Introduction
Worldwide, the incidence of cancer is increasing,
with lung cancer representing the most commonly diagnosed type,
accounting for 11.6% of the total number of cancer cases (1). Lung cancer continues to be the
foremost cause of worldwide cancer-related mortality, accounting
for 18.4% of overall cancer-related fatalities, resulting in
considerable societal burden and economic detriment (1,2). The
World Health Organization (WHO) states that there are two primary
forms of lung cancer: Non-small cell lung cancer (NSCLC), which
accounts for 80-85% of all lung cancer cases, and small cell lung
cancer, which accounts for 15% of cases (3,4).
Additional subtypes of non-small cell lung cancer include
adenocarcinoma, squamous cell carcinoma and large cell carcinoma
(5).
During the initial decade of the 21st century, the
median overall survival (OS) rate of individuals diagnosed with
stage IV NSCLC was 1 year. The identification of actionable genetic
abnormalities and the advancement of targeted therapeutics resulted
in the significant enhancement of OS rates in a specific group of
patients with NSCLC. Patients with NSCLC who lack driver mutations
do not derive advantages from targeted therapy (5,6). The
prognosis of the majority of patients with NSCLC lacking an
actionable genetic driver was restricted, and platinum-based
chemotherapy constituted the primary first-line treatment for these
individuals (7). The paradigm for
treating lung cancer, particularly NSCLC, has markedly changed as a
result of the discovery of immunological checkpoints and the
subsequent development of immune checkpoint inhibitors (ICIs),
which were awarded the Nobel Prize (8). Targeting the programmed cell death
protein 1 (PD-1)/programmed death ligand 1 (PD-L1) axis, ICIs, such
as PD-1 antibodies (pembrolizumab and nivolumab) and PD-L1
antibodies (atezolizumab) exhibit immense promise due to their
potent antitumor effects, which include restoring T-cell function
and stimulating immune responses against tumors (8,9).
Immune checkpoint molecules provide inhibitory signals to T-cells,
preventing the excessive activation of the immune system and
maintaining immunological homeostasis (10-12).
Consequently, by inhibiting immune checkpoint molecules, ICIs
activate antitumor T-cell responses that subsequently eradicate
cancer cells (13,14).
Unlike conventional chemotherapy and targeted
therapies, the use of ICIs is associated with side-effects, termed
immune-related adverse events (irAEs), due to increased T-cell
activation. Pneumonitis, thyroid dysfunction, skin toxicity,
hepatitis and colitis are common immune-related side-effects, while
other anatomical regions may also be affected (15,16).
Previous studies have investigated the prognostic determinants in
patients with NSCLC treated with ICIs (17-19). Nonetheless,
a dependable predictive biomarker is still lacking (20). Consequently, previous studies have
assessed the association between the adverse events and clinical
benefits in patients undergoing anti-PD-1 antibody therapy
(14,15). In recent years, it has become
increasingly evident that the adverse events may serve as
predictive markers for patients who experience them; nevertheless,
the patient features linked to responses to immunotherapy remain
ambiguous (20).
Despite the encouraging antitumor effects of ICIs in
patients with NSCLC, a thorough understanding of immunotherapy and
the improvement of treatment outcomes are hampered by the paucity
of literature detailing the safety and effectiveness of both
licensed and emergent ICIs for first-line NSCLC treatment. Thus,
the present study aimed to investigate the therapeutic relevance of
irAEs as a prognostic indicator in patients with NSCLC treated with
pembrolizumab.
Patients and methods
Patient selection
The Research Ethics Committee of Mustansiriyah
University, Baghdad, Iraq reviewed and approved the study (approval
no. BCSMU/1024/0050Z). All data were retrospectively obtained from
medical records in compliance with the principles of the
Declaration of Helsinki. From January, 2020 and April, 2022, the
present study included patients with stage IV NSCLC who had
received a minimum of one dose of pembrolizumab at the Oncology
Teaching Hospital/Medical City, in Baghdad, Iraq. All patients
included were aged ≥18 years, and had received at least one dose of
pembrolizumab, had previously untreated stage IV NSCLC, and a PD-L1
tumor proportion score (TPS) of at least 50%, measurable disease by
irRECIST v1.1(21), and an Eastern
Cooperative Oncology Group performance status of 0 or 1. Patients
received 200 mg intravenous pembrolizumab monotherapy once every 3
weeks for up to 45 cycles. Treatment was continued for the
specified number of cycles until progressive disease, as per the
irRECIST v1.1, adverse events (AEs) of unacceptable severity, or
patient withdrawal. The exclusion criteria encompassed individuals
who were administered pembrolizumab alongside other ICIs or
therapeutic drugs, including standard chemotherapeutics and
targeted therapies, as well as patients who did not have a
follow-up visit following treatment with a single dose of
pembrolizumab. Patients with sensitizing EGFR or ALK alterations,
untreated brain metastases, or active autoimmune disease requiring
systemic treatment or receiving systemic glucocorticoids or
immunosuppressive therapy were also excluded. Patient-specific
information was safeguarded and remains undisclosed. Data were
retrospectively collected from January 1, 2024 to May 1, 2024.
Data collection and variables
Data were gathered from the initiation of
pembrolizumab treatment until the transition to an alternative
medicine, the occurrence of mortality, or the conclusion of the
study period by retrospective medical record analysis. Upon
initiating treatment with pembrolizumab, demographic information,
such as sex, age and tobacco use was obtained. Additional obtained
data encompassed the specifics of pembrolizumab medication (e.g.,
cycle), irAEs (e.g., occurrence, degree, type, treatment and
progression). irAEs, including thyroiditis, hepatitis and renal
insufficiency, were documented during each cycle. Thyroid function
was also documented at each treatment cycle. Objective tumor
response was evaluated every four to six cycles via PET or CT
scans, in accordance with the irRECIST, version 1.1(21). AEs were graded using the Common
Terminology Criteria for Adverse Events version 4.0(22). The grading of irAEs was determined
by treating physicians (hematologists and oncologists). The present
retrospective study was designed and reported in accordance with
the Strengthening the Reporting of Observational Studies in
Epidemiology (STROBE) guidelines. All retrospectively collected
data were stored using Microsoft Office Excel software on a
computer belonging to the Oncology Teaching Hospital (Medical City
Center, Ministry of Health, Baghdad, Iraq). The data was
password-protected. For the purpose of the present study, no
identifying information was collected. Instead, all patients
recruited for the study were allocated study codes, beginning from
0001, representing the first observed patient.
Data analysis
The objective response rate (ORR) was established as
the sum of the complete response (CR) rate and the partial response
(PR) rate. The disease control rate (DCR) was defined as the ORR
plus the stable disease rate. Progression-free survival (PFS) was
defined as the interval from the initiation of immunotherapy to the
onset of progressive illness. PFS was examined between individuals
with irAEs and those without such events. PFS was also examined
between patients with early- and late-onset irAEs. For
retrospective analysis, pre-existing PD-L1 expression data were
compared between patients who developed irAEs and those who did
not.
Statistical analysis
All statistical analyses were carried out using the
Statistical Package for Social Sciences (IBM Corp.) software
version 25. Categoric variables are expressed as frequency and
percentage. Comparisons between groups were performed using the
Chi-squared test. Fisher's exact test was applied when expected
value in a cell was <5. Sample normality was tested using the
Shapiro-Wilk test and a visual inspection of their histograms and
normal Q-Q plots and box blots revealed that PD-L1 data were not
normally distributed. Comparisons between groups were conducted
using the Mann-Whitney U test. PFS was assessed using Kaplan-Meier
curves, and comparisons between different groups were performed
using the log-rank test. P-values <0.05 were considered to
indicate statistically significant differences.
Results
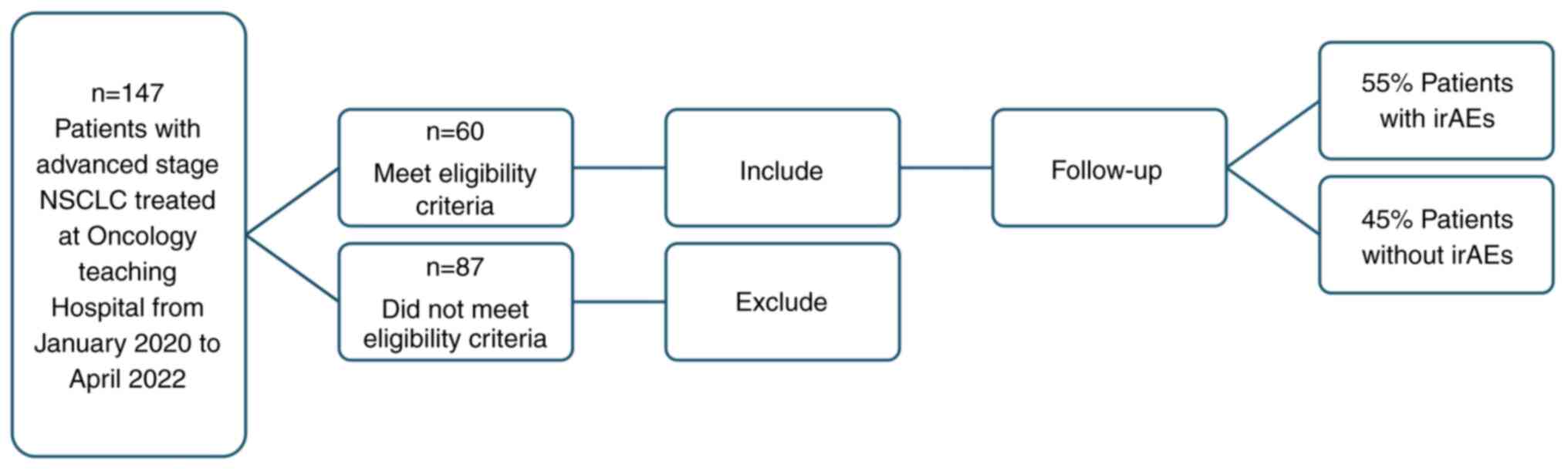
A total of 60 patients considered eligible were
recruited in the present study (Fig.
1). The mean age of the patients was 64±11.9 years. Males
constituted the majority of this cohort. Never smokers represented
(23.4%) of all patients. Adenocarcinoma was the main
histopathological type (70.0%); the remaining patients had squamous
cell carcinoma. The patient characteristics are presented in
Table I.
 | Table ICharacteristics of the patients in
the present study (n=60). |
Table I
Characteristics of the patients in
the present study (n=60).
| Characteristic | No | % |
|---|
| Age, years | | |
|
≤50 | 14 | 23.3 |
|
>50 | 46 | 76.7 |
| Sex | | |
|
Female | 16 | 26.7 |
|
Male | 44 | 73.3 |
| Smoking status | | |
|
Never
smoker | 14 | 23.4 |
|
Ex-smoker | 26 | 43.3 |
|
Current
smoker | 20 | 33.3 |
| Subtype | | |
|
Adenocarcinoma | 42 | 70.0 |
|
Squamous
cell carcinoma | 18 | 30.0 |
| No. of
pembrolizumab cycles | | |
|
2-5 | 18 | 30.0 |
|
6-10 | 20 | 33.3 |
|
11-18 | 22 | 36.7 |
| Response | | |
|
Stable | 8 | 13.3 |
|
Partial
response | 32 | 53.3 |
|
Complete
response | 4 | 6.7 |
|
Progressive
disease | 16 | 26.7 |
The mean follow-up period was 26±3.3036 months.
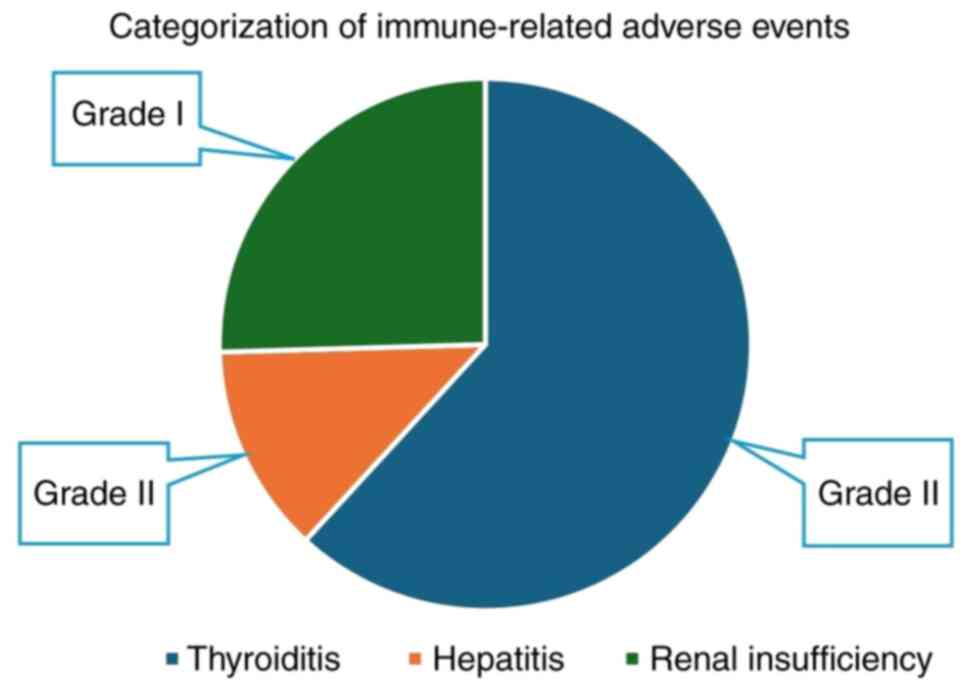
During this period, more than half of the patients (55%)
experienced irAEs. Thyroid-related irAEs accounted for 34%,
followed by renal insufficiency 14% and hepatitis (7%).
Hypothyroidism was the most frequently reported form of
thyroiditis, accounting for 70% of cases, while hyperthyroidism
accounted for 30% of all thyroiditis cases. All thyroiditis and
hepatitis cases were grade II, while renal insufficiency cases were
grade I (Fig. 2). There were no
treatment-related deaths. irAEs were significantly more frequent
after the tenth cycle, observed in 91.7% of the patients (P=0.003);
however, early occurrence in the first five cycles was also
observed (Table II). The median
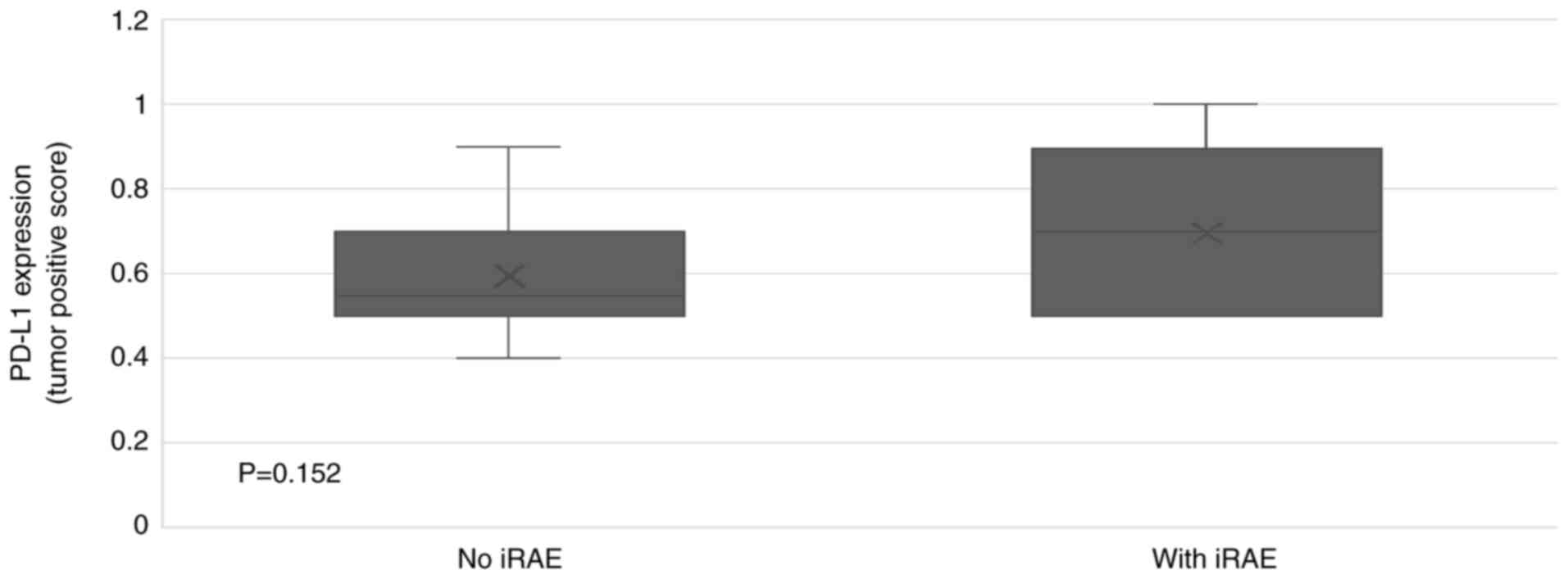
PD-L1 expression in the tumors of patients who experienced irAEs
was 70% compared to 55% in those who did not have irAEs (Fig. 3). The ORR was 60.0%, with 53.3% of
patients demonstrating a PR and 6.7% of patients exhibiting a CR
(Table I). When all irAEs were
considered, the DCR was significantly higher in the group with
irAEs (72.7 vs. 27.3%; P=0.033) compared with the group with no
irAEs. No significant difference was observed when early-onset
irAEs (after the second cycle) and late-onset irAEs (after the
sixth cycle) were analyzed separately (Table III).
 | Table IIPatient characteristics in the
presence and absence of irAEs. |
Table II
Patient characteristics in the
presence and absence of irAEs.
| | No irAEs | With irAEs | |
|---|
| Characteristic | Total no. | No. | % | No. | % | P-value |
|---|
| Age groups | | | | | | |
|
≤50 | 14 | 2 | 14.3 | 12 | 85.7 | 0.354 |
|
>50 | 46 | 22 | 47.8 | 24 | 52.2 | |
| Sex | | | | | | |
|
Female | 16 | 6 | 37.5 | 10 | 62.5 | 0.999 |
|
Male | 44 | 19 | 43.2 | 25 | 56.8 | |
| Smoking status | | | | | | |
|
Never
smoker | 14 | 7 | 50.0 | 7 | 50.0 | 0.999 |
|
Ex-smoker | 26 | 10 | 38.5 | 16 | 61.5 | |
|
Current
smoker | 20 | 8 | 40.0 | 12 | 60.0 | |
| Subtype | | | | | | |
|
Adenocarcinoma | 42 | 17 | 40.5 | 25 | 59.5 | 0.999 |
|
Squamous
cell carcinoma | 18 | 8 | 44.4 | 10 | 55.6 | |
| No. of
pembrolizumab cycles | | | | | | |
|
2-5 | 16 | 14 | 87.5 | 2 | 12.5 | 0.003 |
|
6-10 | 20 | 8 | 40.0 | 12 | 60.0 | |
|
11-18 | 24 | 2 | 8.3 | 22 | 91.7 | |
 | Table IIIResponse to treatment in the two
study groups (with and without irAEs). |
Table III
Response to treatment in the two
study groups (with and without irAEs).
| | No irAEs, n
(%) | irAEs, n (%) | P-value |
|---|
| All irAEs | | | |
|
ORR | 10 of 36
(27.8) | 26 of 36
(72.2) | 0.148 |
|
DCR | 12 of 44
(27.3) | 32 of 44
(72.7) | 0.033 |
| Early-onset
irAEs | | | |
|
ORR | 24 of 36
(66.7) | 12 of 36
(33.3) | 0.999 |
|
DCR | 26 of 44
(59.1) | 18 of 44
(40.9) | 0.371 |
| Late-onset
irAEs | | | |
|
ORR | 14 of 36
(38.9) | 22 of 36
(61.1) | 0.44 |
|
DCR | 16 of 44
(36.4) | 28 of 44
(63.6) | 0.077 |
Out of the 60 patients who received the treatment,
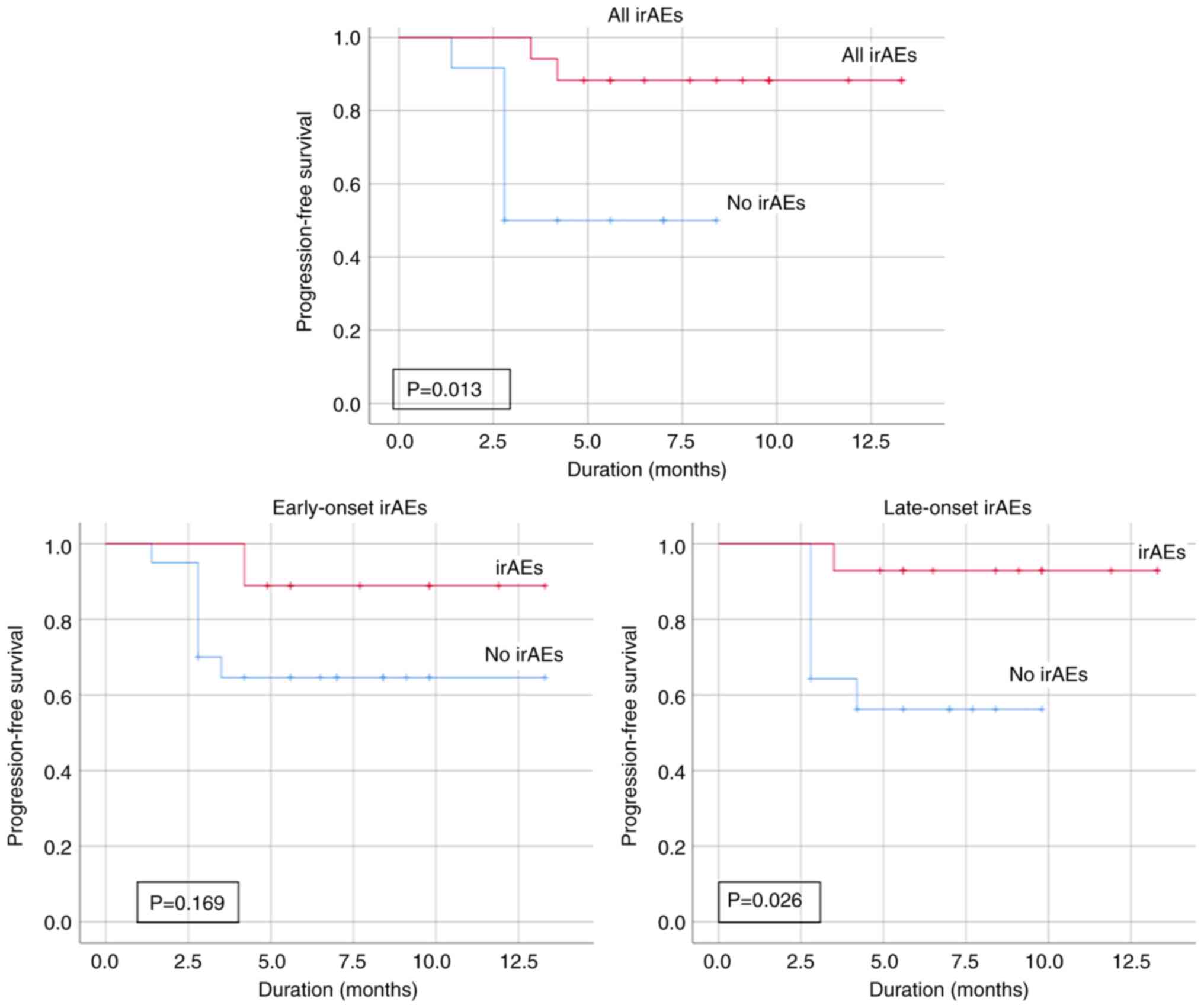
26.7% developed progressive disease. The mean PFS rate of the
patients with irAEs was 12.19 months, while that of patients
without irAEs was markedly shorter at 5.48 months, with the
difference being statistically significant (P=0.013). This
significant difference was maintained when the patients with irAEs
were stratified to late-onset (P=0.026; Fig. 4). While the Kaplan-Meier analysis
focused on the 13-month where progressions occurred, the mean
follow-up of 26 months reflects the complete monitoring period
including censored patients with prolonged surveillance.
Discussion
The present study demonstrated the predictive value
of irAEs in patients with NSCLC treated with pembrolizumab. irAEs
manifested in 55% of patients, with the prevalence in observed
being parallel to that observed in clinical trials (23). The incidence of irAEs induced by
pembrolizumab was associated with a clinical advantage in patients
with NSCLC. This aligns with the findings of other retrospective
studies that demonstrated an association between the incidence of
irAEs and the clinical efficacy of ICIs (13,17).
While any organ system may be affected, irAEs
primarily affect the gastrointestinal tract, hormonal glands, the
skin and liver (24,25). The predominant irAEs in NSCLC
linked to the use of pembrolizumab are thyroid-related toxicities
(26). In the present study,
thyroid-related irAEs accounted for 34%, renal insufficiency for
14% and hepatitis accounted for 7%. Hypothyroidism was the most
frequently reported form of thyroiditis, accounting for 70%, while
hyperthyroidism accounted for ~30% of all thyroiditis cases; this
finding is consistent with the findings of previous studies
(27,28). In the present study, all
thyroiditis and hepatitis cases were grade II, while the renal
insufficiency cases were grade I. The patients received appropriate
treatment tailored to each of the mentioned conditions. During this
period, there were no interruptions to the administration schedule
of the immunotherapy. There were no treatment-related deaths.
Nevertheless, the mechanisms behind the development
of irAEs remain to be fully elucidated. The function of
immunological checkpoints in preserving immunological homeostasis
is considered to be connected to irAEs. Increased T-cell activity
against antigens found in tumors and healthy tissue, elevated
levels of pre-existing autoantibodies, elevated levels of
inflammatory cytokines (such as interleukin-17), and enhanced
complement-mediated inflammation as a result of direct antibody
binding against cytotoxic T-lymphocyte antigen 4 are some possible
mechanisms that may be linked to the occurrence of irAEs (29). The activation of the ‘ideal immune
system’ must equilibrate the immunological response to the tumor
with the self-immune response. However, the precise pathophysiology
underlying irAEs warrants further investigation. The severity of
the irAE, the affected system or organ, and the number of sites
involved determine the equilibrium between its advantages and
disadvantages (30). Additionally,
as irAEs frequently manifest suddenly and can even result in fatal
toxicities, it is critical that clinicians identify and treat the
events as soon as possible (31).
In terms of the timeline of occurrence, the time of
onset of irAEs is also a critical element in determining the
association between irAEs and the clinical results of ICI
treatment. Previous studies have indicated that the majority of
irAEs occur early, typically within the first 6 months of treatment
(32,33). The majority of irAEs occur within
the first 6 months of treatment, according to another retrospective
cohort study that included a large number of hospitalized patients
with irAEs (34). However, a
consensus on whether irAEs, their timing, or specific types are
indicative of positive therapeutic outcomes is still lacking
(35). In the present study, irAEs
were significantly more frequent after the tenth cycle, observed in
~91.7% of patients (P=0.003); however, early occurrence in the
first five cycles was also observed; this result is consistent with
the aforementioned previous studies.
In patients with NSCLC receiving ICI therapy, the
incidence of irAEs and the timing of the events are key indicators
of prognosis (35). The present
study defined early-onset irAEs (after the second cycle, 1.4
months) and late-onset irAEs (after the sixth cycle, 4.2 months)
based on the observed patterns within the study cohort. These
cut-offs reflect marked changes in the frequency and timing of irAE
occurrences. These intervals also align with common practices in
similar studies, ensuring consistency and comparability in the
analysis. Some studies have reported that early-onset irAEs are
associated with improved outcomes following immunotherapy (36,37).
However, in another study on 154 patients with NSCLC receiving ICI
therapy, those who encountered later irAEs (defined as occurring
after 3 months of commencing ICI medication) had a significantly
longer OS and PFS than those who suffered irAEs at an early stage
(38). Furthermore, Cortellini
et al (30) revealed that
patients who acquire irAEs are those who undergo prolonged therapy
with ICIs and consequently exhibit a more favorable prognosis than
those who do not. One possible confounding issue is that patients
with late-onset irAE may have optimal outcomes as they have lived
long enough to develop them. In the present study, there was no
significant difference between early- and late-onset irAE; this may
be attributed to the small sample size and the short follow-up
period. The observed differences between the findings of the
present study and those of previous studies further highlight the
need for additional research to explore this topic in depth and
understand the underlying factors contributing to these
variations.
Consistent with the available data (39,40),
the observations of the present study suggest a connection between
the clinical benefits of anti-PD-1 immunotherapy and the
development of irAEs. In the present study, DCR was significantly
higher in the group with irAEs (72.7 vs. 27.3%, P=0.033). Moreover,
the patients with irAEs were divided into two groups according to
the timeline of occurrence; the early-onset irAEs group (after the
second cycle) and late-onset irAEs group (after the sixth cycle).
The DCR was higher in late-onset group (63.6 vs. 36.4; P-value
0.077). Recent studies have indicated that patients exhibit
improved prognosis when they encounter irAEs during ICI therapy
(41). In the present study, the
non-highly significant results may be attributed to the
investigation of a small number of patients.
The findings of the present study suggest an
association between the development of irAEs and improved clinical
outcomes in patients with advanced stage NSCLC treated with
pembrolizumab. The occurrence of irAEs was substantially associated
with an extended PFS. In the present study, the median PFS rate of
patients with irAEs was 12.19 months, whereas for those without
irAEs, this was markedly lower at 5.48 months, with a statistically
significant difference (P=0.013). This is consistent with the
findings of several recent retrospective studies. For example, Chen
et al (42) and Chen et
al (43) indicated that the
incidence of irAEs was associated with an improvement in PFS, but
not in OS. In particular, Boulhel et al (44) and Ricciuti et al (45) confirmed that patients who
encountered irAEs exhibited a higher significant survival advantage
than those without irAEs, further indicating a causal association
between irAEs and the efficacy of immunotherapy. The findings of
these investigations contribute to the expanding comprehension that
irAEs enhance patient PFS rates and validate the observation
observed herein that the occurrence of irAEs in patients with NSCLC
is associated with a heightened responsiveness to immunotherapy and
an extended PFS. While the present study focused on pembrolizumab,
its efficacy is often compared with other ICIs, such as nivolumab
(another PD-1 inhibitor) and atezolizumab (a PD-L1 inhibitor)
within previous studies. A recent study indicated that
pembrolizumab may be associated with a higher ORR compared to
nivolumab, particularly in first-line therapy for advanced stage
NSCLC (10). Moreover, in a small
cohort study, the median PFS was 9.6 months for atezolizumab, 12.6
months for nivolumab and 8.5 months for pembrolizumab; however, the
differences were not statistically significant, and survival
outcomes were influenced by the patient performance status
(46). Furthermore, other studies
have also indicated that there is no significantly different
between pembrolizumab and nivolumab across advanced stage NSCLC, by
reporting a similar median PFS (10,47).
It is unknown whether there is a link between PD-L1
expression and the development of IrAEs (48). Additionally, a previous clinical
study found no significant link between PD-L1 expression and the
development of irAEs (49).
Moreover, in the BIRCH trial (50), which examined the effects of ICI,
there was no significant association between a high expression of
PD-L1 and the development of irAEs. Additionally, a previous
multicenter retrospective analysis of pembrolizumab monotherapy
first-line treatment revealed no statistically significant
difference in the incidence of adverse events (irAEs) between the
TPS >90% and TPS 50-89% groups (51). This is consistent with the findings
of the present study. Other research, however, has demonstrated
that TPS ≥90% was only significantly linked to the likelihood of
developing irAEs in patients undergoing ICIs as first-line
treatment (52). In the present
study, the median PD-L1 expression ratio in tumors of patients who
developed irAEs was 70% compared to 55% in those who did not have
irAEs; however, statistically, this was not significant. The lack
of a significant impact of this result may be attributed to the
limited sample size and the brief follow-up duration. For an
understanding of the possible prognostic significance of PD-L1
expression, further research is warranted.
In several malignancies, ICIs are being used in
addition to, or in place of front-line therapy due to their
long-lasting antitumoral effect. As a result, diagnosing and
treating irAEs presents clinicians with increasing difficulties in
the treatment of patients. To solve these issues, basic,
translational and clinical studies are required to elucidate the
complex mechanisms of irAEs and identify biomarkers derived from
these mechanisms (53). However,
identifying broadly applicable biomarkers remains difficult due to
the complexity of irAEs and their varied presentations across
organs (54,55). Furthermore, given the possible link
between irAEs and tumor response, efforts to prevent irAEs before
they affect the immune system changes required for a prolonged
survival time may deny patients the potential long-term and hidden
advantages (56). Even in the
absence of irAEs, efforts should be directed towards modifying the
immune response in order to improve the survival rates (56). The future objectives of the field
are not limited to strategies to increase efficacy. As
aforementioned, separating effectiveness from irAEs requires
immense efforts. In particular, further knowledge needs to be
obtained about the parameters predicting the occurrence of irAEs
(57). In order to provide a
comprehensive understanding of the relative advantages and
disadvantages of ICI therapy, it is necessary to continuously
synthesize data from a variety of sources, including recent
clinical trials and review articles. These initiatives would fill
in the current gaps in the evidence and guide medical decisions in
this area.
In conclusion, the present retrospective study
indicated that irAEs may positively influence the therapeutic
outcomes of patients with NSCLC undergoing immunotherapy. This
underscores the prospective application of irAEs as a clinical
indicator for survival in patients with advanced stage NSCLC
treated with ICIs. Although a priori statistical power analysis was
performed to determine the sample size needed to detect clinically
significant differences, the timeframe of the study limited the
authors' ability to include the optimal number of participants.
This may have affected the statistical power to detect significant
differences between the groups. While the generalizability of the
results may be attributed to the aforementioned point, the strength
of the observed discrepancies highlights the robustness and
usefulness of the results of the study. Additional research is
necessary to enhance the prediction of which individuals will
experience irAEs and to determine optimal management strategies for
their occurrence.
Acknowledgements
The authors would like to thank Dr Areege M. Kamal
at the Oncology Teaching Hospital (Medical City Center, Ministry of
Health, Baghdad, Iraq) for providing assistance and guidance.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TJT contributed to the conception and design of the
study, and wrote and edited the manuscript. SMJ collected and
analyzed data. FAAS reviewed and edited the manuscript, and was
also involved in data curation, and supervised the study All
authors have read and approved the final manuscript. TJT and SMJ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Research Ethics Committee of Mustansiriyah University (approval No.
1024/0050Z). and subsequently authorized by Oncology Teaching
Hospital/Medical City administration per standard institutional
collaboration procedures. All data were retrospectively extracted
from medical records in accordance with the principles of the
Declaration of Helsinki. All patients who participated in this
study provided written informed consent for the publication of
their data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mohammed Bakheet M, Mohssin Ali H and
Jalil Talab T: Evaluation of some proinflammatory cytokines and
biochemical parameters in pre and postmenopausal breast cancer
women. Cytokine. 179(156632)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Q, Yuan D, Ma C, Liu Y, Ma L, Lv T and
Song Y: A new hope: the immunotherapy in small cell lung cancer.
Neoplasma. 63:342–350. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
World Health Organization (WHO): Lung
cancer. WHO, Geneva, 2025. https://www.who.int/news-room/fact-sheets/detail/lung-cancer.
|
|
5
|
Inamura K: Lung cancer: Understanding its
molecular pathology and the 2015 WHO classification. Front Oncol.
7(193)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takano N, Ariyasu R, Koyama J, Sonoda T,
Saiki M, Kawashima Y, Oguri T, Hisakane K, Uchibori K, Nishikawa S,
et al: Improvement in the survival of patients with stage IV
non-small-cell lung cancer: Experience in a single institutional
1995–2017. Lung Cancer. 131:69–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mamdani H, Matosevic S, Khalid AB, Durm G
and Jalal SI: Immunotherapy in lung cancer: Current landscape and
future directions. Front Immunol. 13(823618)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dahl O and Brydøy M: The pioneers behind
immune checkpoint blockers awarded the Nobel Prize in physiology or
medicine 2018. Acta Oncol. 58:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xia L, Liu Y and Wang Y: PD-1/PD-L1
blockade therapy in advanced non-small-cell lung cancer: Current
status and future directions. Oncologist. 24 (Suppl 1):S31–S41.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cui P, Li R, Huang Z, Wu Z, Tao H, Zhang S
and Hu Y: Comparative effectiveness of pembrolizumab vs. nivolumab
in patients with recurrent or advanced NSCLC. Sci Rep.
10(13160)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang N, Tu J, Wang X and Chu Q:
Programmed cell death-1/programmed cell death ligand-1 checkpoint
inhibitors: differences in mechanism of action. Immunotherapy.
11:429–441. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mohammed MQ, Alwan AH and Almukhtar AA:
Estimation of Serum TLR-9, TNF-α, and IL-6 Levels in the Iraqi
Patients Diagnosed as Acute Myelogenous Leukemia. Baghdad Sci J.
21(2182)2024.
|
|
13
|
Azoury SC, Straughan DM and Shukla V:
Immune checkpoint inhibitors for cancer therapy: Clinical efficacy
and safety. Curr Cancer Drug Targets. 15:452–462. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elshahidi MH: Immune checkpoint inhibitors
and health-related quality of life: A systematic review of the
current literature. Mustansiriya Med J. 17:1–13. 2018.
|
|
15
|
Hua C, Boussemart L, Mateus C, Routier E,
Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, et
al: Association of vitiligo with tumor response in patients with
metastatic melanoma treated with pembrolizumab. JAMA Dermatol.
152:45–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jouda J, Mohammed IK, Asad SS, Falah YF
and Salih KM: Effect of alteration in nutritional style on liver
function tests and general stool examination. In: AIP Conference
Proceedings. AIP Publishing, 2020.
|
|
17
|
Musaelyan AA, Moiseyenko FV, Emileva TE,
Oganesyan AP, Oganyan KA, Urtenova MA, Odintsova SV, Chistyakov IV,
Degtyarev AM, Akopov AL, et al: Clinical predictors of response to
single-agent immune checkpoint inhibitors in
chemotherapy-pretreated non-small cell lung cancer. Mol Clin Oncol.
20(32)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jin CX, Liu YS, Qin HN, Teng YB, Sun R, Ma
ZJ, Wang AM and Liu JW: Peripheral inflammatory factors as
prognostic predictors for first-line PD-1/PD-L1 inhibitors in
advanced non-small cell lung cancer. Sci Rep.
15(11206)2025.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tong W, Xu H, Tang J, Zhao N, Zhou D, Chen
C and Cao D: Inflammatory factors are associated with prognosis of
non-small cell lung cancer patients receiving immunotherapy: A
meta-analysis. Sci Rep. 14(26102)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sonehara K, Tateishi K, Araki T, Komatsu
M, Yamamoto H, Koizumi T and Hanaoka M: The role of immune-related
adverse events in prognosis and efficacy prediction for patients
with non-small cell lung cancer treated with immunotherapy: A
retrospective clinical analysis. Oncology. 99:271–279.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e52.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
National Institute of Health (NIH): Common
Terminology Criteria for Adverse Events (CTCAE) v4.0. NIH,
Bethesda, 2009. Accessed May 28, 2010.
|
|
23
|
Rizwan S, Rai M, Bakalov V, Abel S, Lo H,
Niranjan S, Sethi A, Khan T, Alhamad K, Attah A, et al: Impact of
immune-related adverse events on survival in patients with advanced
non-small cell lung cancer treated with immune checkpoint
inhibitors: A real-world perspective. SSRN, J Clin Oncol. 39 (Suppl
15)(e21213)2021.
|
|
24
|
Morimoto K, Yamada T, Takumi C, Ogura Y,
Takeda T, Onoi K, Chihara Y, Taniguchi R, Yamada T, Hiranuma O, et
al: Immune-related adverse events are associated with clinical
benefit in patients with non-small-cell lung cancer treated with
immunotherapy plus chemotherapy: A retrospective study. Front
Oncol. 11(630136)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Couey MA, Bell RB, Patel AA, Romba MC,
Crittenden MR, Curti BD, Urba WJ and Leidner RS: Delayed
immune-related events (DIRE) after discontinuation of
immunotherapy: Diagnostic hazard of autoimmunity at a distance. J
Immunother Cancer. 7(165)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Owen DH, Wei L, Bertino EM, Edd T,
Villalona-Calero MA, He K, Shields PG, Carbone DP and Otterson GA:
Incidence, risk factors, and effect on survival of immune-related
adverse events in patients with non–small-cell lung cancer. Clin
Lung Cancer. 19:e893–e900. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gridelli C, Rossi E, De Chiara G,
Ciardiello F and Sgambato A: Thyroid-induced toxicity of
check-point inhibitors immunotherapy in the treatment of advanced
non-small cell lung cancer. J Endocrinol Diabe. 3:1–10. 2016.
|
|
28
|
Delivanis DA, Gustafson MP, Bornschlegl S,
Merten MM, Kottschade L, Withers S, Dietz AB and Ryder M:
Pembrolizumab-induced thyroiditis: Comprehensive clinical review
and insights into underlying involved mechanisms. J Clin Endocrinol
Metab. 102:2770–2780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cortellini A, Chiari R, Ricciuti B, Metro
G, Perrone F, Tiseo M, Bersanelli M, Bordi P, Santini D, Giusti R,
et al: Correlations between the immune-related adverse events
spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients.
Clin Lung Cancer. 20:237–247.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Alrabadi NN, Abushukair HM, Ababneh OE,
Syaj SS, Al-Horani SS, Qarqash AA, Darabseh OA, Al-Sous MM,
Al-Aomar SR, Ahmed YB, et al: Systematic review and meta-analysis
efficacy and safety of immune checkpoint inhibitors in advanced
melanoma patients with anti-PD-1 progression: A systematic review
and meta-analysis. Clin Transl Oncol. 23:1885–1904. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K: ESMO Guidelines Committee.
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28
(suppl 4):iv119–iv142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Durbin SM, Zubiri L, Perlman K, Wu CY, Lim
T, Grealish K, Hathaway N, LoPiccolo J, Wang M, Falade A, et al:
Late-onset immune-related adverse events after immune checkpoint
inhibitor therapy. JAMA Netw Open. 8(e252668)2025.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sayer MR, Mambetsariev I, Lu KH, Wong CW,
Duche A, Beuttler R, Fricke J, Pharoan R, Arvanitis L, Eftekhari Z,
et al: Predicting survival of NSCLC patients treated with immune
checkpoint inhibitors: Impact and timing of immune-related adverse
events and prior tyrosine kinase inhibitor therapy. Front Oncol.
13(1064169)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Teraoka S, Fujimoto D, Morimoto T, Kawachi
H, Ito M, Sato Y, Nagata K, Nakagawa A, Otsuka K, Uehara K, et al:
Early immune-related adverse events and association with outcome in
advanced non-small cell lung cancer patients treated with
nivolumab: A prospective cohort study. J Thorac Oncol.
12:1798–1805. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khoja L, Day D, Chen TWW, Siu LL and
Hansen AR: Tumour-and class-specific patterns of immune-related
adverse events of immune checkpoint inhibitors: A systematic
review. Ann Oncol. 28:2377–2385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hsiehchen D, Naqash AR, Espinoza M, Von
Itzstein MS, Cortellini A, Ricciuti B, Owen DH, Laharwal M, Toi Y,
Burke M, et al: Association between immune-related adverse event
timing and treatment outcomes. Oncoimmunology.
11(2017162)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hosoya K, Fujimoto D, Morimoto T, Kumagai
T, Tamiya A, Taniguchi Y, Yokoyama T, Ishida T, Hirano K, Matsumoto
H, et al: Association between early immune-related adverse events
and clinical outcomes in patients with non-small cell lung cancer
treated with immune checkpoint inhibitors. Clin Lung Cancer.
21:e315–e328. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aso M, Toi Y, Sugisaka J, Aiba T, Kawana
S, Saito R, Ogasawara T, Tsurumi K, Ono K, Shimizu H, et al:
Association between skin reaction and clinical benefit in patients
treated with anti-programmed cell death 1 monotherapy for advanced
non-small cell lung cancer. Oncologist. 25:e536–e544.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sugano T, Seike M, Saito Y, Kashiwada T,
Terasaki Y, Takano N, Hisakane K, Takahashi S, Tanaka T, Takeuchi
S, et al: Immune checkpoint inhibitor-associated interstitial lung
diseases correlate with better prognosis in patients with advanced
non-small-cell lung cancer. Thorac Cancer. 11:1052–1060.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen X, Nie J, Dai L, Hu W, Zhang J, Han
J, Ma X, Tian G, Han S and Wu D: , et al: Immune-related
adverse events and their association with the effectiveness of
PD-1/PD-L1 inhibitors in non-small cell lung cancer: A real-world
study from China. Front Oncol. 11(607531)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen M, Li Q, Xu Y, Zhao J, Zhang L, Wei
L, Zhong W and Wang M: Immunotherapy as second-line treatment and
beyond for non-small cell lung cancer in a single center of China:
Outcomes, toxicities, and clinical predictive factors from a
real-world retrospective analysis. Thorac Cancer. 11:1955–1962.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bouhlel L, Doyen J, Chamorey E, Poudenx M,
Ilie M, Gal J, Guigay J, Benzaquen J, Marquette CH, Berthet JP, et
al: Occurrence and number of immune-related adverse events are
independently associated with survival in advanced non-small-cell
lung cancer treated by nivolumab. Bull Cancer. 107:946–958.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ricciuti B, Genova C, De Giglio A,
Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi
F and Chiari R: Impact of immune-related adverse events on survival
in patients with advanced non-small cell lung cancer treated with
nivolumab: Long-term outcomes from a multi-institutional analysis.
J Cancer Res Clin Oncol. 145:479–485. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Burgos-San José A, Colomer-Aguilar C,
Martínez-Caballero D and Massutí-Sureda B: Effectiveness and safety
of atezolizumab, nivolumab and pembrolizumab in metastatic
non-small cell lung cancer. Farm Hosp. 45:121–125. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
47
|
Torasawa M, Yoshida T, Yagishita S,
Shimoda Y, Shirasawa M, Matsumoto Y, Masuda K, Shinno Y, Okuma Y,
Goto Y, et al: Nivolumab versus pembrolizumab in previously-treated
advanced non-small cell lung cancer patients: A propensity-matched
real-world analysis. Lung Cancer. 167:49–57. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sugisaka J, Toi Y, Taguri M, Kawashima Y,
Aiba T, Kawana S, Saito R, Aso M, Tsurumi K, Suzuki K, et al:
Relationship between programmed cell death protein ligand 1
expression and immune-related adverse events in non-small-cell lung
cancer patients treated with pembrolizumab. JMA J. 3:58–66.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lisberg A, Tucker DA, Goldman JW, Wolf B,
Carroll J, Hardy A, Morris K, Linares P, Adame C, Spiegel ML, et
al: Treatment-related adverse events predict improved clinical
outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer
Immunol Res. 6:288–294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Peters S, Gettinger S, Johnson ML, Jänne
PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE,
Carcereny Costa E, et al: Phase II trial of atezolizumab as
first-line or subsequent therapy for patients with programmed
death-ligand 1–selected advanced non–small-cell lung cancer
(BIRCH). J Clin Oncol. 35:2781–2789. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Edahiro R, Kanazu M, Kurebe H, Mori M,
Fujimoto D, Taniguchi Y, Suzuki H, Hirano K, Yokoyama T, Morita M,
et al: Clinical outcomes in non-small cell lung cancer patients
with an ultra-high expression of programmed death ligand-1 treated
using pembrolizumab as a first-line therapy: A retrospective
multicenter cohort study in Japan. PLoS One.
14(e0220570)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Akazawa Y, Yoshikawa A, Kanazu M, Yano Y,
Yamaguchi T and Mori M: Non-small cell lung cancer with tumor
proportion score>90% could increase the risk of severe
immune-related adverse events in first-line treatments with immune
checkpoint inhibitors: A retrospective single-center study. Thorac
Cancer. 13:2450–2458. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bracamonte-Baran W and Kim ST: The current
and future of biomarkers of immune related adverse events. Rheum
Dis Clin. 50:201–227. 2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Townsend MJ, Benque IJ, Li M and Grover S:
Contemporary management of gastrointestinal, pancreatic and hepatic
toxicities of immune checkpoint inhibitors. Aliment Pharmacol Ther.
59:1350–1365. 2024.PubMed/NCBI View Article : Google Scholar
|
|
55
|
O'Leary CL, Pierce N, Patel SP and Naidoo
J: Immune-related toxicity in NSCLC: Current state-of-the-art and
emerging clinical challenges. J Thorac Oncol. 19:395–408.
2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cardeña-Gutiérrez A and López Barahona M:
Predictive biomarkers of severe immune-related adverse events with
immune checkpoint inhibitors: Prevention, underlying causes,
intensity, and consequences. Front Med (Lausanne).
9(908752)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mor A and Strazza M: Bridging the gap:
Connecting the mechanisms of immune-related adverse events and
autoimmunity through PD-1. Front cell Dev Biol.
9(790386)2022.PubMed/NCBI View Article : Google Scholar
|