalga,
is part of the Chlorophyta division. Initially, it was considered
to be a thermotolerant mutant of Chlorella pyrenoidosa
(17). However, chloroplast 16S
rDNA and 18S rRNA profiling in the late 1980s and early 1990s
identified C. sorokiniana as a separate species (10). This tiny, single-celled alga,
ranging in size from 2 to 4.5 µm in diameter, can grow
mixotrophically on various carbon and nitrogen sources, rendering
it ideal for waste feedstock cultivation (18).
This chlorophyte has several advantages over other
microalgae species. Previous studies have demonstrated that C.
sorokiniana can achieve optimal growth at temperatures ranging
from 35 to 40˚C (19), with
phototrophic doubling times as short as 4-6 h (20). It can also grow under harsh
conditions, including wastewater or heavy metal wastes (21). It has been reported to have a more
rapid growth in mixotrophic and even heterotrophic conditions, with
a preference for sugars, such as glucose or simple organic acids,
such as acetate (22).
The typical physicochemical composition and
biological value (BV) indicators of the dry biomass of C.
sorokiniana, obtained by cultivation in a pilot bioreactor, are
presented in Tables I and II. The dry biomass of C.
sorokiniana contains an average of 48% protein, which is
markedly higher than that of soy, peanut, wheat germ and other
animal sources such as beef, fish and chicken (23,24).
The lipid content of the biomass sample is 13-22%, comparable to or
slightly higher than that of other aquacultures, such as
Spirulina spp (25). The
biomass is rich in physiologically active phytochemicals, the most
abundant of which are chlorophylls (22.13 mg/g dry biomass) and
carotenoids (6.04 mg/g dry biomass) (23) (Table
III). C. sorokiniana consists of 30-38% carbohydrates on
a dry weight basis (26), which is
higher than that C. vulgaris, with ~15% carbohydrate content
on a dry basis (27).
 | Table IPhysicochemical characteristics of
Chlorella sorokiniana on a dry basis. |
Table I
Physicochemical characteristics of
Chlorella sorokiniana on a dry basis.
| Components | Content |
|---|
| Appearance,
dry | Free-flowing
powder |
| Color, dry | Green |
| Taste and
smell | Fishy,
characteristic of algae |
| External
admixtures | Absent/none |
| Moisture content,
% | 4% |
| Protein, g/100 g
dry biomass | 47.82±2.30 |
| Lipids, g/100 g dry
biomass | 17.50±4.50 |
| Carbohydrates,
g/100 g dry biomass | 30.00±4.00 |
| Minerals, mg | 4.36±0.56 |
 | Table IIIndicators of the biological value
(BV) of the dry biomass of Chlorella sorokiniana
microalgae. |
Table II
Indicators of the biological value
(BV) of the dry biomass of Chlorella sorokiniana
microalgae.
| Polyunsaturated
fatty acids (PUFAs) | Concentration
(mg/g) | Carbohydrates
(sugars) | Concentration
(mg/g) | Phytochemicals | Concentration
(mg/g) |
|---|
| ω3 | 26.6±1.16 | Sucrose | 204.0±2.00 | Chlorophyll | 22.13±2.20 |
| Eicosapentanoic
acid | 0.53±0.02 | | | | |
| α-linolenic
acid | 16.1±0.60 | Glucose | 133.0±1.20 | Phenolic
compounds | 0.05±0.02 |
| Docosahexaenic
acid | 10.0±0.20 | | | | |
| ω6 | 25.7±1.00 | Xylose | 58.0±0.50 | Carotenoids | 6.04±0.60 |
| E-linoleic
acid | 3.3±0.05 | | | | |
| Z-linoleic
acid | 14.8±0.50 | Fructose | 20.0±0.30 | Organic acids | 2.70±0.40 |
| Octadecatrienoic
acid | 7.5±0.10 | | | | |
 | Table IIIPER, BV, NPU and DC of various
protein sources. |
Table III
PER, BV, NPU and DC of various
protein sources.
| Protein
sources | PERa | BVa | NPUa | DCa |
|---|
| Beef | 116.0 | 103.9 | 96.1 | 100.0 |
| Soy protein | 88.0 | 96.1 | 80.3 | 99.0 |
| Wheat
gluten/cereal | 32.0 | 83.1 | 88.2 | 91.9 |
| Chlorella
spp. | 80.0 | 87.2 | 81.5 | 93.6 |
Factors such as temperature, nutritional composition
and light availability can influence the quantities of biomass,
macro- and micronutrients, and other useful bioactive substances,
including antioxidants, in Chlorella cells (28).
4. Macronutrients in C. sorokiniana
biomass
The average macronutrient content of C.
sorokiniana biomass based on previous studies (23,31,32)
is summarized in Table IV. The
analysis of C. sorokiniana dry biomass has revealed that it
contains an average of 45% protein, which is much greater than the
protein content of foods such as peanut (26%), wheat germ (27%) and
other animal sources, such as beef (22%), fish (22%) and chicken
(24%) (23,24,33).
 | Table IVMacronutrient content of Chlorella
sorokiniana biomass on a dry basis. |
Table IV
Macronutrient content of Chlorella
sorokiniana biomass on a dry basis.
| Macronutrients | Content (g/100 g
dry weight) |
|---|
| Proteins | 44.75±1.88 |
| Lipids | 16.42±5.18 |
| Carbohydrates | 37.61±1.81 |
The essential amino acid composition of C.
sorokiniana biomass is presented in Table V. Notably, these amino acid
profiles, which mammals do not synthesize, are present in notable
concentrations in C. sorokiniana microalgae; thus, when
compared to the recommendations of the World Health Organization
for the use of essential amino acids in food, this microalga could
be used in the food industry (32). The high protein content and
favorable amino acid composition of C. sorokiniana
microalgae suggest that this microalga may serve as a potential
protein source.
 | Table VEssential amino acid composition of
Chlorella sorokiniana microalgae. |
Table V
Essential amino acid composition of
Chlorella sorokiniana microalgae.
| Essential amino
acid | Content (mg/g) |
|---|
| Histidine | 6.1±0.60 |
| Threonine | 19.0±2.20 |
| Isoleucine | 4.53±0.04 |
| Valine | 20.0±1.20 |
| Methionine | 1.8±0.20 |
| Tryptophan | 0.2±0.02 |
| Phenylalanine | 18.0±1.00 |
| Leucine | 30.0±2.10 |
| Lysine | 21.0±1.60 |
According to Gouveia and Oliveira (25), the lipid content of C.
sorokiniana biomass on a dry basis ranges from 11.24-21.6% dry
matter. It is equivalent to or slightly higher than other
aquacultures, such as Spirulina spp. (4-9% dry matter). As
shown in Table VI, ~80% of the
total fatty acid content of C. sorokiniana lipids is
polyunsaturated fatty acids (PUFAs) (23). Since this microalga biomass
contains sufficient amounts of omega-3 PUFAs (~25-28% of total
fatty acids) and omega-6 PUFAs (~25-27% of total fatty acids), it
can effectively prevent and treat several diseases. Indeed, omega-3
PUFAs, such as alpha-linolenic acid (ALA, C18:3), eicosapentaenoic
acid (EPA, C20:5), docosapentaenoic acid (DPA, C22:5) and
docosahexaenoic acid (DHA, C22:6) are effective in preventing or
treating cardiovascular disorders (34), lowering hypertension (35) as well as preventing cancer
(36,37), type 2 diabetes (38), inflammatory bowel disorder
(39), asthma (13), arthritis (40), kidney and skin disorders (38,39),
depression (13) and schizophrenia
(41). Furthermore, due to its
lipid composition, C. sorokiniana may also play an essential
function in preventing atherosclerosis, hypercholesterolemia and
tumors (40).
 | Table VIPUFAs and carbohydrates in
Chlorella sorokiniana microalgae dry biomass. |
Table VI
PUFAs and carbohydrates in
Chlorella sorokiniana microalgae dry biomass.
| Components | Content (mg/g dry
weight) |
|---|
| PUFAs | |
|
Alpha-linolenic
acid (ALA) | 16.1±0.60 |
|
Eicosapentanoic
acid (EPA) | 0.53±0.02 |
|
Docosahexaenoic
acid (DHA) | 10.0±0.20 |
|
Total
omega-3 | 26.6±1.16 |
|
E-linoleic
acid (LA) | 3.3±0.05 |
|
Z-linoleic
(LA) | 14.8±0.50 |
|
Octadecatrienoic
acid | 7.5±0.10 |
|
Total
omega-6 | 25.7±1.00 |
| Carbohydrates | |
|
Sucrose | 204.0±2.00 |
|
Glucose | 133.0±1.20 |
|
Xylose | 58.0±0.50 |
|
Fructose | 20.0±0.30 |
Another key class of macronutrient substances found
in the hydrophilic fraction of C. sorokiniana microalgae
biomass comprises carbohydrates. Effectively, 36-39% dry weight of
carbohydrates found in C. sorokoniana microalgae biomass are
found in the form of sucrose, glucose, xylose and fructose
(23,31,42).
Moreover, the cell wall of C. sorokinana is also composed of
many insoluble polysaccharides, primarily mannose and glucose
(43). Polysaccharides are
polymeric carbohydrate structures commonly employed in the food
industry (44). Owing to their
high polysaccharide concentration, microorganisms such as
Chlorella microalgae have been actively investigated in the
search for novel natural antioxidants (44,45).
In the study by Pugh et al (46), immurella polysaccharides, a high
molecular weight polysaccharide, were isolated from C.
sorokiniana, which presented a higher activity against cancer.
Polysaccharides derived from C. sorokiniana microalgae have
also been shown to exhibit substantial antioxidant, tumor-fighting,
immunomodulatory characteristics, and the ability to remove
superoxide and hydroxyl peroxide radicals (47).
5. Protein quality of C. sorokiniana
biomass
The considerable protein content of C.
sorokiniana has positioned it as a promising alternative
protein source. The protein content in microalgae is typically
determined by measuring the total nitrogen and multiplying it by
the factor Nx6.25(48).
Experimentally determined N-protein factors for specific microalgal
species range from Nx3.06 to 5.95 (49,50).
This variation in the N-protein factor is attributed to non-protein
nitrogen, which comprises up to ~10% of the nitrogen in microalgae
and includes substances, such as amines, glucosamines, nucleic
acids and cell wall components (48).
Of note, four important quality parameters are
considered to assess the nutritional value of algal protein:
Protein efficiency ratio (PER), BV, digestibility coefficient (DC)
or true digestibility, and net protein utilization (NPU) (14). PER is a fundamental method for
evaluating protein quality, involving animal feeding trials with
weanling rats throughout 3 to 4 weeks (48). It measures the weight gain per unit
of protein consumed, expressed as PER=weight gain (g)/protein
intake (g) (48).
BV, another metric for assessing protein quality, is
based on the proportion of retained nitrogen to absorbed nitrogen
(48). The calculation for BV is
BV=[I-(F-F0)-(U-U0)]/[I-(F-F0)], where I represents nitrogen
intake, F is fecal nitrogen, U is urinary nitrogen, and F0 and U0
are the amounts of fecal and urinary nitrogen excreted when animals
are fed a nitrogen-free or low-nitrogen diet (51).
DC, also known as real digestibility, is another
indicator of protein quality. It reflects the proportion of meal
nitrogen absorbed by the animal and is calculated using the same
parameters as BV: DC=[I-(F-F0)]/I (48). NPU, on the other hand, is
determined by dividing nitrogen retained by nitrogen intake. The
measure of both digestibility and BV is provided by the NPU, which
is calculated using the formula NPU=(B-Bk)/I. Herein, B represents
the body nitrogen assessed at the end of the test period in animals
fed the test food. Bk represents the body nitrogen measured in
another group of animals fed a protein-free or low-protein diet
(48).
A comparison between Chlorella spp.
microalgae and traditional protein sources based on four quality
parameters (PER, BV, DC and NPU) is presented in Table III. The values in the table were
compared to casein, the primary protein found in milk, which serves
as a reference for scoring. Overall, the values presented in
Table III indicate that the
nutritional value of Chlorella spp. algal protein is lower
than that of beef and casein. However, it is comparable to that of
soy protein and superior to that of wheat gluten (52).
Amino acid composition
Protein quality can vary significantly based on the
digestion and the availability of essential amino acids (EAAs)
(53). Animal protein sources are
often considered complete proteins as they contain a high
concentration of EAAs that the human body cannot synthesize
(3). By contrast, plant proteins
are frequently observed as incomplete protein sources as they may
lack one or more EAAs, such as histidine, isoleucine, leucine,
lysine, methionine, phenylalanine, threonine, tryptophan and valine
(54). For example, soy protein,
which is increasingly popular in the human diet, is deficient in
methionine, an essential antioxidant in the body (55). However, the specific EAAs acids
lacking in plant-based proteins can vary. Therefore, consuming a
varied diet of plant proteins from fruits, vegetables, grains and
legumes can provide an adequate quantity of all EAAs (56).
Plant-based proteins are often more challenging to
digest than animal proteins due to differences in their protein
structure (57). Plant-based
proteins have a higher proportion of β-sheet conformation and a
relatively lower α-helix conformation than animal proteins
(58). The high concentration of
β-sheet conformation in plant proteins renders them more resistant
to proteolysis in the gastrointestinal system, reducing their
digestibility (58). Additionally,
plant-based sources may contain non-starch polysaccharides or
fibers that hinder enzyme access to proteins and can further reduce
protein digestion (59).
Despite these differences, there is an increasing
concern about the high levels of saturated fats and cholesterol
present in animal-derived foods, which have been linked to
cardiovascular disease and diabetes. As a result, nutritionists and
organizations such as the Food and Agriculture Organization (FAO)
recommend a diverse diet rich in plant-based proteins (60).
Analyzing the amino acid composition is crucial for
understanding the value of food raw materials. Algae, including
microalgae such as Chlorella spp. and Spirulina spp.,
are widely recognized as viable protein sources with EAA
compositions that meet the FAO standards (61,62).
WHO/FAO/UNU has recommended these microalgae species for their
adequate EAA content for human consumption (62). Microalgae, including Chlorella
spp., have been found to contain amino acids, such as
isoleucine, valine, lysine, tryptophan, methionine, threonine and
histidine in amounts equivalent to or greater than protein-rich
sources, such as eggs and soybeans (33). Notably, the dry biomass of C.
sorokiniana microalgae provides all the amino acids necessary
for the growth and development of living organisms (Table III). Research has shown that the
quality of Chlorella products, including C.
sorokiniana (Table V), is high
based on the essential amino acid index, with values higher than
that of soybean protein (63).
These findings suggest that Chlorella proteins are of high
quality.
Microalgae protein digestibility
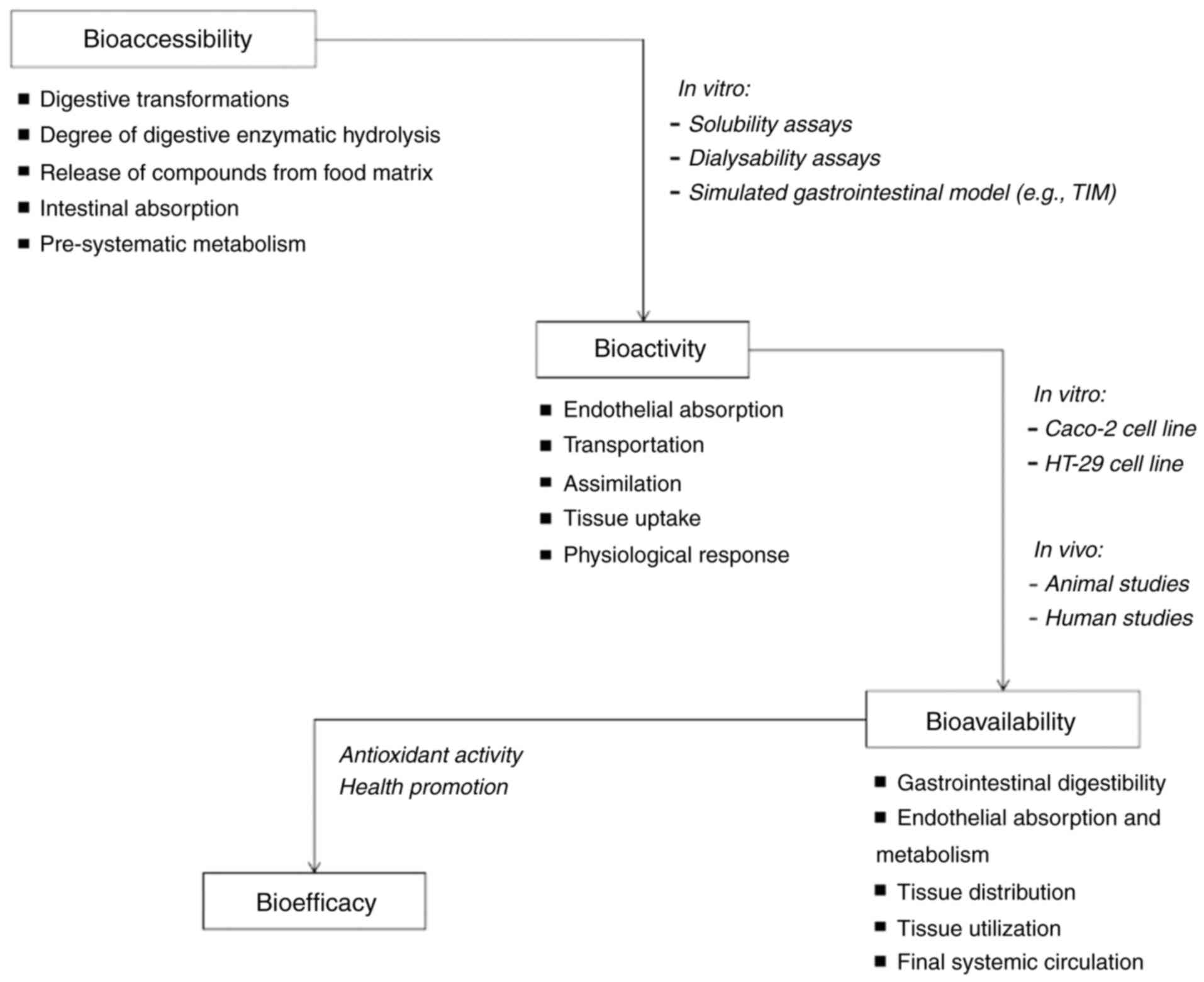
The FDA defines bioavailability as the rate and
extent to which the active ingredient is absorbed and becomes
accessible at the site of action. It encompasses the entire process
after consuming a food element, including its digestibility and
solubility in the gastrointestinal tract, absorption into the
circulatory system through intestinal epithelial cells, and
eventual incorporation into the target site of utilization
(Fig. 1). The bioavailability of
food nutrients is considered crucial for maintaining optimal
health. It also plays a crucial role in developing functional foods
and health claims based on food components, as understanding the
circulating metabolites and mechanisms of action is essential.
Bioavailability is further divided into two stages:
Bioaccessibility and bioactivity. Bioaccessibility refers to the
proportion of a component in a meal that is released from the food
matrix during digestion and becomes available for intestinal
absorption or undergoes biotransformation by gut bacteria (64). On the other hand, bioactivity
involves the assimilation of a food element across intestinal
cells, transport to the target site, interaction with the target
site, any necessary biotransformation, and the resulting
physiological response (65). The
activity of ingested substances or their metabolites in metabolic
pathways leads to biological effects on the body.
Several factors influence digestibility, rendering
in vitro research challenging. These factors include the
composition of macronutrients, enzyme specificity, anti-nutritional
substances, fiber content and variations in absorptive capabilities
along the gastrointestinal tract. Additionally, unraveling the
bioavailability of food constituents is more complex than
pharmaceutical drugs due to the diverse nature of food compounds,
the various factors affecting their transition during digestion,
and the different absorption mechanisms for water-soluble and
lipid-soluble molecules (66).
In vitro research can be an initial screening technique to
identify potential food matrices, growing conditions, and
processing methods.
In addition to understanding the definition of the
two stages of bioavailability, it is crucial to define
digestibility to comprehend the metabolism dynamics of different
dietary matrices. Digestibility refers to the amount of nutrients
an individual absorbs and is typically calculated as the difference
between the nutrients ingested and the amount retained in
feces.
Assessing the potential of novel food sources
involves determining their biochemical composition. In vitro
and in vivo studies on digestibility, bioaccessibility and
bioavailability are crucial in gaining comprehensive knowledge
about nutrient interactions with food components. Such studies also
investigate the effects of pH and enzymes on absorbability,
providing insight into the potential nutrients that can be absorbed
(Fig. 1) (67). Therefore, understanding the
digestibility and bioaccessibility of algal compounds is essential
to maximize the potential of algal food products and
co-products.
Researching algal digestibility is particularly
critical, considering the complexity of the polysaccharide cell
wall. The cell wall composition of some algae, including
microalgae, typically consists of cellulose, hemicellulose, pectin
compounds and glycoproteins (68).
These robust wall structures can restrict the accessibility of
digestive enzymes to intracellular algae compounds, limiting
nutrient and algae component bioaccessibility or bioavailability.
Extraction or pre-treatment procedures, such as enzymatic
hydrolysis or chemical, mechanical and physical approaches, can
promote cell disruption, enhancing algal nutrient digestibility and
bioaccessibility (69). Approaches
can encourage disruption cells, thereby enhancing algal nutrient
digestibility and bioaccessibility.
There are limited studies available in the
literature on microalgae digestibility, including investigations on
in vitro bioaccessibility, primarily due to the
aforementioned factors. However, certain microalgae species exhibit
distinct characteristics regarding digestibility. For instance,
Arthrospira platensis has a fragile cell wall, indicating
easily digestible biomass. On the other hand, Chlorophyta species
such as Chlorella vulgaris and Haematococcus
lacustris are tiny microorganisms (5-10 µm) with rigid cell
walls that require prior disruption to enhance the digestibility
and bioaccessibility of microalgal compounds (70).
A recent study conducted by Gómez-Jacinto et
al (71) focused on the in
vitro selenium bioaccessibility, in vivo bioavailability
and bioactivity of the Se-enriched microalga C. sorokiniana
for potential use as a functional food. The in vitro
gastrointestinal digestion of the selenized microalga demonstrated
81% bioaccessibility. In vivo experiments on mice treated
with Se-enriched C. sorokiniana revealed significant
selenium concentrations in the kidney, indicating a potential
excretion pathway through urine (71). This suggests that Se-enriched C.
sorokiniana can serve as a nutraceutical ingredient and a
dietary selenium supplement for humans.
When comparing the digestibility of traditional
protein sources, milk and eggs have the highest true digestibility
values, ~97% (72). They are
followed by meats, fish and poultry (73). By contrast, microalgae species such
as Scenedesmus obliquus, Arthrospira platensis
(Cyanobacteria), Spirulina sp. and Chlorella sp.
exhibit apparent DC values of 88.0, 86, 77.6 and 76.6%,
respectively (14,68).
6. Micronutrients in C. sorokiniana
biomass
Chlorella is an excellent source of vitamins
and minerals that adults and children can easily consume to meet
their daily vitamin requirements (12). As demonstrated in Table VII, C. sorokiniana
microalgae biomass contains the balanced mineral content required
in humans. In acceptable amounts, iron, copper and zinc are trace
elements necessary for the human body to function normally
(74). It is important to note
that C. sorokiniana biomass contains substantial amounts of
iron (18 mg/100 g dry weight) and zinc (20.2 mg/100 g dry weight),
of which adequate intake prevents anemia (75) and muscular weakness (76), respectively. Iron is required for
respiration, energy production, DNA synthesis and cell
proliferation (77). In previous a
study conducted by Nakano et al (78), oral Chlorella
supplementation (6 g/day) for 12-18 weeks lowered markers of anemia
in a cohort of 32 women in their second and third trimesters of
pregnancy compared to the control group. This suggests that
Chlorella supplementation considerably reduces the risk of
developing pregnancy-associated anemia (79).
 | Table VIIMineral composition of Chlorella
sorokiniana dry biomass. |
Table VII
Mineral composition of Chlorella
sorokiniana dry biomass.
| Metals | Content (mg/100 g
dry weight) |
|---|
| Iron (Fe) | 18.00±2.00 |
| Copper (Cu) | 3.00±0.07 |
| Zinc (Zn) | 20.20±2.02 |
| Manganese (Mn) | 8.00±0.15 |
| Cobalt (Co) | 1.00±0.01 |
| Magnesium (Mg) | 91.00±5.00 |
| Calcium (Ca) | 31.00±2.50 |
Moreover, some of the vitamins found in notable
quantities in Chlorella are vitamin A (in the form of
β-carotene), vitamin C, vitamin E, Vitamins K and vitamin B, such
as thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid
(B5), pyridoxine (B6), folic acid (B9) and cobalamin (B12)
(12,44). These vitamins fuel the body,
detoxify and restore digestive function, activate the immune system
and renew cells (44). Moreover,
the vitamin E content of green microalgae Chlorella
contributes to its high antioxidant activity (80,81).
7. Bioactive compounds in C.
sorokiniana biomass
Bioactive compounds are nutrients and non-nutrients
found in food (both plant and animal sources) that have
physiological effects in addition to their nutritional qualities
(82). Foods high in bioactive
chemicals that are routinely consumed positively impact human
health by performing antioxidant activity, reducing the risk of
developing diseases such as heart disease, diabetes, cancer,
cataracts and stroke (83,84). Microalgae are highly diverse and
contain significant bioactive compounds, including polyphenols,
carotenoids and organic acids, as presented in Table VIII.
 | Table VIIIIndicators of the biological value of
Chlorella sorokiniana dry biomass. |
Table VIII
Indicators of the biological value of
Chlorella sorokiniana dry biomass.
|
Phyto-chemicals | Content (mg/g dry
weight) |
|---|
| Chlorophyll | 22.13±2.20 |
| Carotenoids | 6.04±0.60 |
| Total phenolic
compounds (GAE) | 12.27±2.31 |
| Total flavonoid
content (QE) | 15.05±3.83 |
Pigments are essential for the metabolism of
photosynthetic microalgae and have antioxidant, anti-carcinogenic,
anti-inflammatory, anti-obesity, anti-angiogenic and
neuroprotective properties in different metabolisms, including
human metabolism (44).
Chlorella contains substantial levels of chlorophyll,
similar to higher plants, although in markedly higher
concentrations than terrestrial plants, with the potential to
collect chlorophyll to a concentration of 2.5% (86). Chlorophyll is the principal natural
green pigment found in microalgae and plants, where it is needed
for oxygenic photosynthesis (87).
As presented in Table VIII,
C. sorokiniana biomass contains a notable amount of
biologically active phytochemicals, with chlorophylls (22.13 mg/g
dry biomass) dominating (23).
Azaman et al (88) also
discovered that under photoautotrophic conditions, C.
sorokiniana exhibited a total chlorophyll concentration of
24.37 µg/mg dry weight of the sample, which was more significant
than that of Chromochloris zofingiensis.
Carotenoid is a pigment in algae, plants, fungi and
bacteria that ranges in hue from yellow to red (84). It has health-promoting qualities
are mostly linked to its antioxidant activity that scavenges
singlet molecular oxygen and radicals (84). Previous research has shown that
carotenoids significantly contribute to the total antioxidant
capacity of microalgae (89,90).
In particular, Diprat et al (91) identified the carotenoid types
present in C. sorokiniana microalgae powder, which includes
violaxanthin, lutein, zeaxanthin, β-carotene and α-carotene. Using
high-performance liquid chromatography coupled to diode array and
mass spectrometry detectors, Fernandes et al (92) also identified 11 carotenoids in
C. sorokiniana, with the greatest significant quantities of
all-trans-lutein (831.18±1.18 g/g dry weight),
all-trans-β-carotene (156.21±0.22 g/g dry weight) and
all-trans-α-carotene (71.47±0.10 g/g dry weight). Indeed,
including C. sorokiniana biomass as a raw material in
functional foods will allow goods to be enriched with essential
nutritional components while obtaining the appropriate color range
without synthetic dyes (23).
Phenolic compounds, or polyphenolics, are abundant
secondary metabolites with antioxidant activity and numerous
biological functions that can be derived from microalgae (93). Due to their importance as one of
the most significant classes of natural antioxidants, phenolic
compounds are gaining popularity among consumers and food
manufacturers. They function as reducing agents and hydrogen
donors, capable of scavenging free radicals (94). Phenolic compounds have two basic
chemical structures formed by one or more aromatic rings with
hydroxyl (-OH) groups (93). They
are chemically classified into several classes, including phenolic
acids (hydroxybenzoic acids, hydroxycinnamic acids), flavonoids
(flavones, flavonols, flavanones, flavanonols, flavonols and
anthocyanins), isoflavonoids (isoflavones and coumestans),
stilbenes, lignans and phenolic polymers (proanthocyanidin)
(95).
Based on the Folin-Ciocalteu method, Olasehinde
et al (85) reported that
C. sorokiniana dry biomass contains a total phenolic content
of 12.27±2.31 gallic acid equivalent (GAE) mg/g, which is higher
compared to C. minutissima (10.53±2.82 GAE mg/g dry weight)
(Table VIII). Likewise, the
same authors revealed that C. sorokiniana dry biomass
contains a total flavonoid content of 15.05±3.83 quercetin
equivalent (QE) mg/g dry weight (85). Furthermore, Safafar et al
(96) also presented that C.
sorokiniana dry biomass contains 5.84±0.04 GAE mg/g total
phenolics and 2.45±0.04 QE mg/g total flavonoid content.
Furthermore, they found that C. sorokiniana, grown in
different light intensities, had the same phenolic acid profile
(96). Still, the total detected
phenolic acids in the sample grown in average light intensity was
somewhat higher. Thus, generating phenolics and other antioxidant
chemicals in microalgae depends on growth circumstances and
challenges such as oxidative stress (96).
Apart from having antioxidant properties, phenolic
compounds have therapeutic qualities such as anti-tumor and
antibacterial activities (84).
Chlorella phenolic chemicals may also prevent liver cell
carcinogenesis by blocking lipid peroxidation on the cell membrane,
neutralizing cellular free radicals and preventing DNA damage
(84).
8. C. sorokiniana biomass-based food
products
Given the abundance of nutrients and bioactive
substances found in C. sorokiniana, it is no surprise that
it can be regarded as one of the most promising new food and
functional goods sources. C. sorokiniana has a well-balanced
chemical composition and provides a source of beneficial bioactive
chemicals (13). As a result of
its potential, C. sorokiniana can represent an alternative
and novel source of natural components that can be used as
functional ingredients to improve the nutritional content of
foods.
Chlorella sp. has been extensively utilized
as a dietary supplement for an extended period of time. The
microalgal market offers Chlorella sp. in powder and tablet
forms, primarily due to its ability to effectively sequester heavy
metals, such as mercury, recognized as toxic substances, and
facilitate their elimination from the body (84). In recent times, there has been a
surge in research efforts focused on advancing food products
derived from C. sorokiniana biomass. Pasta (23) and gluten-free bread (91) have been incorporated with C.
sorokiniana biomass for baked food products.
Bazarnova et al (23) examined the effects of incorporating
the dry biomass of microalgae C. sorokiniana as a
replacement for flour mixture to enrich pasta effectively. In their
study, it was shown that flour replacement with C.
sorokiniana should not be >5% due to the formation of a
distinct fish flavor of the product. Moreover, substituting 5%
C. sorokiniana biomass to pasta flour increased proteins to
15.7±0.50% and lipids to 4.1±0.06%. Furthermore, the addition of
C. sorokiniana dry biomass to pasta has helped improve the
polyunsaturated fatty acids, chlorophyll and carotenoid content of
the product (23).
Diprat et al (91) also examined the partial replacement
of pea flour with C. sorokiniana powder at 2.5 and 5% to
increase the nutritional quality of gluten-free bread. The
replacement of 5% C. sorokiniana powder vs. the control with
pea flour increased the protein content (67 to 85 mg/g), lutein
content (1.6 to 57.5 µg/g) and omega-3 content in the fatty acids
(5.0-6.1%) of the gluten-free bread. Furthermore, it is interesting
to note that compared with the pasta food product studied by
Bazarnova et al (23), the
sensory analysis of the gluten-free bread with 5% replacement of
C. sorokiniana powder had an acceptance rate >70%, with
no observed distinct fishy flavor being identified. Furthermore,
the addition of C. sorokiniana powder had no effect on the
texture and specific volume of the bread product.
The expanding number of studies on Chlorella
as a food ingredient have revealed that Chlorella sp. has a
significant potential to be an alternative protein source and
future food. However, further product application of C.
sorokiniana biomass in food needs to be conducted in order to
assess its effects on the physicochemical, sensory,
techno-functional and antioxidant properties as a food ingredient.
Consumers also strongly demand food to prevent illness, improve
mental health and increase life quality, as well as a desire to
consume alternative proteins to be healthy and more sustainable. As
a result, the worldwide market for Chlorella ingredients is
predicted to expand and meet human nutritional requirements
(84).
9. Conclusions and future perspectives
C. sorokiniana microalgae has shown promise
as an alternative protein ingredient to meet the increasing demand
for sustainable food products. There is a growing consumer interest
and awareness regarding alternative protein sources, rendering the
use of microalgal protein in developing healthier food products
highly relevant. The high protein content, balanced amino acid
profiles and bioactive substances in C. sorokiniana make it
a potential dietary source with various health benefits. As
numerous high-value microalgae products are intended for human
consumption or other applications, they are subject to food laws
and regulatory standards set by organizations such as the FDA.
Collaborative efforts from researchers across disciplines are
necessary to expand the market for microalgal food products
further. These efforts should focus on identifying novel microalgae
species and their unique characteristics, developing more
cost-effective cultivation and biorefinery systems, improving the
sensory properties of microalgal food products and assessing the
availability of algal compounds.
Acknowledgements
The authors would like to express their gratitude
to the DOST-SEI for providing the scholarship to JLZF (ASTHRDP) and
the collaborative project between DOST-PCAARRD and SEI for
supporting the scholarship of MSA (GREAT Program).
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JLZF and MSA performed the literature review. JLZF
and MSA wrote the manuscript. Furthermore, both authors have
thoroughly reviewed, and have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Hertzler SR, Lieblein-Boff JC, Weiler M
and Allgeier C: Plant proteins: Assessing their nutritional quality
and effects on health and physical function. Nutrients.
12(3704)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sharif M, Zafar MH, Aqib AI, Saeed M,
Farag MR and Alagawany M: Single cell protein: Sources, mechanism
of production, nutritional value and its uses in aquaculture
nutrition. Aquac. 531(735885)2021.
|
|
3
|
Bleakley S and Hayes M: Algal proteins:
Extraction, application, and challenges concerning production.
Foods. 6(33)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bahi A, Ramos-Vega A, Angulo C,
Monreal-Escalante E and Guardiola FA: Microalgae with
immunomodulatory effects on fish. Rev Aquac. 15:1522–1539.
2023.
|
|
5
|
Schenk PM: Phycology: Algae for food,
feed, fuel and the planet. Phycol. 1:76–78. 2021.
|
|
6
|
Martins CF, Ribeiro DM, Costa M, Coelho D,
Alfaia CM, Lordelo M, Almeida AM, Freire JPB and Prates JAM: Using
microalgae as a sustainable feed resource to enhance quality and
nutritional value of pork and poultry meat. Foods.
10(2933)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Madeira MS, Cardoso C, Lopes PA, Coelho D,
Afonso C, Bandarra NM and Prates JAM: Microalgae as feed
ingredients for livestock production and meat quality: A review.
Livest Sci. 205:111–121. 2017.
|
|
8
|
Sathasivam R, Radhakrishnan R, Hashem A
and Abd Allah EF: Microalgae metabolites: A rich source for food
and medicine. Saudi J Biol Sci. 26:709–722. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Beyerinick MW: Cultivation experiments
with Zoochlorella, Lichengonidia, and other lower algae. Bot Ztg.
47:725–739, 741-754, 757-768, 781-785. 1890.http://img.algaebase.org/pdf/AC100CF003338161AAoHt43C4207/35049.pdf.
|
|
10
|
Wu HL, Hseu RS and Lin LP: Identification
of Chlorella spp. isolates using ribosomal DNA sequences. Bot Bull
Acad Sin. 42:115–121. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ortiz Montoya EY, Casazza AA, Aliakbarian
B, Perego P, Converti A and de Carvalho JC: Production of Chlorella
vulgaris as a source of essential fatty acids in a tubular
photobioreactor continuously fed with air enriched with
CO2 at different concentrations. Biotechnol Prog.
30:916–922. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rani K, Sandal N and Sahoo PK: A
comprehensive review on chlorella-its composition, health benefits,
market and regulatory scenario. Pharma Innovation. 7:584–589.
2018.
|
|
13
|
Matos J, Cardoso C, Bandarra NM and Afonso
C: Microalgae as healthy ingredients for functional food: A review.
Food Funct. 8:2672–2685. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Becker EW: Microalgae as a source of
protein. Biotechnol Adv. 25:207–210. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ferdous UT, Nurdin A, Ismail S and Balia
Yusof ZN: Evaluation of the antioxidant and cytotoxic activities of
crude extracts from marine Chlorella sp. Biocatal Agric Biotechnol.
47(102551)2023.
|
|
16
|
van Eck NJ and Waltman L: Software survey:
VOSviewer, a computer program for bibliometric mapping.
Scientometrics. 84:523–538. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kunz WF: Response of the alga Chlorella
sorokiniana to 60 Co gamma radiation. Nature. 236:178–179.
1972.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramanna L, Guldhe A, Rawat I and Bux F:
The optimization of biomass and lipid yields of Chlorella
sorokiniana when using wastewater supplemented with different
nitrogen sources. Bioresour Technol. 168:127–135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
de-Bashan LE, Trejo A, Huss VA, Hernandez
JP and Bashan Y: Chlorella sorokiniana UTEX. 2805, a heat and
intense, sunlight-tolerant microalga with potential for removing
ammonium from wastewater. Bioresour Technol. 99:4980–4989.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Janssen M, Kuijpers TC, Veldhoen B,
Ternbach MB, Tramper J, Mur LR and Wijffels RH: Specific growth
rate of Chlamydomonas reinhardtii and Chlorella sorokiniana under
medium duration light/dark cycles: 13-87 s. J Biotechnol.
70:323–333. 1999.
|
|
21
|
León-Vaz A, León R, Díaz-Santos E, Vigara
J and Raposo S: Using agro-industrial wastes for mixotrophic growth
and lipids production by the green microalga Chlorella sorokiniana.
N Biotechnol. 51:31–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wan MX, Wang RM, Xia JL, Rosenberg JN, Nie
NY, Kobayashi N, Oyler GA and Betenbaugh MJ: Physiological
evaluation of a new Chlorella sorokiniana isolate for its biomass
production and lipid accumulation in photoautotrophic and
heterotrophic cultures. Biotechnol Bioeng. 109:1958–1964.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bazarnova J, Nilova L, Trukhina E,
Bernavskaya M, Smyatskaya Y and Aktar T: Use of microalgae biomass
for fortification of food products from grain. Foods.
10(3018)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koyande AK, Chew KW, Rambabu K, Tao Y, Chu
D and Show P: Microalgae: A potential alternative to health
supplementation for humans. Food Sci Hum Wellness. 8:16–24.
2019.
|
|
25
|
Gouveia L and Oliveira AC: Microalgae as a
raw material for biofuels production. J Ind Microbiol Biotechnol.
36:269–274. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lizzul AM, Lekuona-Amundarain A, Purton S
and Campos LC: Characterization of Chlorella sorokiniana, UTEX
1230. Biology (Basel). 7(25)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Andreeva A, Budenkova E, Babich O, Sukhikh
S, Dolganyuk V, Michaud P and Ivanova S: Influence of carbohydrate
additives on the growth rate of microalgae biomass with an
increased carbohydrate content. Mar Drugs. 19(381)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Panahi Y, Yari Khosroushahi A, Sahebkar A
and Heidari HR: Impact of cultivation condition and media content
on chlorella vulgaris composition. Adv Pharm Bull. 9:182–194.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Petkov G and Garcia G: Which are fatty
acids of the green alga chlorella? Biochem Syst Ecol. 35:281–285.
2007.
|
|
30
|
Otles S (Ed.): Methods of analysis of food
components and additives. CRC Press, 2011.
|
|
31
|
Illman AM, Scragg AH and Shales SW:
Increase in Chlorella strains calorific values when grown in low
nitrogen medium. Enzyme Microb Technol. 27:631–635. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Smyatskaya Y, Toumi A, Atamaniuk I,
Vladimirov I, Donaev FK and Akhmetova IG: Influence of the drying
method on the sorption properties the biomass of chlorella
sorokiniana microalgae. E3S Web Conf. 124(01051)2019.
|
|
33
|
Christaki E, Florou-Paneri P and Bonos E:
Microalgae: A novel ingredient in nutrition. Int J Food Sci Nutr.
62:794–799. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hamed I, Özogul F, Özogul Y and Regenstein
JM: Marine bioactive compounds and their health benefits: A review.
Compr Rev Food Sci Food Saf. 14:446–465. 2015.
|
|
35
|
Bao DQ, Mori TA, Burke V, Puddey IB and
Beilin LJ: Effects of dietary fish and weight reduction on
ambulatory blood pressure in overweight hypertensives.
Hypertension. 32:710–717. 1998.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Amaro HM, Barros R, Guedes AC, Sousa-Pinto
I and Malcata FX: Microalgal compounds modulate carcinogenesis in
the gastrointestinal tract. Trends Biotechnol. 31:92–98.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kagan ML, Levy A and Leikin-Frenkel A:
Comparative study of tissue deposition of omega-3 fatty acids from
polar-lipid rich oil of the microalgae Nannochloropsis oculata with
krill oil in rats. Food Funct. 6:186–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gouveia L, Batista AP, Raymundo A,
Bandarra N and Blades M and Blades M: Spirulina maxima and
Diacronema vlkianum microalgae in vegetable gelled desserts. Nutr
Food Sci. 38:492–501. 2008.
|
|
39
|
Mata TM, Martins AA and Caetano NS:
Microalgae for biodiesel production and other applications: A
review. Renew Sustain Energy Rev. 14:217–232. 2010.
|
|
40
|
Gouveia L, Marques AE, Sousa JM, Moura P
and Bandarra NM: Microalgae - source of natural bioactive molecules
as functional ingredients. Food Sci Technol Bull Funct Foods.
7:21–37. 2010.
|
|
41
|
Priyadarshani I and Rath B: Commercial and
industrial applications of micro algae-A review. J Algal Biomass
Utln. 3:89–100. 2012.
|
|
42
|
Lizzul AM, Lekuona-Amundarain A, Purton S
and Campos LC: Characterization of chlorella sorokiniana, UTEX
1230. Biology (Basel). 7(25)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ishiguro S, Robben N, Burghart R, Cote P,
Greenway S, Thakkar R, Upreti D, Nakashima A, Suzuki K, Comer J and
Tamura M: Cell wall membrane fraction of Chlorella sorokiniana
enhances host anti-tumor immunity and inhibits colon carcinoma
growth in mice. Integr Cancer Ther.
19(153473541990055)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Andrade LM, Andrade CJ, Das M, Nascimento
CAO and Mendes MA: Chlorella and Spirulina microalgae as sources of
functional foods, nutraceuticals, and food supplements; an
overview. MOJ Food Process Technol. 6(00144)2018.
|
|
45
|
Spolaore P, Joannis-Cassan C, Duran E and
Isambert A: Commercial applications of microalgae. J Biosci Bioeng.
101:87–96. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pugh N, Ross SA, ElSohly HN, ElSohly MA
and Pasco DS: Isolation of three high molecular weight
polysaccharide preparations with potent immunostimulatory activity
from Spirulina platensis, Aphanizomenon flos-aquae, and Chlorella
pyrenoidosa. Planta Med. 67:737–742. 2001.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sheng J, Yu F, Xin Z, Zhao L, Zhu X and Hu
Q: Preparation, identification and their anti-tumor activities in
vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem.
105:533–539. 2007.
|
|
48
|
Matos ÂP: Microalgae as a potential source
of proteins. In: Proteins: Sustainable Source, Processing and
Applications. Academic Press, Brookline, MA, pp63-96, 2019.
|
|
49
|
López CV, García Mdel C, Fernández FG,
Bustos CS, Chisti Y and Sevilla JM: Protein measurements of
microalgal and cyanobacterial biomass. Bioresour Technol.
101:7587–7591. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lourenço SO, Barbarino E, Lavín PL, Lanfer
Marquez UM and Aidar E: Distribution of intracellular nitrogen in
marine microalgae: calculation of new nitrogen-to-protein
conversion factors. Eur J Phycol. 39:17–32. 2004.
|
|
51
|
Becker EW: Microalgae for human and animal
nutrition. In: Handbook of Microalgal Culture: Applied Phycology
and Biotechnology. Richmond A (ed). Blackwell Science, Oxford,
pp461-503, 2013.
|
|
52
|
Fu Y, Chen T, Chen SHY, Liu B, Sun P, Sun
H and Chen F: The potentials and challenges of using microalgae as
an ingredient to produce meat analogues. Trends Food Sci Technol.
112:188–200. 2021.
|
|
53
|
Boisen S and Eggum BO: Critical evaluation
of in vitro methods for estimating digestibility in simple-stomach
animals. Nutr Res Rev. 4:141–162. 1991.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Young VR and Pellett PL: Plant proteins in
relation to human protein and amino acid nutrition. Am J Clin Nutr.
59 (Suppl 5):1203S–1212S. 1994.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Friedman M and Brandon DL: Nutritional and
health benefits of soy proteins. J Agric Food Chem. 49:1069–1086.
2001.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hoffman JR and Falvo MJ: Protein-which is
best? J Sports Sci Med. 3:118–130. 2004.
|
|
57
|
Berrazaga I, Micard V, Gueugneau M and
Walrand S: The role of the anabolic properties of plant-versus
animal-based protein sources in supporting muscle mass maintenance:
A critical review. Nutrients. 11(1825)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nguyen TTP, Bhandari B, Cichero J and
Prakash S: Gastrointestinal digestion of dairy and soy proteins in
infant formulas: An in vitro study. Food Res Int. 76:348–358.
2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Duodu KG, Taylor JRN, Belton PS and
Hamaker BR: Factors affecting sorghum protein digestibility. J
Cereal Sci. 38:117–131. 2003.
|
|
60
|
Food and Agriculture Organization (FAO):
Dietary protein quality evaluation in human nutrition: report of an
FAO Expert Consultation. Food and nutrition paper 92. FAO: Rome,
Italy, 2013.
|
|
61
|
Fleurence J: Seaweed proteins:
Biochemical, nutritional aspects and potential uses. Trends Food
Sci Technol. 10:25–28. 1999.
|
|
62
|
Chronakis IS and Madsen M: Algal proteins.
In Handbook of food proteins. Woodhead Publishing.
353-394:2011.
|
|
63
|
Waghmare AG, Salve MK, LeBlanc JG and Arya
SS: Concentration and characterization of microalgae proteins from
Chlorella pyrenoidosa. Bioresour Bioprocess. 3(16)2016.
|
|
64
|
Rodrigues DB, Marques MC, Hacke A, Loubet
Filho PS, Cazarin CBB and Mariutti LRB: Trust your gut:
Bioavailability and bioaccessibility of dietary compounds. Curr Res
Food Sci. 5:228–233. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Alegría A, Garcia-Llatas G and Cilla A:
Static digestion models: General introduction. In: The Impact of
Food Bioactives on Health: In vitro and ex vivo
models. Verhoeckx K, Cotter P and López-Expósito I (eds). Springer,
New York, NY, pp3-12, 2015.
|
|
66
|
Rein MJ, Renouf M, Cruz-Hernandez C,
Actis-Goretta L, Thakkar SK and da Silva Pinto M: Bioavailability
of bioactive food compounds: A challenging journey to bioefficacy.
Br J Clin Pharmacol. 75:588–602. 2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Dima C, Assadpour E, Dima S and Jafari SM:
Bioavailability and bioaccessibility of food bioactive compounds;
overview and assessment by in vitro methods. Compr Rev Food Sci
Food Saf. 19:2862–2884. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Niccolai A, Zittelli GC, Rodolfi L, Biondi
N and Tredici MR: Microalgae of interest as food source:
Biochemical composition and digestibility. Algal Res.
42(101617)2019.
|
|
69
|
Maehre HK, Edvinsen GK, Eilertsen KE and
Elvevoll EO: Heat treatment increases the protein bioaccessibility
in the red seaweed dulse (Palmaria palmata), but not in the brown
seaweed winged kelp (Alaria esculenta). J Appl Phycol. 28:581–590.
2016.
|
|
70
|
Bernaerts TMM, Verstreken H, Dejonghe C,
Gheysen L, Foubert I, Grauwet T and Van Loey AM: Cell disruption of
Nannochloropsis sp. improves in vitro bioaccessibility of
carotenoids and ω3-LC-PUFA. J Funct Foods. 65(103770)2020.
|
|
71
|
Gómez-Jacinto V, Navarro-Roldán F,
Garbayo-Nores I, Vílchez-Lobato C, Borrego AA and García-Barrera T:
In vitro selenium bioaccessibility combined with in vivo
bioavailability and bioactivity in Se-enriched microalga (Chlorella
sorokiniana) to be used as functional food. J Funct Foods.
66(103817)2020.
|
|
72
|
Demarco M, Oliveira de Moraes J, Matos ÂP,
Derner RB, de Farias Neves F and Tribuzi G: Digestibility,
bioaccessibility and bioactivity of compounds from algae. Trends
Food Sci Technol. 121:114–128. 2022.
|
|
73
|
Moughan P: Digestion and absorption of
proteins and peptides. Designing Funct Foods. 148-170:2008.
|
|
74
|
World Health Organization: Trace Elements
in Human Nutrition and Health. 1996. Available from: https://www.who.int/publications/i/item/9241561734.
|
|
75
|
Camaschella C: Iron-deficiency anemia. N
Engl J Med. 372:1832–1843. 2025.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ozturk H, Niazi P, Mansoor M, Monib AW,
Alikhail M and Azizi A: The function of zinc in animal, plant, and
human nutrition. J Res Appl Sci Biotechnol. 2:35–43. 2023.
|
|
77
|
Hentze MW, Muckenthaler MU, Galy B and
Camaschella C: Two to tango: Regulation of mammalian iron
metabolism. Cell. 142:24–38. 2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Nakano S, Takekoshi H and Nakano M:
Chlorella pyrenoidosa supplementation reduces the risk of anemia,
proteinuria and edema in pregnant women. Plant Foods Hum Nutri.
65:25–30. 2010.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Bito T, Okumura E, Fujishima M and
Watanabe F: Potential of Chlorella as a dietary supplement to
promote human health. Nutrients. 12(2524)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Batista AP, Niccolai A, Fradinho P,
Fragoso S, Bursic I, Rodolfi L, Biondi N, Tredici MR, Sousa I and
Raymundo A: Microalgae biomass as an alternative ingredient in
cookies: Sensory, physical and chemical properties, antioxidant
activity and in vitro digestibility. Algal Res. 26:161–171.
2017.
|
|
81
|
Chini Zittelli G, Rodolfi L, Biondi N and
Tredici MR: Productivity and photosynthetic efficiency of outdoor
cultures of Tetraselmis suecica in annular columns. Aquac.
261:932–943. 2006.
|
|
82
|
Cazarin CBB, Bicas JL, Pastore GM and
Marostica Junior MR: Bioactive Food Components Activity in
Mechanistic Approach. Elsevier, Amsterdam, pp1-3, 2022.
|
|
83
|
Carbonell-Capella JM, Buniowska M, Barba
FJ, Esteve MJ and Frígola A: Analytical methods for determining
bioavailability and bioaccessibility of bioactive compounds from
fruits and vegetables: A review. Compr Rev Food Sci Food Saf.
13:155–171. 2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Widyaningrum D and Prianto AD: Chlorella
as a source of functional food ingredients: Short review. IOP Conf
Ser Earth Environ Sci. 794(012148)2021.
|
|
85
|
Olasehinde TA, Odjadjare EC, Mabinya LV,
Olaniran AO and Okoh AI: Chlorella sorokiniana and Chlorella
minutissima exhibit antioxidant potentials, inhibit cholinesterases
and modulate disaggregation of β-amyloid fibrils. Electron J
Biotechnol. 40:1–9. 2019.
|
|
86
|
Beale SI and Appleman D: Chlorophyll
synthesis in Chlorella: Regulation by degree of light limitation of
growth. Plant Physiol. 47:230–235. 1971.PubMed/NCBI View Article : Google Scholar
|
|
87
|
da Silva JC and Lombardi AT: Chlorophylls
in microalgae: occurrence, distribution, and biosynthesis. In:
Pigments from Microalgae Handbook. Springer International
Publishing, Switzerland, pp1-18, 2020.
|
|
88
|
Azaman SNA, Nagao N, Yusoff FM, Tan SW and
Yeap SK: A comparison of the morphological and biochemical
characteristics of Chlorella sorokiniana and Chlorella zofingiensis
cultured under photoautotrophic and mixotrophic conditions. PeerJ.
5(e3473)2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Goiris K, Muylaert K, Fraeye I, Foubert I,
De Brabanter J and De Cooman L: Antioxidant potential of microalgae
in relation to their phenolic and carotenoid content. J Appl
Phycol. 24:1477–1486. 2012.
|
|
90
|
Takaichi S: Carotenoids in algae:
Distributions, biosyntheses and functions. Mar Drugs. 9:1101–1118.
2011.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Diprat AB, Silveira Thys RC, Rodrigues E
and Rech R: Chlorella sorokiniana: A new alternative source of
carotenoids and proteins for gluten-free bread. LWT.
134(109974)2020.
|
|
92
|
Fernandes AS, Petry FC, Mercadante AZ,
Jacob-Lopes E and Zepka LQ: HPLC-PDA-MS/MS as a strategy to
characterize and quantify natural pigments from microalgae. Curr
Res Food Sci. 3:100–112. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Machu L, Misurcova L, Ambrozova JV,
Orsavova J, Mlcek J, Sochor J and Jurikova T: Phenolic content and
antioxidant capacity in algal food products. Molecules.
20:1118–1133. 2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wojdylo A, Oszmianski J and Czemerys R:
Antioxidant activity and phenolic compounds in 32 selected herbs.
Food Chem. 105:940–949. 2007.
|
|
95
|
Manach C, Scalbert A, Morand C, Rémésy C
and Jiménez L: Polyphenols: food sources and bioavailability. Am J
Clin Nutr. 79:727–747. 2004.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Safafar H, van Wagenen J, Møller P and
Jacobsen C: Carotenoids, phenolic compounds and tocopherols
contribute to the antioxidative properties of some microalgae
species grown on industrial wastewater. Mar Drugs. 13:7339–7356.
2015.PubMed/NCBI View Article : Google Scholar
|