Introduction
Dental caries is a disease resulting from multiple
factors, beginning with changes in the microbiological composition
of the intricate biofilm and influenced by sugar intake, fluoride
exposure, saliva flow and composition, as well as dental hygiene
practices. Dental caries results from the transformation of
fermentable sugars by bacteria in dental plaque into acids on the
surface of teeth. Therefore, it is crucial to emphasize the
reduction of sugar intake and the management of plaque (1). Dental caries is widely recognized to
develop when the biofilm microbiota that typically exists in a
state of balance in the oral cavity shifts to an acid-producing,
acid-tolerant, and cavity-causing community as a result of regular
sugar intake. The outcome of this change may be undetectable in
clinical settings or cause a net loss of minerals in the hard
tissues of the tooth, leading to the appearance of a cavity
(2,3). Caries is recognized as the most
common oral health issue globally, affecting children, teenagers
and adults, and it is the leading cause of tooth loss in the
population. In addition to caries, there are various other diseases
of the mouth that can be identified and tracked using cytokines,
which play a critical role in regulating the immune and
inflammatory responses (4).
Cytokines play a pivotal role in the immunopathogenesis of dental
caries. Among the different cytokines under examination for their
potential inclusion in caries conclusion are interleukin (IL)-4,
IL-6, IL-8 and tumor necrosis factor-α (TNF-α) (5).
Cytokines play a major role in modulating the immune
response, and are either pro-inflammatory or anti-inflammatory.
They are critical for regulating immune responses. Any cell nuclei
can release cytokines, but helper T cells and macrophages are the
primary producer (6). The
chemokine class prototype molecule is IL-8, a cytokine that
provokes inflammatory reactions. Additionally, IL-8 is a key player
in the acute inflammatory reaction and remains at the site of
inflammation for a considerable amount of time (7). The protein TNF-α plays a role in
alert responses and host defense. White blood cell molecules stick
to endothelial cells and trigger phagocytic killing processes,
which encourages cell proliferation. In addition to cytotoxic and
cytostatic effects on cancerous cells. IL-8 and TNF-α products of
immune cells also play an critical role in diseases of the oral
mucosa. However, patients with dental caries higher levels of IL-8
and TNF-α, leading to a lower number of osteoblasts and
fibroblasts, and they support the demineralization of teeth and
development of the dental caries process. These cytokines, which
are produced locally by osteoclasts, have been shown to be a
critical factor in regulating the differentiation of these cells
and participating in resorption processes (6). The inflammatory aspect of caries
disease, a known source for molecular diagnosis, remains unknown,
and the diagnosis for the condition contuse to depend on clinical,
visual and radiographic techniques (8). The aim of the present study was to
assess the diagnostic precision of cytokines (IL-8 and TNF-α) in
distinguishing between subjects with caries and those without.
Subjects and methods
Study design and ethics approval. The present study
was a case-control study. The subjects included in the study were
provided with a comprehensive explanation of the purpose and
methods of the study. Their permission was gained via a document
vetted via the Ethic Committee of the College of Dentistry,
University of Baghdad (Reference no. 1000 on 7-1-2025).
Study subjects
A total of 44 subjects were enrolled in the present
study (22 adults subjects with dental caries and 22 of the same age
and sex without caries were randomly selected as the control
group). There was a specific case sheet used to obtain information
about the name, age, sex and general health of the adult and
whether the subject was a smoker or alcoholic or not. A
questionnaire about the general and oral health habits of the
selected patients was prepared to ensure that they were free from
any systematic diseases and did not take any medications for at
least the past 1 month.
Inclusion and exclusion criteria
All participants recruited in the study as the test
subjects had dental caries, >20 years, had no systemic disease
with no antibiotic consumption for the past 3 months. The control
group were subjects with healthy gingiva, exhibiting good oral
hygiene without any history of dental caries or related symptoms.
The present study excluded children and patients who had the
following conditions: Smoking or alcohol consumption, unapproved
consent, pregnant or lactating women, or those taking medications
(antibiotics or any medications).
Oral examination
The assessment of clinical caries was performed by
an examination of teeth and evaluation of decayed, missing and
filled surfaces (DMFS) and plaque index (the amount of dental
plaque at four surfaces of each tooth was assessed). The assessment
of dental plaque was performed according to the plaque index system
(9). The scores and criteria for
this system were followed as proposed by authors, as follows: Score
0, no plaque in the gingival area; score 1, a film of plaque
adhering to the free gingival margin and adjacent area of the tooth
surface; score 2, moderate accumulation of soft deposits within the
gingival pocket on the gingival margin and/or adjacent tooth
surface which can be seen by naked eye; score 3, abundance of soft
matter within the gingival pocket and/or on the gingival margin and
the adjacent tooth surface.
Gingival index
The occurrence of gingival inflammation at four
surfaces of each tooth was assessed using the criteria of gingival
index system (9), as follows:
Score 0, normal gingiva; score 1, mild inflammation, slight change
in color, slight edema, no bleeding on probing; score 2, moderate
inflammation, redness, edema and glazing, bleeding on probing;
score 3, severe inflammation, marked redness and edema, ulceration,
tendency for spontaneous bleeding.
Saliva sample collection
At least 1 h prior to saliva collection, all
participants were requested to not consume anything other than
water. The subjects then repeatedly rinsed their mouths with
sterile water and waited 1 to 2 min for the water to drain; up to 2
ml whole unstimulated saliva was collected into tubes. The
collected saliva was then centrifuged at 804.96 x g for 10 min at
room temperature (~20˚C) and the resulting supernatant was stored
at -20˚C in Eppendorf tubes until further processing (10,11).
Detection of IL-8 and TNF-α
levels
The ELISA procedure for the present study was the
sandwich-ELISA technique. According to the manufacturer of the kit
(Shanghai YL Biotech Co., Ltd.), a capture antibody that is highly
specific for the biomarker (IL-8 and TNF-α) was attached to the
wells of the strip plate. During the incubation time (60 min at
37˚C), each biomarker sample and known standards were bound to
capture antibodies, followed by binding the biotinylated anti-IL-8
(Human IL-8 ELISA KIT, cat no. YLA1210HU, Shanghai YL Biotech Co.,
Ltd.) and anti-TNF-α (Human receptor superfamily member 13B ELISA
KIT, cat no. YLA0576HU, Shanghai YL Biotech Co., Ltd.) secondary
antibody (to the analyte to create the captured complex. The
secondary antibody and any extra unbound analyte were rinsed away.
The HRP conjugation solution (provided with the aforementioned
kits) was added to each well, even the zero wells, to attach to the
complex. The excess conjugate was then carefully washed away
following incubation (60 min at 37˚C). Adding a chromogen substrate
solution (provided with the aforementioned kits) to the wells
gradually caused the conjugate and chromogen to produce a
blue-colored compound. The addition of acid then halted color
development. After the sulfuric acid reaction was terminated, the
yellow hue was produced. The concentration of each biomarker in the
samples and standards directly relates to the intensity of the
colorful formed complex. The absorbances were assessed
spectrophotometrically at 450 nm using an ELISA plate reader
(Glomax; Promega Corporation).
The data were analyzed using G power 3.1.9.7
software from Franz-Faul (Kiel University, Germany). Given these
conditions, the sample size was determined to be 40 participants in
total, 20 in each of the two groups, assuming a medium effect size
of 0.6 between them. This gave the statistical test an overall
power of 85% and an α error of probability of 0.05. Accordingly, 44
subjects were included in the study.
Statistical analysis
All statistical analyses of the data were performed
and processed with the computerized analysis statistical package
for the social sciences (SPSS) software program (version 25, IBM
Corp.) and GraphPad Prism software (version 9.0, Dotmatics). A
value of P<0.05 was considered to indicate a statistically
significant difference. The distribution of clinical and
immunological data was determined using the Shapiro-Wilk test.
Since the variables in the dataset were normally distributed,
parametric tests were preferred. The data are presented as
descriptive statistics involving the mean and standard deviation.
The Chi-squared test was preferred to analyze sex distributions.
When comparing two groups, the (Student's t-test) was utilized.
Pearson' rank correlation analysis was used to analyze the
correlations between clinical and immunological parameters. In
addition, to determine the diagnostic potential of the cytokines, a
receiver operating characteristic (ROC) curve was established.
Results
Patient demographics and clinical
parameters
The mean and standard deviation values were
calculated for the demographic variables and clinical parameters of
the two study groups (Table I).
The demographic data distributions for patients were determined for
sex (12 females, 10 males and age (range, 20-35 years). The DMFS
ranged from 9 to 70 with a mean value of 32.12±19.05, and the
sample site analysis of the clinical parameters plaque index (PLI)
and gingival index (GI) were revealed to be all significantly
higher for dental caries (study) group compared to the healthy
group (P<0.05) (Table I).
 | Table IDemographic characteristics and
clinical parameters in study groups. |
Table I
Demographic characteristics and
clinical parameters in study groups.
| Demographic
characteristics | Study group n=22 | Control group
n=22 | P-value |
|---|
| Age | | | 0.258
(NS)a |
|
Range
(years) | 20-35 | 20-33 | |
|
Mean ±
SD | 29.90±5.42 | 28.68±5.16 | |
| Sex | | | 0.762
(NS)b |
|
Female | 12 (54.54%) | 11 (50.0%) | |
|
Male | 10 (45.45%) | 11 (50.0%) | |
| Clinical
parameters | | | |
|
PLI (mean ±
SD) | 1.56±0.52 | 0.45±0.05 |
<0.001c |
|
GI (mean ±
SD) | 1.57±0.57 | 0.90±0.29 |
<0.001c |
|
DMFS | 32.12±19.05 | - | - |
Salivary cytokine levels
The present study revealed that the mean values of
IL-8 and TNF-α were significantly higher (P<0.05) in the dental
caries group in comparison to the group without caries (Table II). Moreover, as demonstrated in
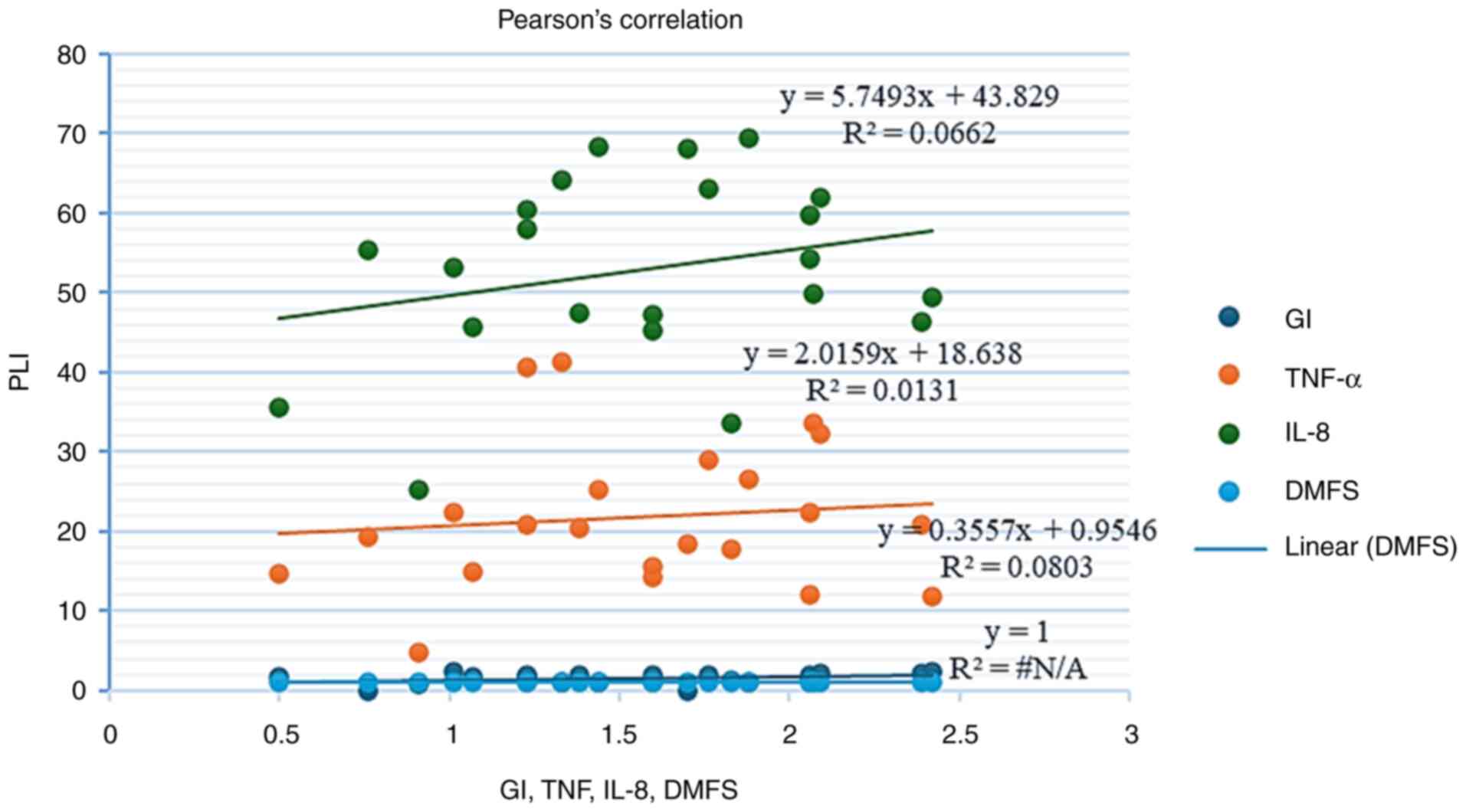
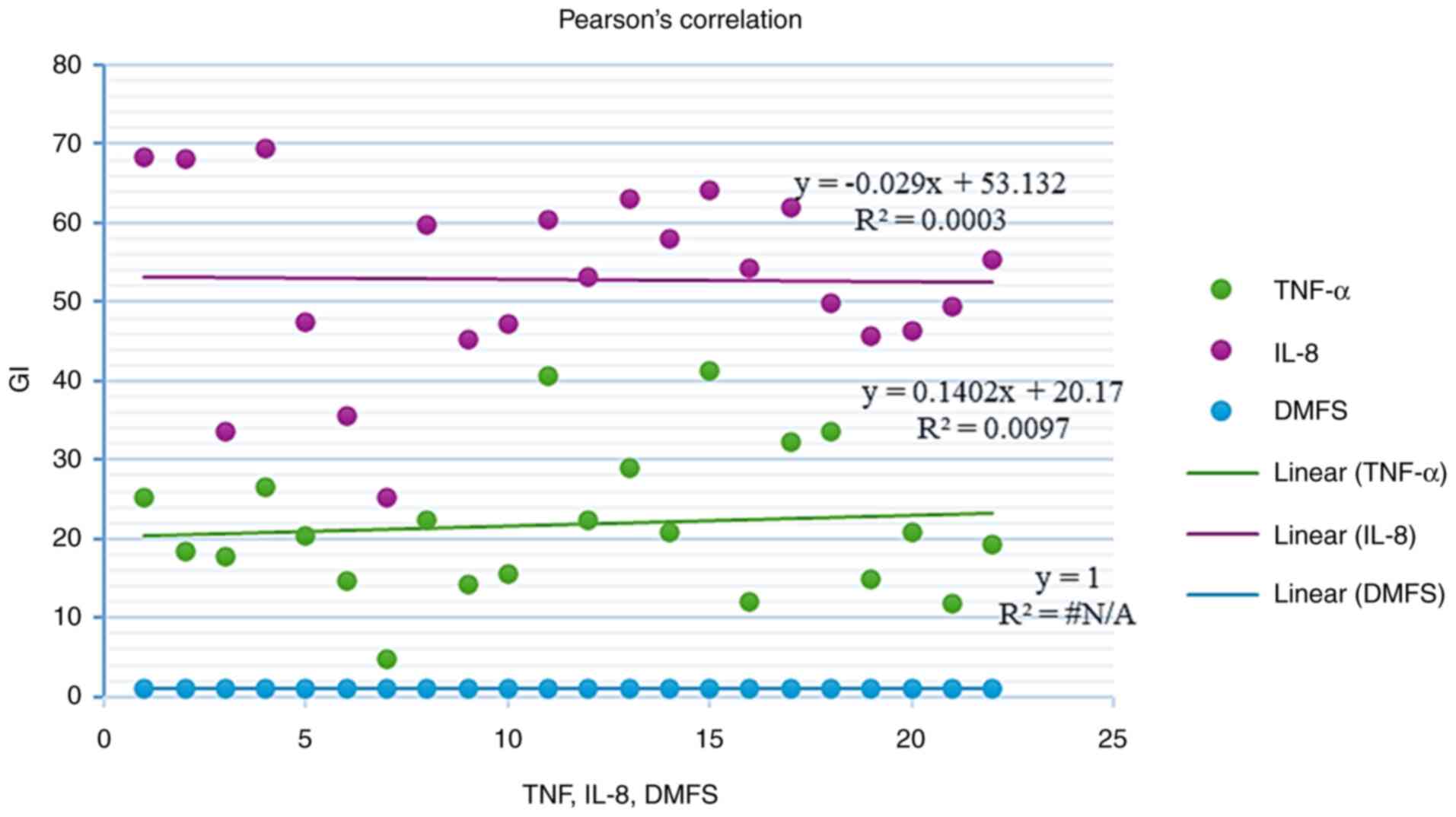
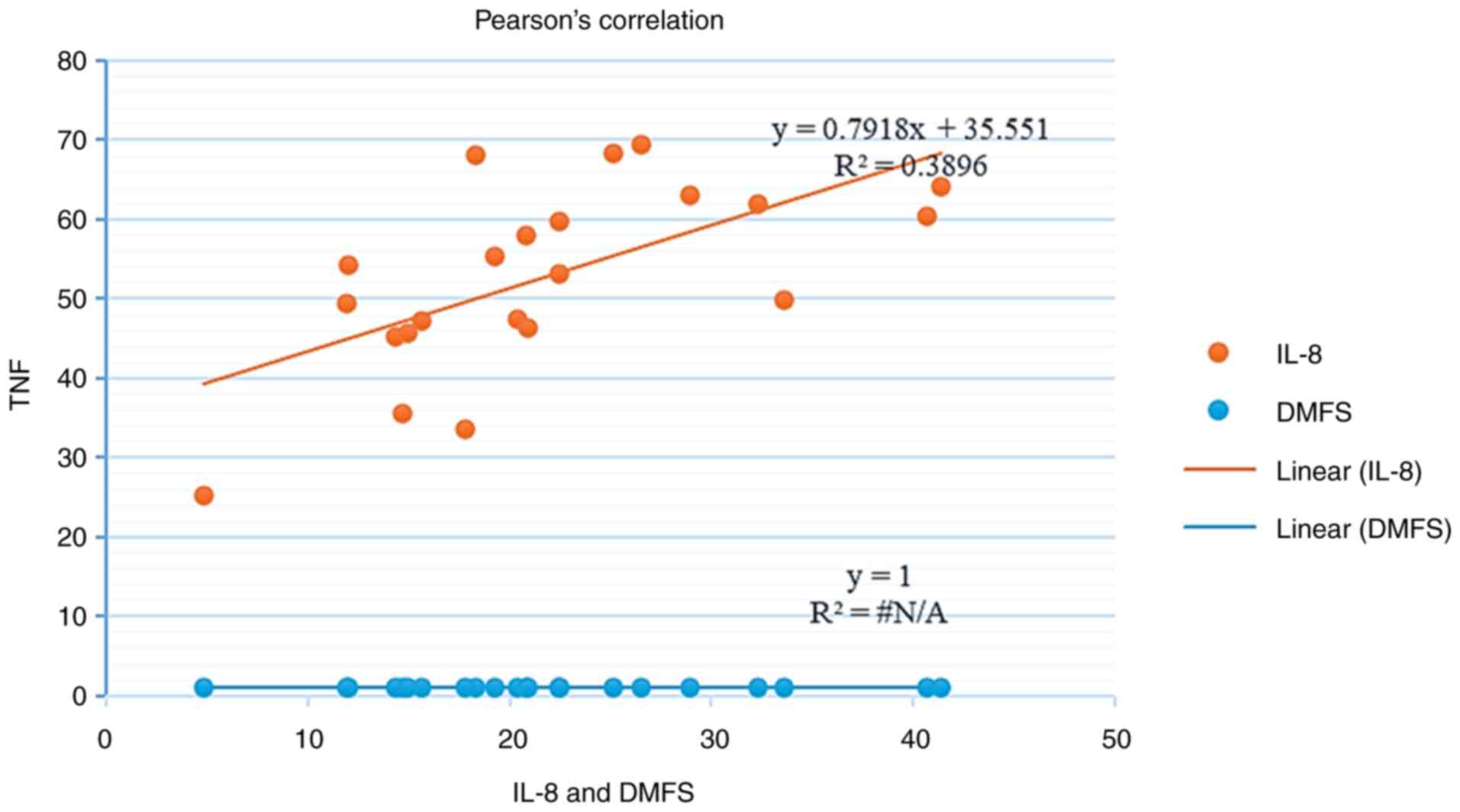
Table III and Fig. 1, 2
and 3, the results revealed that
there was a significant positive correlation between IL-8 and TNF-α
in the patient group (r=0.624, P=0.001), whereas there are no
significant correlations between clinical parameters and cytokines
in caries group.
 | Table IIMean salivary levels of IL-8 and TNF-α
in the study groups. |
Table II
Mean salivary levels of IL-8 and TNF-α
in the study groups.
| Salivary
cytokines | Study group n=22 | Control group
n=22 | t-test (P-value) |
|---|
| IL-8 (pg\ml) | | | 0.002a |
|
Range | 22.24-69.54 | 20.55-65.28 | |
|
Mean ±
SD | 52.79±11.69 | 42.63±11.08 | |
| TNF-α (pg\ml) | | | 0.011a |
|
Range | 4.83-41.35 | 7.57-33.77 | |
|
Mean ±
SD | 21.78±9.22 | 16.39±6.55 | |
 | Table IIIPearson's correlation analysis of the
correlation between cytokines and clinical parameters in the caries
group. |
Table III
Pearson's correlation analysis of the
correlation between cytokines and clinical parameters in the caries
group.
| | Parameter |
|---|
| Parameter | PLI | GI | DMFS | IL-8 | TNF-α |
|---|
| PLI | - | r=0.217 P=0. 330 | r=0.089 P=0. 723 | r=0.257 P=0.247 | r=0.114 P=0. 612 |
| GI | r=0.217 P=0. 330 | - | r=0.070
P=0.726 | r=0.183 P=0.
413 | r=-0.018
P=0.936 |
| DMFS | r=0.089 P=0.
723 | r=0.070 P=0.
726 | - | r=0.157 P=0.
484 | r=0.217 P=0.
332 |
| IL-8 | r=0.257
P=0.247 | r=0.183 P=0.
413 | r=0.157
P=0.484 | - | r=0.624 P=0.
001a |
| TNF-α | r=0.114
P=0.612 | r=-0.018
P=0.936 | r=0.217
P=0.332 | r=0.624 P=0.
001a | - |
Diagnostic accuracy of salivary
cytokines
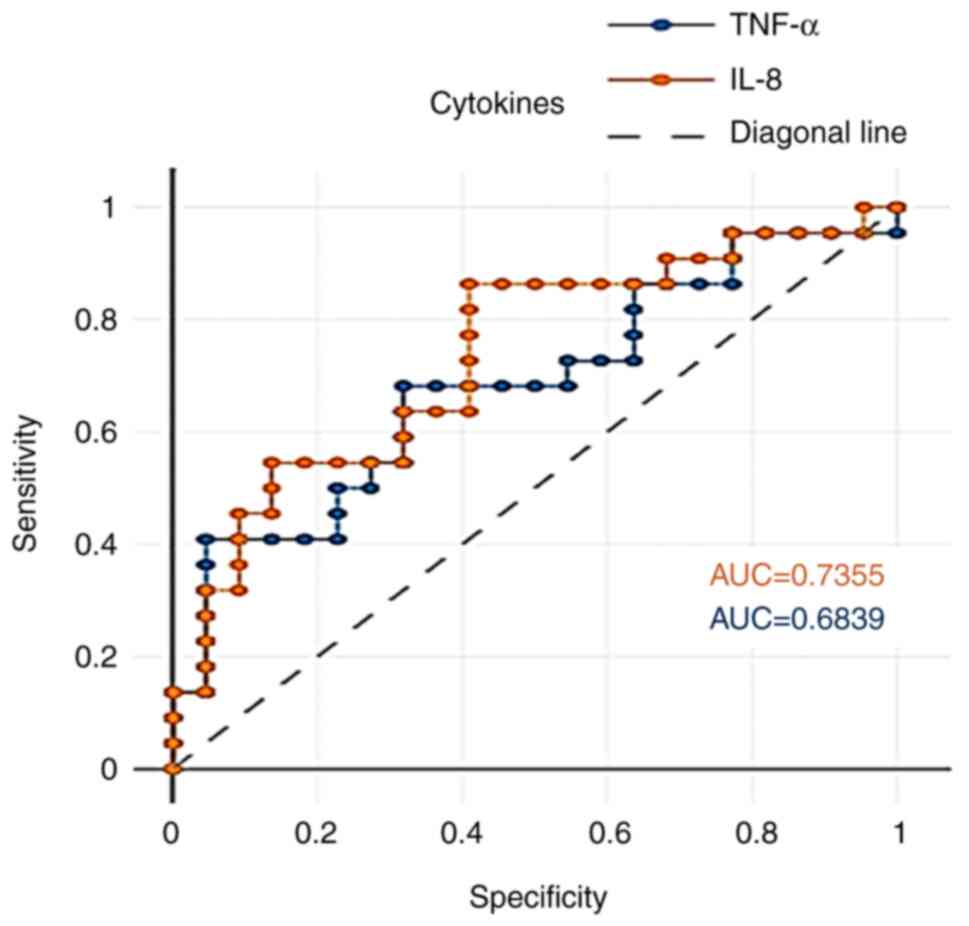
ROC analysis was performed to determine the
sensitivity and specificity of salivary IL-8 and TNF-α to
discriminate caries patients from healthy controls. IL-8 in saliva
exhibited acceptable potentials in differentiating patients from
healthy controls (Fig. 4).
Furthermore, the area under the curve (AUC) values for salivary
IL-8 and TNF between the study and control groups were 0.735 and
0.683, respectively, as shown in Table IV.
 | Table IVComparison of the diagnostic
properties of cytokines among the study groups. |
Table IV
Comparison of the diagnostic
properties of cytokines among the study groups.
| Comparison | Test result
variables (s) | AUC | P-value | Optimal cut of
point | Sensitivity | Specificity |
|---|
| Dental caries vs.
controls | IL-8 | 0.735 | 0.01a | 45.16 | 0.95 | 0.95 |
| | TNF-α | 0.683 | 0.01a | 22.43 | 0.95 | 0.95 |
Discussion
In dental pulp, dental caries promote a host
inflammatory reaction that is evidenced by the buildup of
inflammatory leucocytes and the subsequent produce of
pro-inflammatory cytokines (12).
Cytokines were essential for inflammatory process and immune
response. Saliva could be utilized as a non-invasive diagnostic
liquid to evaluate biomarkers in the early and developed phases of
the disease, and cytokines and other factors are helpful diagnostic
and monitoring tools for the oral cavity (13). The present study assessed IL-8
levels in the participants and found that the dental caries group
had higher levels than the control group. The IL-8 is a less
commonly investigated interleukin among inflammatory biomarkers
associated with dental caries. The present study demonstrated that
the salivary levels of IL-8 exhibited a discriminative ability
between healthy controls and patients with dental caries, with a
sensitivity value of 0.95 and specificity value of 0.95. The
diagnostic potential AUC value, as measured in the present study,
was 0.735 with a cut-off point of 45.16 pg/ml. This finding is
consistent with the findings of previous studies (14-16), which found
increased levels of this cytokine among conditions with active
dental caries compared to their control groups. According to
Gornowicz et al (17),
adolescents with dental caries had elevated amounts of this
pro-inflammatory cytokine than those without dental decay,
verifying the function of IL-8 as a pivotal chemokine in
granulocytes chemotaxis. Likewise, Zhao et al (18) reported that patients with active
carious lesions had a noticeably higher IL-8 concentration.
Cariogenic pathogens are primarily Gram-positive, and their
products, such as lipoteichoic acid, which is ubiquitous in
cariogenic streptococci, promote Toll-like receptor 2 and
inflammasomes peptides, which results in the marked production of
this chemokine. This could explain the increase in the level of
this mediator (19,20). However, two other studies (12,21)
did not find any key variations between the caries and caries-free
groups in terms of the salivary levels of IL-8.
Apart from IL-8, TNF-α levels was also analyzed in
the present study, which were shown to be higher in the patients
with caries. The salivary levels of TNF-α exhibited a
discriminative ability between healthy controls and dental caries
patients with a sensitivity value of 0.95 and specificity value of
0.95. The diagnostic potential AUC value, as measured herein, was
0.683 with a cut-off point of 22.43 pg/ml. Likewise, as regards the
TNF-α level, previous studies (22-25) have
demonstrated that the levels of this cytokine in the saliva of
children and adults with dental caries are increased, and are
associated with disease progression and severity. Higher levels of
this cytokine may indicate an inflammatory response in the defenses
of the body, leading to a production of TNF-α through damaging
mechanisms. TNF-α is involved in various disease processes,
including caries development (26,27).
TNF-α is an empirical indicator that quantifies the amount of
inflammation, perhaps beneficial for diagnosing caries (28). Kurtiş et al (29) found that increased levels of TNF-α
in patients with dental caries reduced the total amount of
osteoblasts and fibroblasts, promoting tooth demineralization and
the occurrence of dental disease. However, only one study (16) reported higher levels of this
cytokine in normal subjects than in individuals with the disease.
The discrepancy between prior results and the current results may
be attributable to variations in age or the use of non-parametric
approaches for the analysis of data rather than the less
conservative parametric approaches used in the present study.
Furthermore, salivary TNF-α levels were found to be positively
related to IL-8. This finding is not surprising, as TNFα and IL-8
are released by the same cell types, interact frequently and share
numerous mechanisms, including inflammatory bone resorption.
Moreover, These cytokines contribute to oral cavity immunity
(17). TNF-α is an operational
molecule that causes dental illnesses and increases IL-8 levels
(6). In addition, the present
study revealed that there was no significant association between
cytokines levels and clinical parameters; however, according to
Giudice et al (30),
cytokine levels in children may increase as a result of poor plaque
control, plaque buildup and periodontal inflammation.
The present study had a main limitation which should
be mentioned. The present study did not include the severity of
disease and anti-inflammatory cytokines were also not examined.
In conclusion, according to the findings of the
present study, the salivary levels of the IL-8 and TNF-α cytokines
were higher in patients with dental caries, suggesting a possible
involvement of these cytokines in the etiology of dental caries.
Moreover, IL-8 exhibited a moderate clinical accuracy in
differentiating the patients from the controls. Thus, IL-8 and
TNF-α may prove to be useful as diagnostic biomarkers for dental
caries. The identification of IL-8 and TNF-α as potential
biomarkers for dental caries opens new avenues for early detection,
risk assessment and personalized treatments. These findings
underscore the importance of inflammatory pathways in the
pathogenesis of dental caries and highlight the need for further
research to validate these biomarkers and their association with
caries progression, in order to enhance diagnostic and therapeutic
strategies. Moreover, the findings of the present study may pave
the way for innovative, biomarker-driven approaches in preventive
dentistry, ultimately reducing the global burden of dental
caries.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BHAG and AAHA were involved in the conception and
design of the study, in the literature search, in the clinical
analysis, data analysis, statistical analysis, and in the
preparation and reviewing of the manuscript. HKM, SSJ and FAMAK
were involved in the conception and design of the study, as well as
in data analysis, and in the preparation and reviewing of the
manuscript. BHAG and HKM confirm the authenticity of all the raw
data. The final manuscript has been read and approved by all
authors.
Ethics approval and consent to
participate
The Institutional Review Board's Ethics Committee of
the Ethics Committee of the Dentistry School at the University of
Baghdad, Baghdad, Iraq, gave its approval to this study (Reference
no. 1000 on 7-1-2025). A written informed consent to participate in
the study, as specified in the Declaration of Helsinki, was sought
from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ali AA and Belgasem KA: Dental caries
experience of primary school children in Sirte-libya. Saudi J Oral
Dent Res. 9:1–6. 2024.
|
|
2
|
Meyer F, Schulze Zur Wiesche E, Amaechi
BT, Limeback H and Enax J: Caries etiology and preventive measures.
Eur J Dent. 18:766–776. 2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duangthip D, Gao SS, Chen KJ, Lo ECM and
Chu CH: Oral health-related quality of life and caries experience
of Hong Kong preschool children. Int Dent J. 70:100–107.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kareem SJ and Al-Ghurabi BH: Regulatory
role of human neutrophil peptides (HNP1-3) on Interleukin-6
production in early childhood caries. J Emergency Med Trauma Acute
Care. 2023(11)2023.
|
|
5
|
Alarcón-Sánchez MA, Becerra-Ruiz JS,
Avetisyan A and Heboyan A: Activity and levels of TNF-α, IL-6 and
IL-8 in saliva of children and young adults with dental caries: A
systematic review and Meta-analysis. BMC Oral Health.
24(816)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hussein BJ, Atallah HN and Al-Dahhan NAA:
Salivary levels of interleukin-6, interleukin-8 and tumor necrosis
Factor-alpha in smokers aged 35-46 years with dental caries
disease. Medico Legal Update. 20:1464–1470. 2020.
|
|
7
|
Tampa M, Mitran MI, Mitran CI, Sarbu MI,
Matei C, Nicolae I, Caruntu A, Tocut SM, Popa MI, Caruntu C and
Georgescu SR: Mediators of inflammation-a potential source of
biomarkers in oral squamous cell carcinoma. J Immunol Res.
2018(1061780)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abdelaziz M: Detection, diagnosis, and
monitoring of early caries: The future of individualized dental
care. Diagnostics (Basel). 13(3649)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Silness J and Loe H: Periodontal disease
in pregnancy. II. Correlation between oral hygiene and periodontal
condtion. Acta Odontol Scand. 22:121–135. 1964.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Navazesh M: Methods for collecting saliva.
Ann N Y Acad Sci. 694:72–77. 1993.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Hindawi SH, Luaibi NM and Al-Ghurabei
BH: Possible use of saliva as a diagnostic tool in hypothyroidism.
J Biosci Biotech. 6:539–542. 2017.
|
|
12
|
Sharma V, Gupta N, Srivastava N, Rana V,
Chandna P, Yadav S and Sharma A: Diagnostic potential of
inflammatory biomarkers in early childhood caries-A case control
study. Clin Chim Acta. 471:158–163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Benabdelmoumene S, Dumont S, Petit C,
Poindron P, Wachsmann D and Klein JP: Activation of human monocytes
by Streptococcus mutans serotype f polysaccharide: Immunoglobulin G
Fc receptor expression and tumor necrosis factor and interleukin-1
production. Infect Immun. 59:3261–3266. 1991.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Biria M, Sattari M, Iranparvar P and
Eftekhar L: Relationship between the salivary concentrations of
proteinase-3 and interleukin-8 and severe early childhood caries.
Dent Med Probl. 60:577–582. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Børsting T, Venkatraman V, Fagerhaug TN,
Skeie MS, Stafne SN, Feuerherm AJ and Sen A: Systematic assessment
of salivary inflammatory markers and dental caries in children: An
exploratory study. Acta Odontol Scand. 80:338–345. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rinderknecht C, Filippi C, Ritz N,
Fritschi N, Simmen U, Filippi A and Diesch-Furlanetto T:
Associations between salivary cytokines and oral health, age, and
sex in healthy children. Sci Rep. 12(15991)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gornowicz A, Bielawska A, Bielawski K,
Grabowska SZ, Wójcicka A, Zalewska M and Maciorkowska E:
Pro-inflammatory cytokines in saliva of adolescents with dental
caries disease. Ann Agric Environ Med. 19:711–716. 2012.PubMed/NCBI
|
|
18
|
Zhao A, Blackburn C, Chin J and Srinivasan
M: Soluble toll like receptor 2 (TLR-2) is increased in saliva of
children with dental caries. BMC Oral Health.
14(108)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Keller JF, Carrouel F, Colomb E, Durand
SH, Baudouin C, Msika P, Bleicher F, Vincent C, Staquet MJ and
Farges JC: Toll-like receptor 2 activation by lipoteichoic acid
induces differential production of pro-inflammatory cytokines in
human odontoblasts, dental pulp fibroblasts and immature dendritic
cells. Immunobiology. 215:53–59. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Al-Ghurabi BH: The role of soluble TLR-2
in the immunopathogenesis of Gingivitis. Int Med J. 28:37–39.
2021.
|
|
21
|
Seyedmajidi M, Khodadadi E, Maliji G,
Zaghian M and Bijani A: Neutrophil count and level of
interleukin-1β and interleukin-8 in the saliva of three to five
year olds with and without dental caries. J Dent (Tehran).
12:662–668. 2015.PubMed/NCBI
|
|
22
|
Leme LAFP, Rizzardi KF, Santos IB and
Parisotto TM: Exploring the relationship between salivary levels of
TNF-α, Lactobacillus acidophilus, Lactobacillus gasseri, obesity,
and caries in early childhood. Pathogens. 11(579)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ali Al-Dahhan HA, Al-Kishwan SS, Dakheel
AH and Dakheel BH: Assessment of some Pro-inflammatory cytokines in
saliva of dental caries. Indian J Forensic Med Toxicol.
15:3035–3040. 2021.
|
|
24
|
Nazemisalman B, Jafari F, Esmaelzadeh A,
Faghihzadeh S, Vahabi S and Moslemi H: Salivary tumor necrosis
factor-alpha and dental caries in children and adolescents. Iranian
J Pediatrics. 29(e80899)2019.
|
|
25
|
Ribeiro CCC, Pachêco CdJB, Costa EL,
Ladeira LLC, Costa JF, da Silva RA and Carmo CDS: Proinflammatory
cytokines in early childhood caries: Salivary analysis in the
Mother/children pair. Cytokine. 107:113–117. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Akcalı A, Çeneli SK, Meriç P, Nalbantsoy
A, Ozçaka Ö and Buduneli N: Altered levels of inhibitory cytokines
in patients with thalassemia major and gingival inflammation.
Brazilian Dental Sci. 22:349–357. 2019.
|
|
27
|
Ali AJ, Al-Juboori JNA and Al-Nimer M:
Concomitant using of topical carrageenan-kappa and oral vitamin D
against 7, 12-dimethylbenz[a]anthracene induced-oral cancer in
rats: A synergism or an antagonism effects. Brazilian Dental Sci.
23(7)2020.
|
|
28
|
Hirsch V, Wolgin M, Mitronin AV and
Kielbassa AM: Inflammatory cytokines in normal and irreversibly
inflamed pulps: A systematic review. Arch Oral Biol. 82:38–46.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kurtiş B, Tüter G, Serdar M, Akdemir P,
Uygur C, Firatli E and Bal B: Gingival crevicular fluid levels of
monocyte chemoattractant protein-1 and tumor necrosis factor-alpha
in patients with chronic and aggressive periodontitis. J
Periodontol. 76:1849–1855. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lo Giudice R, Militi A, Nicita F, Bruno G,
Tamà C, Lo Giudice F, Puleio F, Calapai F and Mannucci C:
Correlation between oral hygiene and il-6 in children. Dent J
(Basel). 8(91)2020.PubMed/NCBI View Article : Google Scholar
|