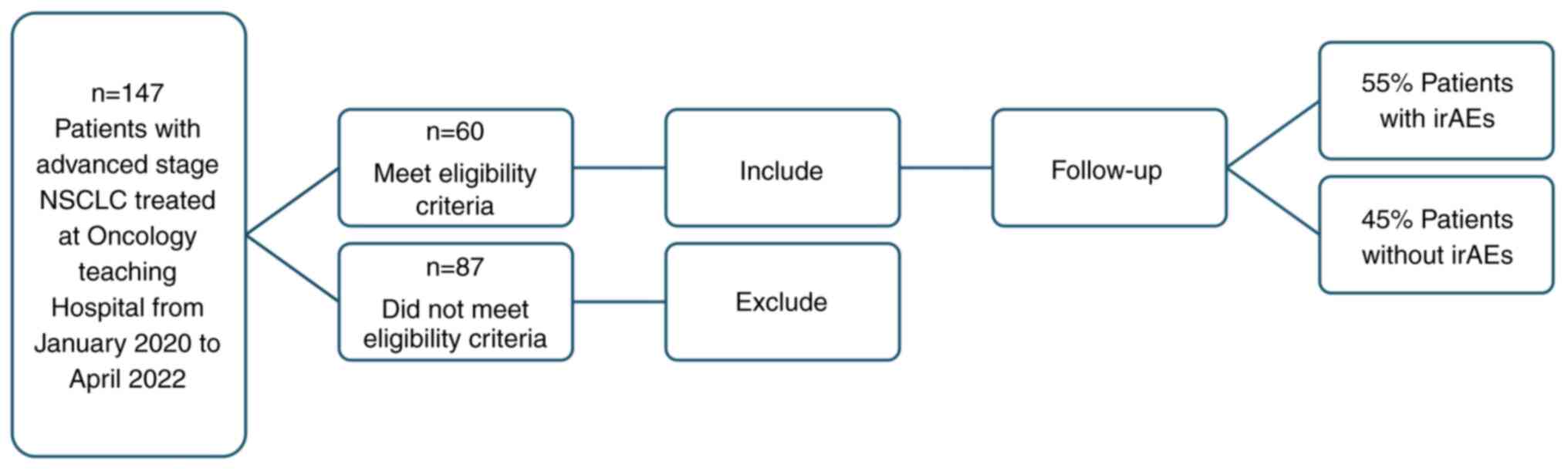
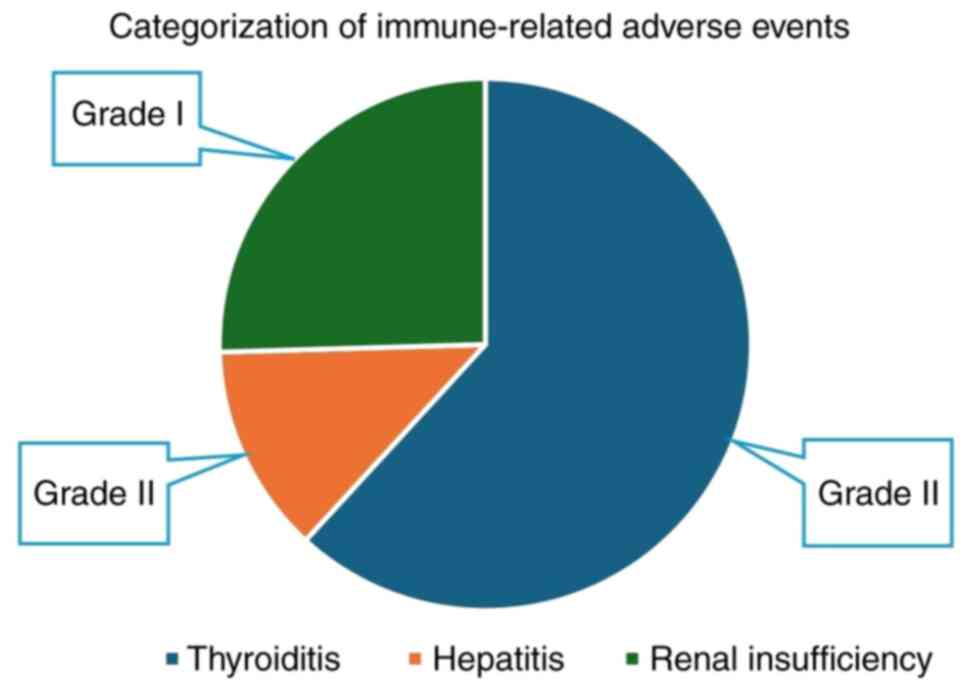
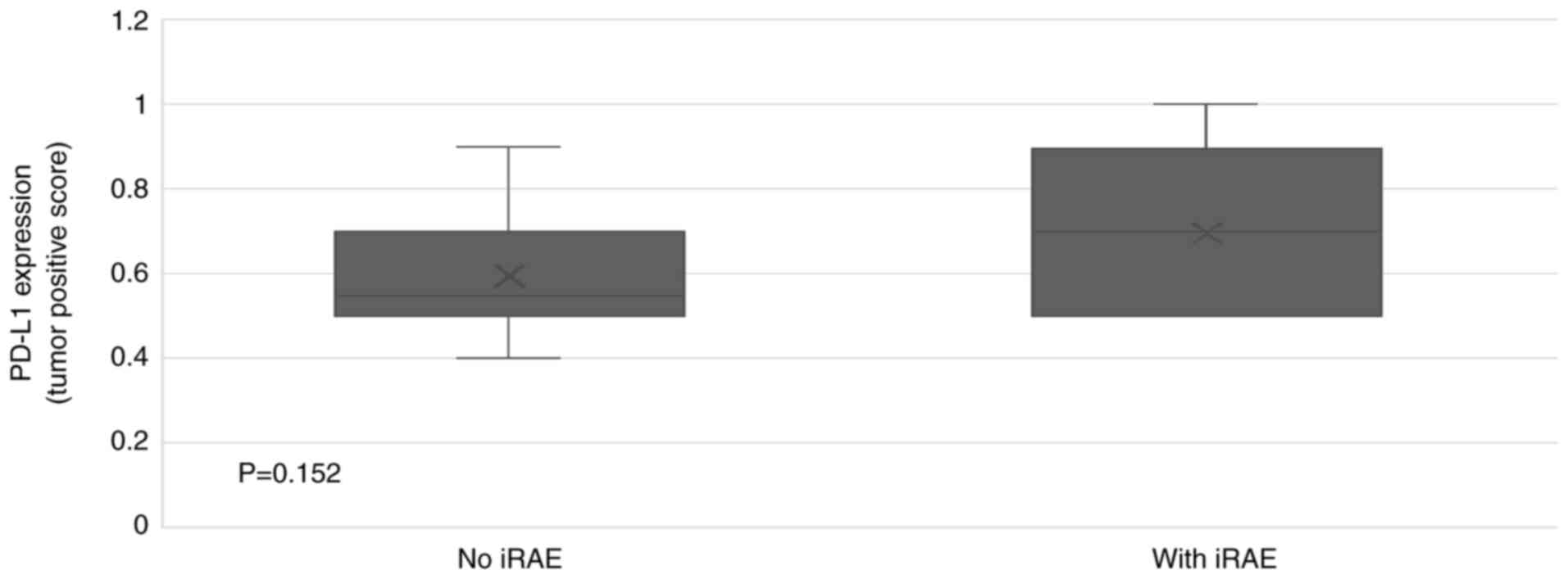
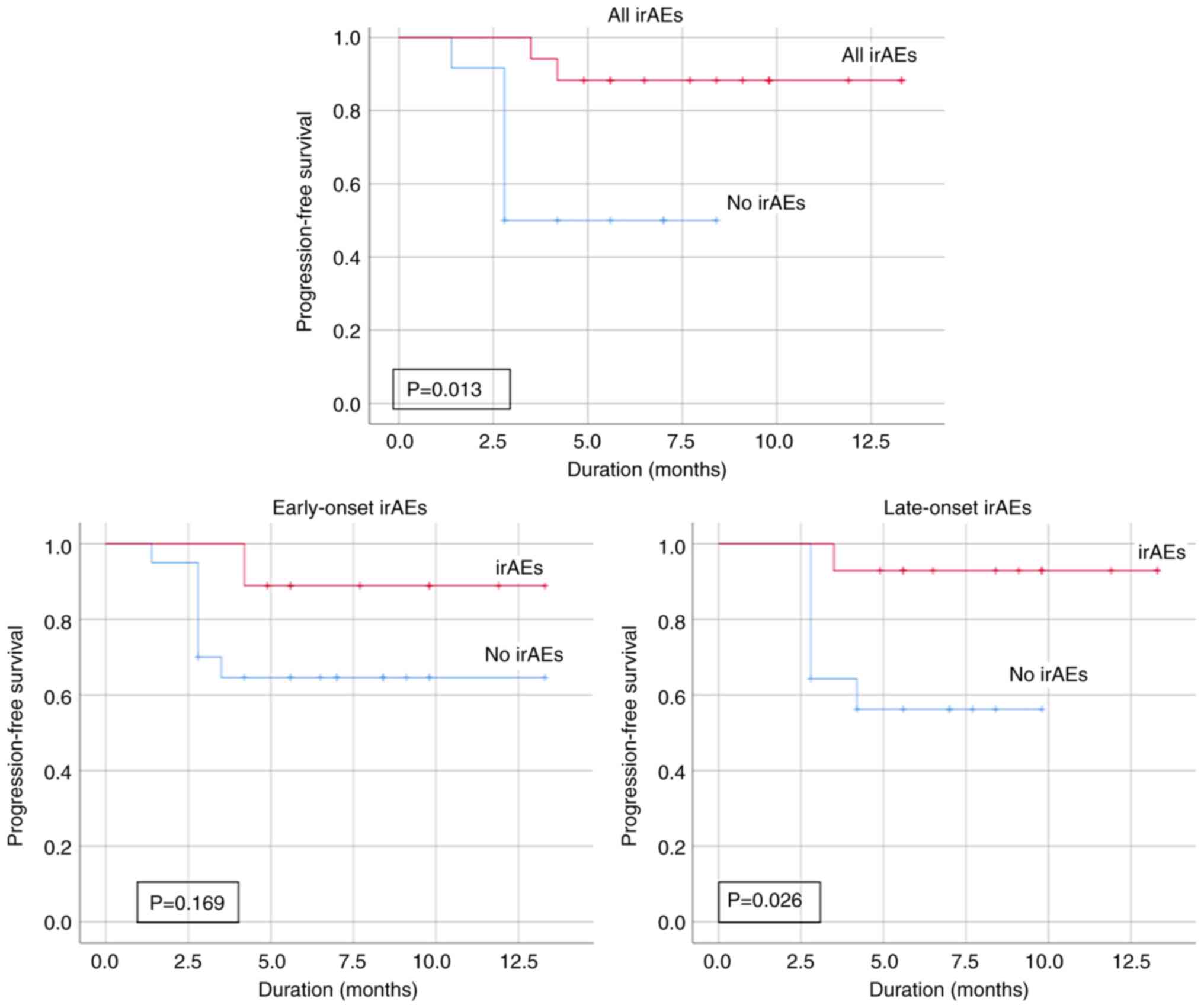
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mohammed Bakheet M, Mohssin Ali H and
Jalil Talab T: Evaluation of some proinflammatory cytokines and
biochemical parameters in pre and postmenopausal breast cancer
women. Cytokine. 179(156632)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Q, Yuan D, Ma C, Liu Y, Ma L, Lv T and
Song Y: A new hope: the immunotherapy in small cell lung cancer.
Neoplasma. 63:342–350. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
World Health Organization (WHO): Lung
cancer. WHO, Geneva, 2025. https://www.who.int/news-room/fact-sheets/detail/lung-cancer.
|
|
5
|
Inamura K: Lung cancer: Understanding its
molecular pathology and the 2015 WHO classification. Front Oncol.
7(193)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takano N, Ariyasu R, Koyama J, Sonoda T,
Saiki M, Kawashima Y, Oguri T, Hisakane K, Uchibori K, Nishikawa S,
et al: Improvement in the survival of patients with stage IV
non-small-cell lung cancer: Experience in a single institutional
1995–2017. Lung Cancer. 131:69–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mamdani H, Matosevic S, Khalid AB, Durm G
and Jalal SI: Immunotherapy in lung cancer: Current landscape and
future directions. Front Immunol. 13(823618)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dahl O and Brydøy M: The pioneers behind
immune checkpoint blockers awarded the Nobel Prize in physiology or
medicine 2018. Acta Oncol. 58:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xia L, Liu Y and Wang Y: PD-1/PD-L1
blockade therapy in advanced non-small-cell lung cancer: Current
status and future directions. Oncologist. 24 (Suppl 1):S31–S41.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cui P, Li R, Huang Z, Wu Z, Tao H, Zhang S
and Hu Y: Comparative effectiveness of pembrolizumab vs. nivolumab
in patients with recurrent or advanced NSCLC. Sci Rep.
10(13160)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang N, Tu J, Wang X and Chu Q:
Programmed cell death-1/programmed cell death ligand-1 checkpoint
inhibitors: differences in mechanism of action. Immunotherapy.
11:429–441. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mohammed MQ, Alwan AH and Almukhtar AA:
Estimation of Serum TLR-9, TNF-α, and IL-6 Levels in the Iraqi
Patients Diagnosed as Acute Myelogenous Leukemia. Baghdad Sci J.
21(2182)2024.
|
|
13
|
Azoury SC, Straughan DM and Shukla V:
Immune checkpoint inhibitors for cancer therapy: Clinical efficacy
and safety. Curr Cancer Drug Targets. 15:452–462. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elshahidi MH: Immune checkpoint inhibitors
and health-related quality of life: A systematic review of the
current literature. Mustansiriya Med J. 17:1–13. 2018.
|
|
15
|
Hua C, Boussemart L, Mateus C, Routier E,
Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, et
al: Association of vitiligo with tumor response in patients with
metastatic melanoma treated with pembrolizumab. JAMA Dermatol.
152:45–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jouda J, Mohammed IK, Asad SS, Falah YF
and Salih KM: Effect of alteration in nutritional style on liver
function tests and general stool examination. In: AIP Conference
Proceedings. AIP Publishing, 2020.
|
|
17
|
Musaelyan AA, Moiseyenko FV, Emileva TE,
Oganesyan AP, Oganyan KA, Urtenova MA, Odintsova SV, Chistyakov IV,
Degtyarev AM, Akopov AL, et al: Clinical predictors of response to
single-agent immune checkpoint inhibitors in
chemotherapy-pretreated non-small cell lung cancer. Mol Clin Oncol.
20(32)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jin CX, Liu YS, Qin HN, Teng YB, Sun R, Ma
ZJ, Wang AM and Liu JW: Peripheral inflammatory factors as
prognostic predictors for first-line PD-1/PD-L1 inhibitors in
advanced non-small cell lung cancer. Sci Rep.
15(11206)2025.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tong W, Xu H, Tang J, Zhao N, Zhou D, Chen
C and Cao D: Inflammatory factors are associated with prognosis of
non-small cell lung cancer patients receiving immunotherapy: A
meta-analysis. Sci Rep. 14(26102)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sonehara K, Tateishi K, Araki T, Komatsu
M, Yamamoto H, Koizumi T and Hanaoka M: The role of immune-related
adverse events in prognosis and efficacy prediction for patients
with non-small cell lung cancer treated with immunotherapy: A
retrospective clinical analysis. Oncology. 99:271–279.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e52.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
National Institute of Health (NIH): Common
Terminology Criteria for Adverse Events (CTCAE) v4.0. NIH,
Bethesda, 2009. Accessed May 28, 2010.
|
|
23
|
Rizwan S, Rai M, Bakalov V, Abel S, Lo H,
Niranjan S, Sethi A, Khan T, Alhamad K, Attah A, et al: Impact of
immune-related adverse events on survival in patients with advanced
non-small cell lung cancer treated with immune checkpoint
inhibitors: A real-world perspective. SSRN, J Clin Oncol. 39 (Suppl
15)(e21213)2021.
|
|
24
|
Morimoto K, Yamada T, Takumi C, Ogura Y,
Takeda T, Onoi K, Chihara Y, Taniguchi R, Yamada T, Hiranuma O, et
al: Immune-related adverse events are associated with clinical
benefit in patients with non-small-cell lung cancer treated with
immunotherapy plus chemotherapy: A retrospective study. Front
Oncol. 11(630136)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Couey MA, Bell RB, Patel AA, Romba MC,
Crittenden MR, Curti BD, Urba WJ and Leidner RS: Delayed
immune-related events (DIRE) after discontinuation of
immunotherapy: Diagnostic hazard of autoimmunity at a distance. J
Immunother Cancer. 7(165)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Owen DH, Wei L, Bertino EM, Edd T,
Villalona-Calero MA, He K, Shields PG, Carbone DP and Otterson GA:
Incidence, risk factors, and effect on survival of immune-related
adverse events in patients with non–small-cell lung cancer. Clin
Lung Cancer. 19:e893–e900. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gridelli C, Rossi E, De Chiara G,
Ciardiello F and Sgambato A: Thyroid-induced toxicity of
check-point inhibitors immunotherapy in the treatment of advanced
non-small cell lung cancer. J Endocrinol Diabe. 3:1–10. 2016.
|
|
28
|
Delivanis DA, Gustafson MP, Bornschlegl S,
Merten MM, Kottschade L, Withers S, Dietz AB and Ryder M:
Pembrolizumab-induced thyroiditis: Comprehensive clinical review
and insights into underlying involved mechanisms. J Clin Endocrinol
Metab. 102:2770–2780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cortellini A, Chiari R, Ricciuti B, Metro
G, Perrone F, Tiseo M, Bersanelli M, Bordi P, Santini D, Giusti R,
et al: Correlations between the immune-related adverse events
spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients.
Clin Lung Cancer. 20:237–247.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Alrabadi NN, Abushukair HM, Ababneh OE,
Syaj SS, Al-Horani SS, Qarqash AA, Darabseh OA, Al-Sous MM,
Al-Aomar SR, Ahmed YB, et al: Systematic review and meta-analysis
efficacy and safety of immune checkpoint inhibitors in advanced
melanoma patients with anti-PD-1 progression: A systematic review
and meta-analysis. Clin Transl Oncol. 23:1885–1904. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K: ESMO Guidelines Committee.
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28
(suppl 4):iv119–iv142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Durbin SM, Zubiri L, Perlman K, Wu CY, Lim
T, Grealish K, Hathaway N, LoPiccolo J, Wang M, Falade A, et al:
Late-onset immune-related adverse events after immune checkpoint
inhibitor therapy. JAMA Netw Open. 8(e252668)2025.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sayer MR, Mambetsariev I, Lu KH, Wong CW,
Duche A, Beuttler R, Fricke J, Pharoan R, Arvanitis L, Eftekhari Z,
et al: Predicting survival of NSCLC patients treated with immune
checkpoint inhibitors: Impact and timing of immune-related adverse
events and prior tyrosine kinase inhibitor therapy. Front Oncol.
13(1064169)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Teraoka S, Fujimoto D, Morimoto T, Kawachi
H, Ito M, Sato Y, Nagata K, Nakagawa A, Otsuka K, Uehara K, et al:
Early immune-related adverse events and association with outcome in
advanced non-small cell lung cancer patients treated with
nivolumab: A prospective cohort study. J Thorac Oncol.
12:1798–1805. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khoja L, Day D, Chen TWW, Siu LL and
Hansen AR: Tumour-and class-specific patterns of immune-related
adverse events of immune checkpoint inhibitors: A systematic
review. Ann Oncol. 28:2377–2385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hsiehchen D, Naqash AR, Espinoza M, Von
Itzstein MS, Cortellini A, Ricciuti B, Owen DH, Laharwal M, Toi Y,
Burke M, et al: Association between immune-related adverse event
timing and treatment outcomes. Oncoimmunology.
11(2017162)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hosoya K, Fujimoto D, Morimoto T, Kumagai
T, Tamiya A, Taniguchi Y, Yokoyama T, Ishida T, Hirano K, Matsumoto
H, et al: Association between early immune-related adverse events
and clinical outcomes in patients with non-small cell lung cancer
treated with immune checkpoint inhibitors. Clin Lung Cancer.
21:e315–e328. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aso M, Toi Y, Sugisaka J, Aiba T, Kawana
S, Saito R, Ogasawara T, Tsurumi K, Ono K, Shimizu H, et al:
Association between skin reaction and clinical benefit in patients
treated with anti-programmed cell death 1 monotherapy for advanced
non-small cell lung cancer. Oncologist. 25:e536–e544.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sugano T, Seike M, Saito Y, Kashiwada T,
Terasaki Y, Takano N, Hisakane K, Takahashi S, Tanaka T, Takeuchi
S, et al: Immune checkpoint inhibitor-associated interstitial lung
diseases correlate with better prognosis in patients with advanced
non-small-cell lung cancer. Thorac Cancer. 11:1052–1060.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen X, Nie J, Dai L, Hu W, Zhang J, Han
J, Ma X, Tian G, Han S and Wu D: , et al: Immune-related
adverse events and their association with the effectiveness of
PD-1/PD-L1 inhibitors in non-small cell lung cancer: A real-world
study from China. Front Oncol. 11(607531)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen M, Li Q, Xu Y, Zhao J, Zhang L, Wei
L, Zhong W and Wang M: Immunotherapy as second-line treatment and
beyond for non-small cell lung cancer in a single center of China:
Outcomes, toxicities, and clinical predictive factors from a
real-world retrospective analysis. Thorac Cancer. 11:1955–1962.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bouhlel L, Doyen J, Chamorey E, Poudenx M,
Ilie M, Gal J, Guigay J, Benzaquen J, Marquette CH, Berthet JP, et
al: Occurrence and number of immune-related adverse events are
independently associated with survival in advanced non-small-cell
lung cancer treated by nivolumab. Bull Cancer. 107:946–958.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ricciuti B, Genova C, De Giglio A,
Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi
F and Chiari R: Impact of immune-related adverse events on survival
in patients with advanced non-small cell lung cancer treated with
nivolumab: Long-term outcomes from a multi-institutional analysis.
J Cancer Res Clin Oncol. 145:479–485. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Burgos-San José A, Colomer-Aguilar C,
Martínez-Caballero D and Massutí-Sureda B: Effectiveness and safety
of atezolizumab, nivolumab and pembrolizumab in metastatic
non-small cell lung cancer. Farm Hosp. 45:121–125. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
47
|
Torasawa M, Yoshida T, Yagishita S,
Shimoda Y, Shirasawa M, Matsumoto Y, Masuda K, Shinno Y, Okuma Y,
Goto Y, et al: Nivolumab versus pembrolizumab in previously-treated
advanced non-small cell lung cancer patients: A propensity-matched
real-world analysis. Lung Cancer. 167:49–57. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sugisaka J, Toi Y, Taguri M, Kawashima Y,
Aiba T, Kawana S, Saito R, Aso M, Tsurumi K, Suzuki K, et al:
Relationship between programmed cell death protein ligand 1
expression and immune-related adverse events in non-small-cell lung
cancer patients treated with pembrolizumab. JMA J. 3:58–66.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lisberg A, Tucker DA, Goldman JW, Wolf B,
Carroll J, Hardy A, Morris K, Linares P, Adame C, Spiegel ML, et
al: Treatment-related adverse events predict improved clinical
outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer
Immunol Res. 6:288–294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Peters S, Gettinger S, Johnson ML, Jänne
PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE,
Carcereny Costa E, et al: Phase II trial of atezolizumab as
first-line or subsequent therapy for patients with programmed
death-ligand 1–selected advanced non–small-cell lung cancer
(BIRCH). J Clin Oncol. 35:2781–2789. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Edahiro R, Kanazu M, Kurebe H, Mori M,
Fujimoto D, Taniguchi Y, Suzuki H, Hirano K, Yokoyama T, Morita M,
et al: Clinical outcomes in non-small cell lung cancer patients
with an ultra-high expression of programmed death ligand-1 treated
using pembrolizumab as a first-line therapy: A retrospective
multicenter cohort study in Japan. PLoS One.
14(e0220570)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Akazawa Y, Yoshikawa A, Kanazu M, Yano Y,
Yamaguchi T and Mori M: Non-small cell lung cancer with tumor
proportion score>90% could increase the risk of severe
immune-related adverse events in first-line treatments with immune
checkpoint inhibitors: A retrospective single-center study. Thorac
Cancer. 13:2450–2458. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bracamonte-Baran W and Kim ST: The current
and future of biomarkers of immune related adverse events. Rheum
Dis Clin. 50:201–227. 2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Townsend MJ, Benque IJ, Li M and Grover S:
Contemporary management of gastrointestinal, pancreatic and hepatic
toxicities of immune checkpoint inhibitors. Aliment Pharmacol Ther.
59:1350–1365. 2024.PubMed/NCBI View Article : Google Scholar
|
|
55
|
O'Leary CL, Pierce N, Patel SP and Naidoo
J: Immune-related toxicity in NSCLC: Current state-of-the-art and
emerging clinical challenges. J Thorac Oncol. 19:395–408.
2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cardeña-Gutiérrez A and López Barahona M:
Predictive biomarkers of severe immune-related adverse events with
immune checkpoint inhibitors: Prevention, underlying causes,
intensity, and consequences. Front Med (Lausanne).
9(908752)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mor A and Strazza M: Bridging the gap:
Connecting the mechanisms of immune-related adverse events and
autoimmunity through PD-1. Front cell Dev Biol.
9(790386)2022.PubMed/NCBI View Article : Google Scholar
|