Introduction
Almost all cases of cervical carcinoma originate
from the ectocervical or endocervical mucosa in the transformation
zone, the area of the cervix between the old and new squamocolumnar
junction (1). In 2020, the number
of new cases of cervical cancer reached 604,127, with related
deaths ranking ninth among all types of cancer worldwide (341,831
individuals) (2). New cases of
cervical cancer in Indonesia alone reached 36,633, with the third
highest number of deaths from total cancer in Indonesia (21,003
individuals) in the same year (2).
Chemotherapy has been applied for follow-up therapy following
surgery since the 20th century (2); however, the high mortality rate still
indicates that existing chemotherapeutic drugs are not optimal.
This description provides the basis for the importance of the
development of novel cancer drugs, particularly for cervical
cancer.
Candidate targets for the development of new drugs
can be determined through the hallmarks (main markers) of cancer,
one of which is the avoidance of apoptosis (3). Apoptosis is programmed cell death
that aims to maintain homeostasis. The process of apoptosis in
cancer cells is inhibited, and the homeostasis of the body becomes
chaotic (4). The loss of control
over apoptosis causes cancer cells to survive for longer time
periods and allows for the accumulation of various mutations that
occur, increasing the invasiveness of cancer cells during tumor
development, stimulating angiogenesis, deregulating cell
proliferation and interfering with the differentiation process
(5). One of the proteins that
regulate the apoptotic mechanism of the intrinsic pathway is the
B-cell lymphoma-2 (BCL-2) family, which includes pro-apoptotic
effector proteins, pro-apoptotic BH3-only and anti-apoptotic
BCL-2(4). This finding supports
the notion that these two molecules have the potential to become
targets for the development of novel cancer drugs.
Streptomyces sennicomposti (S.
sennicomposti) GMY01 is an actinomycetes bacteria obtained from
Krakal Beach, Gunungkidul, Yogyakarta, Indonesia (6). The results of genome mining have
revealed that there are functional genes from S.
sennicomposti GMY01 that encode mannotriose (7). The presence of the mannotriose
extract of GMY01 cell biomass was previously confirmed by liquid
chromatography tandem mass spectrometry, and optimization of the
isolation and purification method of mannotriose from S.
sennicomposti GMY01 was successfully carried out, although it
has not been 100% purified (7).
The methanolic extract containing mannotriose has exhibited
moderate anticancer activity against HeLa cervical carcinoma cells
(IC50, 27.31 µg/ml) and HTB-179 lung carcinoma cells
(IC50=33.75 µg/ml) and exerts minimal toxic effects on
mammalian normal cells (Vero cells) with an IC50 of
823.3 µg/ml (7). The results of
that study indicate the potential of mannotriose to be developed as
a novel cancer drug that induces apoptosis.
Mannotriose is an oligosaccharide that is derived
from raw materials that are easily obtained from various plants;
therefore, its development as a novel cancer drug targeting
anti-apoptotic proteins, one of which is BCL-2, has promising
developmental potential. In silico methods are computational
approaches that examine the molecular structure and quantitative
structure-activity relationship; therefore, such studies can
promote drug discovery and development and reduce preclinical
research (8). The in silico
docking analysis carried out in the present study was performed to
examine the binding of mannotriose molecules from S.
sennicomposti GMY01 to BCL-2 and MCL-1, in order to determine
their potential to induce apoptosis through the inhibition of these
two anti-apoptotic proteins. It was hypothesized that mannotriose
of S. sennicomposti GMY01 has the potential to be developed
as a novel cancer drug. The present study also performed an in
vitro assay to examine the induction of apoptosis by the
methanol extract (ME) and n-hexane-free methanol fraction
(HMF) using HeLa cells. The present study aimed to examine the
potential of mannotriose from S. sennicomposti GMY01 to
induce the apoptosis of cervical cancer cells by inhibiting BCL-2
and MCL-1.
Materials and methods
Reagents
The isolate of S. sennicomposti GMY01 was
obtained from a previous study and deposited in the Indonesian
culture collection (WDCM 769), National Research and Innovation
Agency, Indonesia, encoded as InaCC A147(7). The HeLa (ATCC CRM-CCLL-2) and C2C12
(ATCC CRL-1772) cell lines were obtained from the Cell Culture
Laboratory of the Pharmacology Department, Faculty of Medicine,
Public Health and Nursing, Gadjah Mada University, Yogyakarta,
Indonesia. HeLa cells were grown in Roswell Park Memorial Institute
(RPMI) 1640 medium (Gibco, Thermo Fisher Scientific, Inc.); hence,
the C2C12 cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) (Gibco, Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific,
Inc.).
Fermentation of S. sennicomposti
GMY01
The production and extraction of metabolites from
the cell biomass of S. sennicomposti GMY01 were carried out
using an optimized method described in a previous study (9). The bacterial isolates were
re-cultured in ISP-2 (Difco, BD Biosciences) solid media and
incubated at 30˚C for 7 days or until there were numerous spores.
The cultures were then transferred to TSB (Difco, BD Biosciences)
medium to produce starter cultures. The GMY01 culture in ISP-2 was
then sampled with a transfer loop, placed in 100 ml of starch
nitrate buffer (SNB; modified medium), and incubated in a rotary
shaker incubator at 225 rpm for the following 3 days. The SNB
medium contained 0.5 g NaCl, 1 g KNO3, 0.5 g
K2HPO4, 0.5 g MgSO4, 0.5 g
7H2O, 0.01 g FeSO4 7H2O, and 20 g
soluble starch in 1,000 ml distilled water. All chemical reagents
were obtained from Merck KGaA. Subsequently, 5% (v/v) starter
culture was inoculated into nine 250 ml Erlenmeyer flasks
(Pyrex®, Merck KGaA) containing 100 ml SNB medium,
followed by incubation on a rotary shaker incubator at 225 rpm for
11 days at 30˚C.
Extraction and fractionation
The fermentation culture was then transferred into
50 ml conical tubes (BD, Corning Life Sciences) and centrifuged at
1,000 x g, at 4˚C for 10 min. The cell biomass was separated from
the supernatant following centrifugation by removing the
supernatant. The cell biomass was macerated using 300 ml absolute
methanol for 30 min, with slow stirring, at room temperature.
Following completion, it was filtered using Whatman paper (Merck
KGaA) and the methanolic extract was collected. The ME was
concentrated using a vacuum rotary evaporator. The HMF was prepared
by liquid-liquid extraction at a 1:1 ratio of
methanol:n-hexane as the solvent. The fraction dissolved in
n-hexane was then removed, leaving the remaining fraction in
methanol. Furthermore, the ME and HMF were evaporated using a
rotary evaporator, as previously described (7). The HMF was then prepared at up to
100,000 ppm of the stock solution in the same manner as the ME.
Cytotoxicity assay using HeLa and
C2C12 cells
A stock solution of 100,000 ppm was prepared for
in vitro testing by dissolving 100 mg of the extract in DMSO
to a volume of 1 ml. The two extract stock solutions were then
diluted into seven series of concentrations for MTT assay using
HeLa (3.13-200 µg/ml) and C2C12 cell lines (31.25-20,000 µg/ml).
The HeLa cell culture suspension (100 µl) at a density of
1x105 cells/ml and 100 µl of each extract at various
concentrations was added to a 96-well plate and incubated for 24 h
at 37˚C. The culture medium was then replaced with 100 µl fresh
complete medium (RPMI for HeLa cells and DMEM/FBS 10% for C2C12
cells) and 10 µl of MTT solution following the incubation period.
The plates were incubated for 4 h at 37˚C. A total of 100 liters of
10% SDS solution in 0.01 M HCl was added after incubation was
completed followed by a further incubation in room temperature for
24 h. The absorbance of each well was measured at a wavelength of
595 nm using a microplate reader (Bio-Rad Laboratories, Inc.). The
percentage of cell viability was calculated by comparing the
absorbance values of the wells treated with each extract with the
absorbance of the wells that were not treated (negative control).
The percentage of cell viability was used to calculate the
concentration of the extract that could inhibit 50% of cell growth
(inhibition concentration 50% or IC50). The selectivity
index (SI) was calculated from the ratio of IC50 on
normal to cancer cells (10).
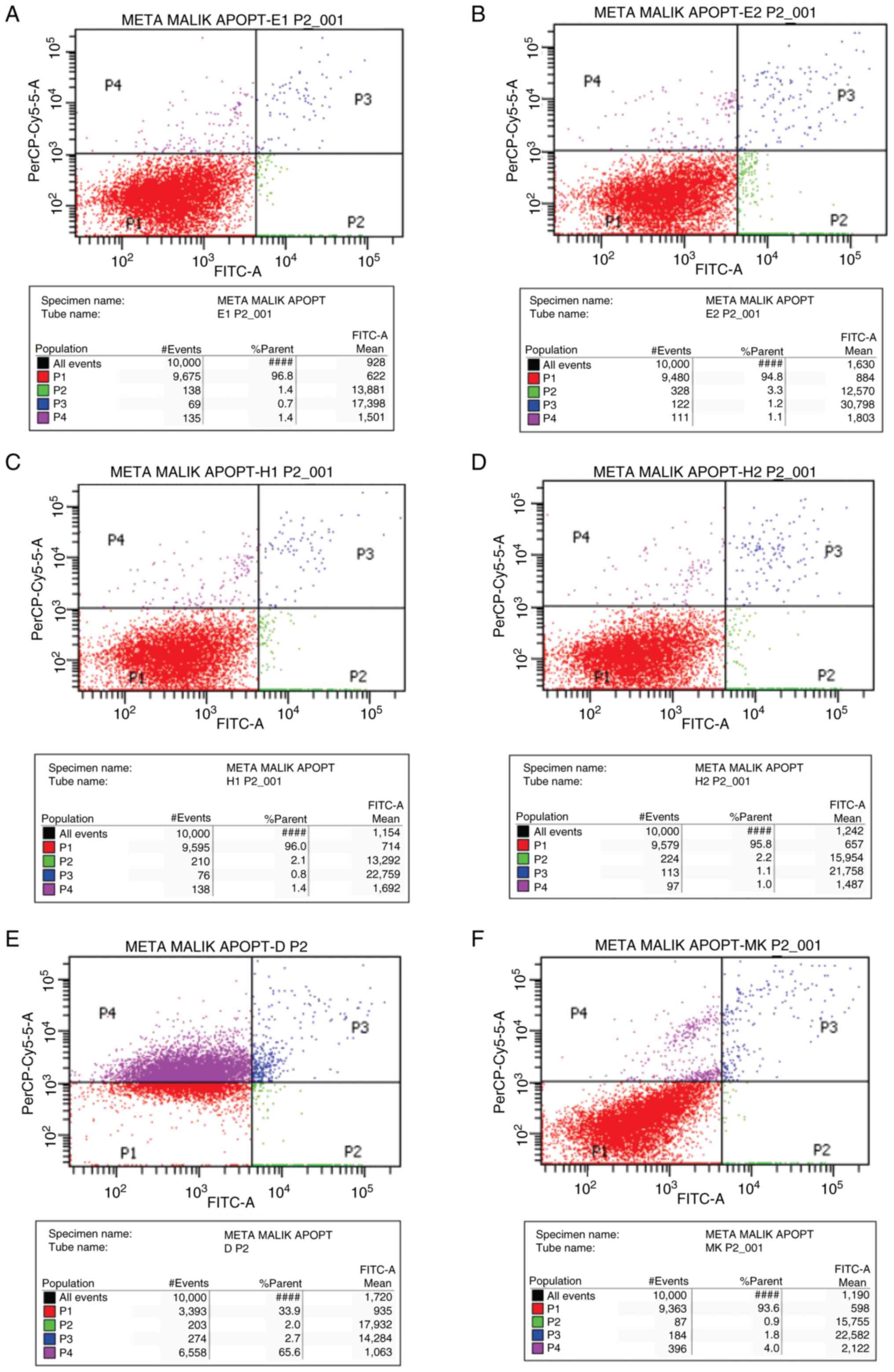
Apoptosis assay
The apoptotic activity of the extract and fraction
was assessed in vitro using flow cytometry with Annexin V-PI
staining. After each well containing cells was induced with both
types of extracts (or only culture media for the negative control
group, and doxorubicin was used as the positive control),
5x105 cells were collected by centrifugation at 1,000 x
g at 4˚C for 5 min. Cell resuspension was then carried out by the
addition of 500 µl Annexin V binding buffer solution, then 5 µl
Annexin V-FITC, and 5 µl PI, and finally incubated at room
temperature for 15 min (in a dark room). Annexin V-FITC binding was
then analyzed by flow cytometry (BD FACSCanto™ II, BD Biosciences)
(Ex: 488 nm; Em: 350 nm) with an FITC signal detector, while PI
staining analysis was carried out with a phycoerythrin emission
signal detector. The results of flow cytometry indicate the
distribution of cell counts in each of the four quadrants in the
Annexin V-FITC test results, which were then analyzed. Quadrant I
(P1) contained a collection of cells that were still alive,
quadrant II (P2) contained a collection of cells that underwent
early-phase apoptosis, quadrant III (P3) contained a cell that
underwent late-phase apoptosis, and quadrant IV (P4) contained a
collection of cells that underwent necrosis. The results in the
control and treatment groups (the given extract) were then compared
to determine the proportion of cells that were still alive,
underwent early-stage apoptosis, advanced-stage apoptosis, or
necrosis.
In silico molecular docking
analysis
In silico molecular docking was initiated by
determining the most optimal conformation of the protein-ligand
complex of PDB for the following steps. The native ligand was
removed to provide space (pocket/cavity) for the ligand-binding
area. The validation of the molecular docking method and the
determination of the grid box for docking were then carried out
before docking with the ligand of the compound to be tested, namely
mannotriose. Validation was performed by re-docking native ligands
on proteins whose native ligand were removed using the AutoDock
program. The Root Mean Square Deviation (RMSD) of the complex of
native ligand and analyzed protein was calculated for docking
validation. The protein can be used further for docking if the RMSD
value is <2.0 Amstrong (Å) in the re-docking process. In the
case that the RMSD value is >2.0 Å, the certain conformation
cannot be used for the docking; thus, it must be replaced with
another conformation with a different PDB ID. The grid box was
determined after obtaining BCL-2 and MCL-1 proteins, which were
used for subsequent docking. The grid is an area where the binding
of ligand and its protein/macromolecule supposed to be, which is
expressed in box coordinates in the AutoDockTools application.
Grids for the binding areas of BCL-2 and MCL-1 were obtained after
the re-docking of proteins associated with their respective native
ligands. Mannotriose 3D structure preparation was performed using
the online application MolView (https://molview.org/) based on the 2D structure. This
3D structure was then docked to BCL-2 and MCL-1 proteins, which
were separated from their native ligands. Furthermore, the docking
process was performed on BCL-2 and MCL-1 proteins using
AutoDockTools. A ligand-receptor complex with a binding energy
below 0 kcal/mol indicates that the ligand can bind to the
associated protein. Both 2D and 3D visualizations of amino acid
residues interacting with the ligand were processed using Discovery
Studio, as previously described (7,9).
Statistical analysis
Statistical analyses were performed using IBM SPSS
16.0 in Windows PC operating system (IBM Corp.). The mean
IC50 values of ME and HMF in both cell lines were
compared using the t-test. The mean comparison of AI for each group
was analyzed using one-way ANOVA with Tukey's post hoc test
(GraphPad Prism 10.0.2, Dotmatics). The IC50 values are
displayed as averages, whereas SI and AI are presented as ratios.
The comparison of the ratio of early and late apoptosis was
performed using the t-test, apart from the HMF 0.5xIC50
group, which was analyzed using the Mann-Whitney U test. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cytotoxicity assay
The results of MTT assay revealed that the HMF had a
lower IC50 value than the ME, which was 93.75 compared
to 78.81 µg/ml; however, the difference was not statistically
significant (P=0.843). In addition, the SI of HMF in the HeLa cells
was significantly higher (117.04) than in C2C12 cells (6.23). The
IC50 values of ME and HMF in HeLa cells were still lower
than those of the positive control, doxorubicin (2.88 µg/ml),
although doxorubicin had a very low SI value of 0.135. The results
of MTT assay are presented in Table
I.
 | Table IIC50 and SI of
Streptomyces sennicomposti GMY01 extract in HeLa and C2C12
cell lines. |
Table I
IC50 and SI of
Streptomyces sennicomposti GMY01 extract in HeLa and C2C12
cell lines.
| | IC50 | |
|---|
| No. | Cell line | Treatment | Estimated | Lower | Upper | SI | P-value |
|---|
| 1. | HeLa | Methanol extract | 93.746 | 50.850 | 281.292 | 6.232 | 0.843 |
| | | n-hexane free
fraction | 78.813 | 43.113 | 218.592 | 117.036 | |
| | | Doxorubicin | 2.876 | 1.007 | 5.502 | 0.135 | - |
| 2. | C2C12 | Methanol
extract | 584.314 | 411.826 | 915.158 | - | 0.001a |
| | | n-hexane free
fraction | 9223.977 | 4100.032 | 55213.755 | | |
| | | Doxorubicin | .388 | .043 | 1.028 | | - |
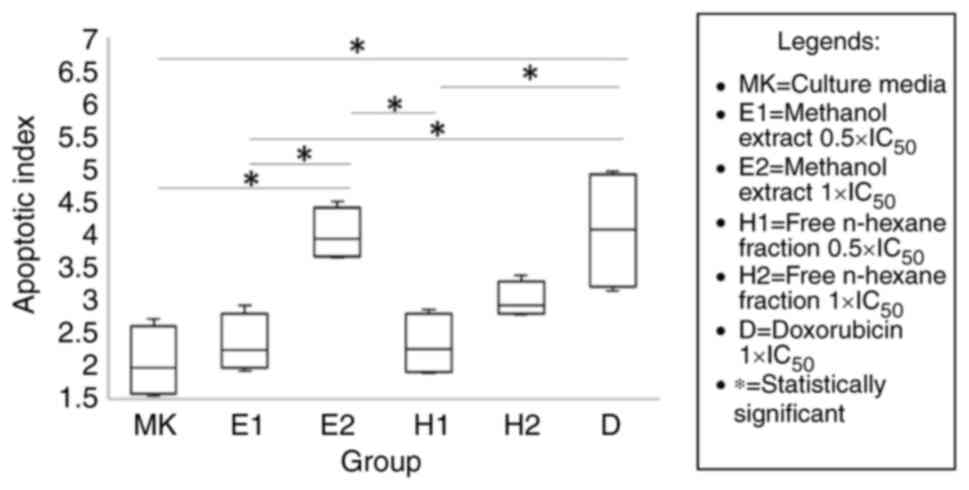
The results of flow cytometry analysis of Annexin V
PI revealed that the mean apoptotic index (AI) differed
significantly between each test group, with ME 1xIC50
having the highest value at 4.01±0.39. Analysis with the post hoc
test revealed that the AI in the ME 1xIC50 and positive
control (doxorubicin) was significantly higher than that in the
negative control (culture medium; both P=0.001). The positive
control also had a significantly higher AI than the two extracts at
a concentration of 0.5xIC50 (P=0.004 and 0.003,
respectively). Higher AI values at 1xIC50 compared to
0.5xIC50 were found with the ME and HMF, although a
significant difference was only found with ME (P=0.005). The HMF
had a lower AI than the ME, but both differences were not
statistically significant (2.32±0.48 compared to 2.33±0.44 at
0.5xIC50 levels with P=1.000, and 2.99±0.27 compared to
4.03±0.39 at 1xIC50 levels with P=0.151). The results of
flow cytometry are presented in Figs.
1 and 2.
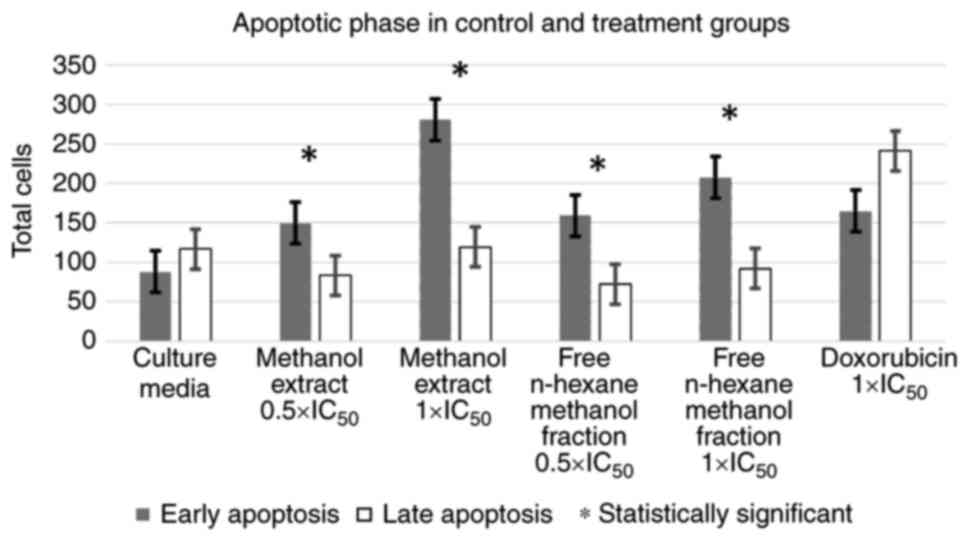
Additional analyses were then performed to compare
the means using a t-test (or Mann-Whitney test for non-parametric
data) between the proportions of apoptotic phases (early vs. late
phase) in each test group. ME and HMF, at concentrations of
0.5xIC50 and 1xIC50, exhibited the same
tendency in the apoptotic phase, which was significantly dominated
by the early phase of apoptosis. Moreover, the positive and
negative controls exhibited the opposite tendency, where late-phase
apoptosis dominated, although the difference was not statistically
significant. The results of this analysis are illustrated in
Figs. 1 and 3.
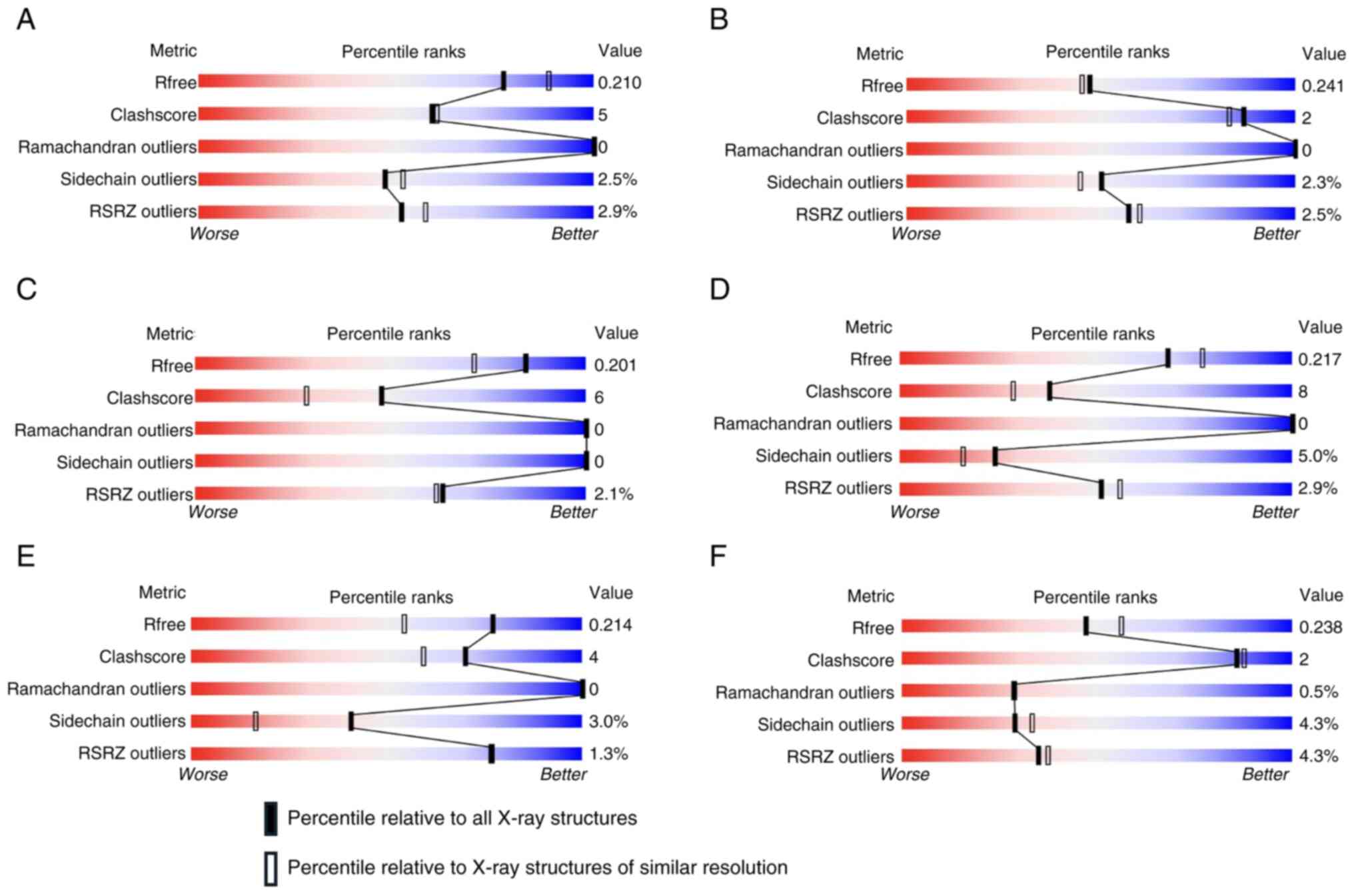
In silico molecular docking
In the present study, the in silico analysis
used several protein conformations of BCL-2 (PDB-ID: 4IEH, 7LHB,
6O0K and 4LVT) and MCL-1 (PDB-ID: 6OQB and 6FS0). These
conformations were selected as all proteins were derived from
Homo sapiens, had a good wwPDB validation value, and bound
to native ligands in the form of inhibitor compounds, as shown in
Fig. 4 and Table II. The docking results of
mannotriose from S. sennicomposti GMY01 for each protein in
all these conformations are presented in Table III.
 | Table IIProtein codes used in docking of
cancer proteins. |
Table II
Protein codes used in docking of
cancer proteins.
| Protein | PDB-ID | Native ligand | Original
species | Mutation |
|---|
| BCL-2 | 4IEH | IE9 | Homo
sapiens | No |
| BCL-2 | 7LHB | ABBV-167 | Homo
sapiens | Yes |
| BCL-2 | 6O0K | Veneto-clax | Homo
sapiens | No |
| BCL-2 | 4LVT | Navito-clax | Homo
sapiens | Yes |
| MCL-1 | 6OQB | N0J | Homo
sapiens | No |
| MCL-1 | 6FS0 | E4W | Homo
sapiens | No |
 | Table IIILigand-protein docking results of
cancer-related proteins. |
Table III
Ligand-protein docking results of
cancer-related proteins.
| | RMSD |
|---|
| Protein
(PDB-ID) | Ligand | Molecular
formula | Molecular weight
(kDa) | Binding energy | Upper | Lower |
|---|
| BCL-2 (4IEH) | IE9 |
C42H44Cl
N7O6S3 | 874.49 | -6.95 | 0 | 1.64 |
| | Mannotriose |
C18H30O16 | 502.42 | -1.10 | 0 | 1.96 |
| BCL-2 (7LHB) | ABBV-167 |
C46H53ClN7O11PS | 978.45 | -10.29 | 0 | 1.97 |
| | Mannotriose |
C18H30O16 | 502.42 | -0.54 | 0 | 1.97 |
| BCL-2 (6O0K) | Venetoclax |
C45H50ClN7O7S | 868.44 | -6.30 | 0 | 1.71 |
| | Mannotriose |
C18H30O16 | 502.42 | -0.42 | 0 | 2 |
| BCL-2 (4LVT) | Navitoclax |
C47H55ClF3N5O6S3 | 974.62 | -11.08 | 0 | 1.91 |
| | Mannotriose |
C18H30O16 | 502.42 | -0.27 | 0 | 1.95 |
| MCL-1 (6OQB) | N0J |
C32H39ClN2O5S | 599.18 | -15.03 | 0 | 0.23 |
| | Mannotriose |
C18H30O16 | 502.42 | -0.75 | 0 | 1.99 |
| MCL-1 (6FS0)s | E4W |
C35H34ClN5O3S2 | 672.26 | -16.21 | 0 | 0.07 |
| | Mannotriose |
C18H30O16 | 502.42 | -1.33 | 0 | 1.94 |
The value of binding energy <0 in the complex
between mannotriose and all proteins in each conformation (the
lowest binding energy values with BCL-2 and MCL-1 were -1.10 and
-1.33 kcal/mol, respectively), indicating that mannotriose could
bind to BCL-2 and MCL-1 in all the selected conformations. However,
these binding energy values were still higher than those of the
native ligand, indicating that the native ligand is better at
binding to the related protein. The complete docking results of
mannotriose on BCL-2 and MCL-1 are presented in Table III, while the visualization of
the most stable bond between mannotriose and BCL-2 and MCL-1 and
the redocking results with native ligands is presented in Figs. 5 and 6, respectively.
Discussion
In the present study, an in vitro MTT assay
was carried out to determine the viability, IC50, and SI
values of both the ME and HMF from the S. sennicomposti
GMY01 cell biomass. The results of the probit analysis of the
viability values obtained indicated that ME and HMF were inhibited
in HeLa cells, which means that they are in accordance with the
proposed hypothesis. The HMF in the present study was found to be
more potent than the ME, as indicated by its lower IC50
value, although the difference between the two extracts was not
statistically significant. These results were considered to be due
to the higher proportion of manotriose in the HMF compared to the
ME, as was the previous hypothesis, which suggested that manotriose
acts as an inducer of apoptosis in cancer cells. On the other hand,
the analysis of C2C12 cell viability revealed that HMF was
non-toxic, while ME was moderately toxic.
The IC50 values of cell lines extracted
from S. sennicomposti GMY01 in the present study were
compared with those of a previous study (7) as that study also carried out an in
vitro assay with ME and HMF from the same cell biomass. The
IC50 results for the HeLa cell line due to the
administration of ME in the present study were still higher than
those in HTB cells (33.56 µg/ml) (7); this suggests that the cytotoxic
activity of this extract was more potent in lung cancer cell line
HTB. Moreover, the IC50 value of HMF and ME in the
present study was lower than of the MCF-7 cells, which were 858 and
245 µg/ml (11), but also lower
than of HeLa cells in another study with an IC50 of
27.31 µg/ml (7). This was the
basis for the assumption that ME cytotoxicity was more potent in
HeLa cells than in MCF-7 cells, but less potent than HTB. The HMF
in the present study was more potent in terms of its cytotoxic
effect on HeLa cells than on MCF-7 in a previous study (11). The SI values of ME and HMF tested
were better than those of the positive control, namely doxorubicin,
where HMF had the highest SI of 117.036. This means that it takes
as much as 117,036 times its concentration in cancer cells, in this
case HeLa cells, to induce an inhibitory effect on normal cells,
such as C2C12 cells, as shown in the present study. This result is
in accordance with the hypothesis that ME had an SI of 6.232 times.
ME and HMF had a higher SI value than the positive control,
doxorubicin; thus, it could still be said that these two had a good
SI value.
Flow cytometry was carried out to determine whether
the cell death due to treatment belonged to the early or late phase
of apoptosis, or even necrosis. Annexin V is a protein complexed
with phosphatidylserine on the outer surface of cell membranes
undergoing apoptosis, even in the early phase of apoptosis.
Necrotizing cells, although also dead, did not react with Annexin V
as there was no phosphatidylserine on the outer surface of the
membrane in cells that did not undergo apoptosis. PI is a nuclear
dye. This molecule can bind when the cell is lysed as the molecule
is large and cannot penetrate the membrane. This underlies the fact
that PI can provide an overview of cells that have been lysed,
either due to late-phase apoptosis or necrosis. The results of the
present study indicate that apoptosis caused by the administration
of the extract and fraction was significantly dominated by the
early phase of apoptosis. Escalating the concentration to
1xIC50 increased the number of cells that underwent
apoptosis, although the dominance of the early phase was still
present. This is different from the culture medium (negative
control) and doxorubicin (positive control), where the apoptosis
that occurs in cells is dominated by the advanced phase, although
the difference was not statistically significant.
The results of the present study could not prove the
hypothesis as the results revealed that the ME had a higher AI than
HMF, both at 0.5xIC50 and 1xIC50 levels,
although the difference was not statistically significant. The
present study demonstrated that the administration of ME and HMF
had a significantly higher AI than the negative control, indicating
that both ME and HMF could induce apoptosis. The extract levels of
0.5xIC50 in both treatments and the HMF
1xIC50 did not exhibit a statistically significant
difference compared to the negative control, although both had
higher AI values. The ME at 1xIC50, as well as the
positive control were found to have a significantly higher AI than
the negative control, indicating that ME at these levels
significantly induced apoptosis. The AI value of the ME at
1xIC50 was not significantly different from that of the
doxorubicin group (positive control). This indicates that the
extract has the potential to induce apoptosis similar to drugs
already on the market, doxorubicin.
The results of the AI calculation also revealed that
1xIC50 ME was significantly higher than its
0.5xIC50 concentration, which indicated that there was
an increase in apoptotic induction activity with increasing extract
concentrations. The HMF at 1xIC50 also had a higher AI
than the same extract at a concentration of 0.5xIC50, in
line with the ME extract, although the difference was not
statistically significant. This is in accordance with the
concentration-effect theory, which states that the concentration of
a substance is directly related to its effect (12).
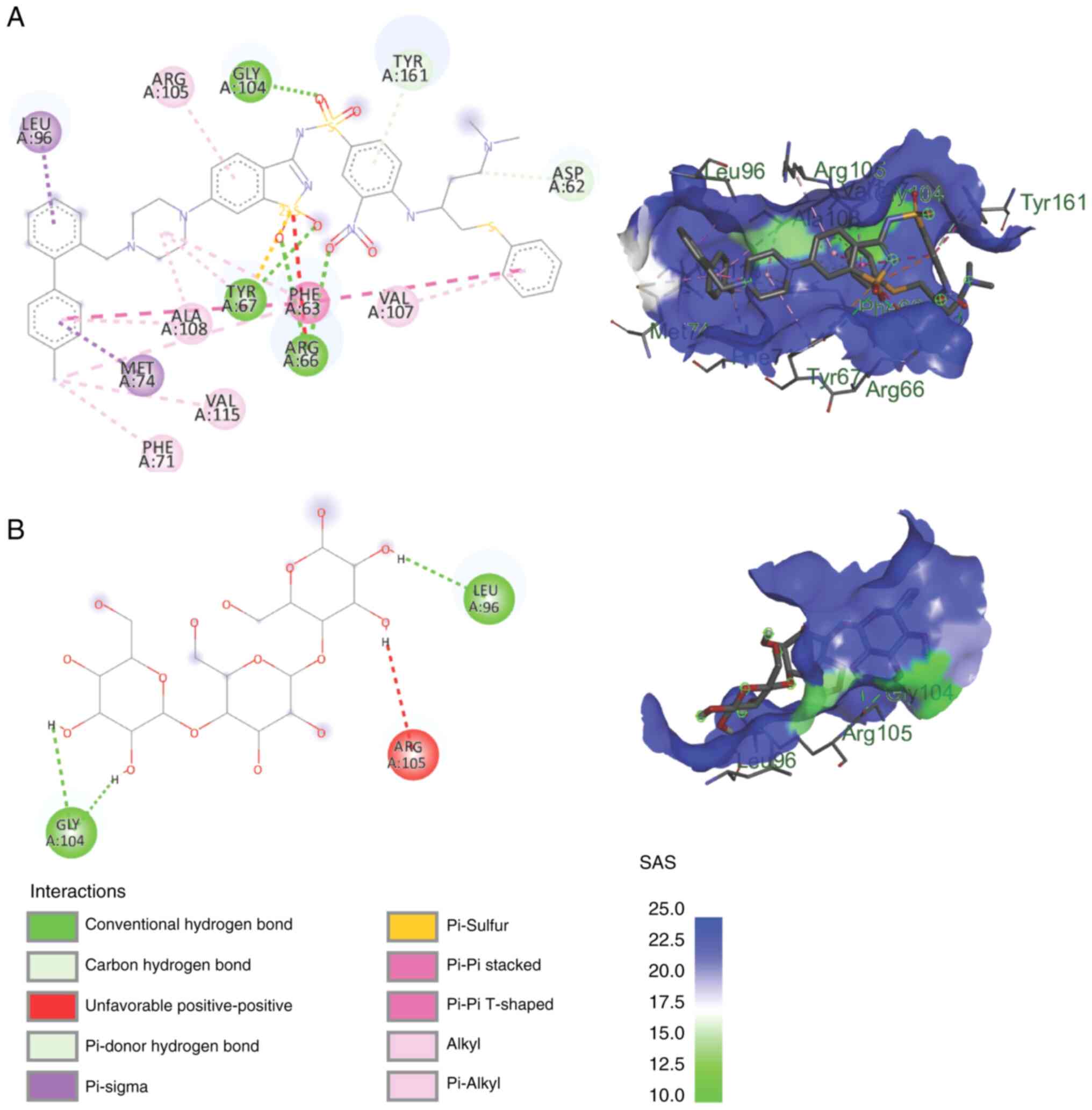
The mannotriose mechanism of action of BCL-2 and
MCL-1 was analyzed in silico by molecular docking. All the
docking results of mannotriose on BCL-2 and MCL-1 revealed a
negative binding energy, although the value was still higher than
that of the native ligand. The most stable conformation of the
mannotriose complex with BCL-2 was with the protein with the code
of 4IEH, with a binding energy of -1.10 kcal/mol (RMSD range,
0-1.96). Van der Waals bonds were found between mannotriose and
BCL-2 in the following six amino acids: GLU(54), PHE(57), THR(60),
PHE(79), VAL(82) and LEU(86). Conventional hydrogen bonds were
found in 3 amino acids: PHE(53), ARG(56) and SER(83). Both Van der
Waals and hydrogen are classified as weak intermolecular
interactions (12), while the
inhibitors that have been developed (native ligands) have multiple
covalent bonds with BCL-2, which are strong intermolecular bonds.
This resulted in, although still able to bind stably, a lower
affinity of the mannotriose complex with BCL-2 than that of the
existing inhibitors. Inhibitors with the code name IE9 have
covalent Pi-sigma, Pi-sulfur, Pi-Pi, alkyl, and Pi-alkyl bonds,
which are much stronger than van der Waals and hydrogen bonds.
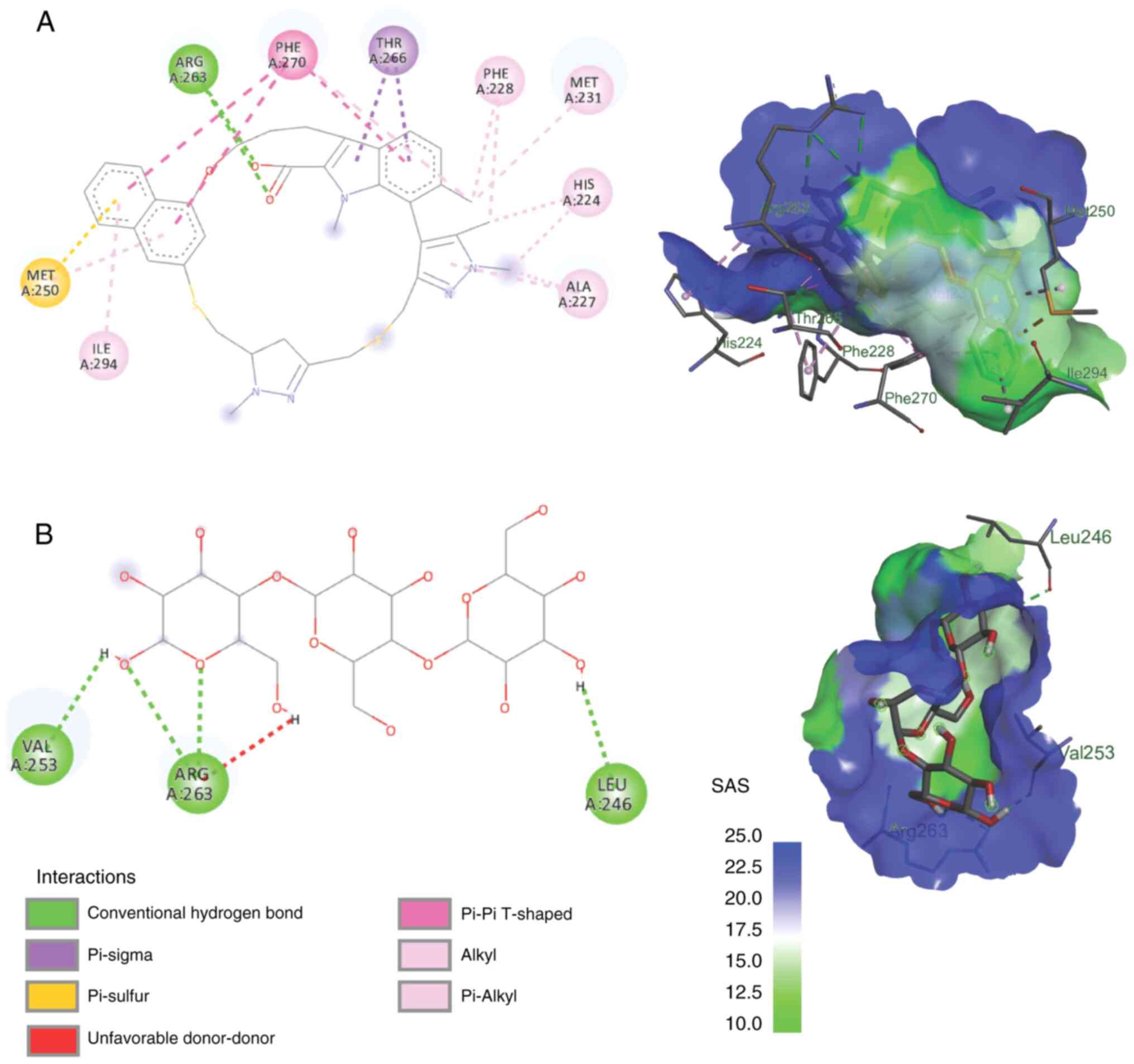
The most stable mannotriose complex with MCL-1 was
with protein corresponding to the 6FS0 code, with a binding energy
of -1.33 kcal/mol. Van der Waals bonds were found in the following
12 amino acids: PHE(228), MET(231), LEU(235), VAL(249), MET(250),
PHE(254), ASP(256), THR(266), LEU(267), PHE(270), LEU(290) and
ILE(294). In addition, there were conventional hydrogen bonds in
the 3 amino acids as follows: LEU(246), VAL(253) and ARG(263). The
arginine amino acid, ARG(263), in MCL-1 also binds to manotriose
via unfavorable donor-donor bonds. Van der Waals and hydrogen are
classified as weak intermolecular interactions (13), whereas inhibitors that have been
developed (native ligands) have multiple covalent bonds with MCL-1
which is a strong intermolecular bond. This explains why the
affinity of mannotriose for MCL-1 is lower than that of existing
inhibitors. The inhibitor with the code name AZD5991 has covalent
bonds of Pi-sigma, Pi-sulfur, Pi-Pi, alkyl and Pi-alkyl types,
which are much stronger than the van der Waals, hydrogen and ionic
bonds (Fig. 6).
Van der Waals bonds are intermolecular bonds formed
owing to the electrical interactions between very close molecules.
This bond is the weakest type of bond, with a bond strength of
0.4-4.0 kK/mol and an intermolecular distance of 0.3-0.5 nm. This
type of bond, although weak, can lead to very strong interactions
between two molecules if there are a large number of them. The most
stable complex conformation in this study had 7 van der Waals bonds
between manotriose and BCL-2 and 12 bonds with MCL-1, and the
number of bonds was sufficient to cause strong interactions between
manotriose and BCL-2 and MCL-1. On the other hand, two molecules
that are too close, on the other hand, will repel each other
strongly, repel each other, even though they have Van der Waals
bonds. This causes the bond to require a large amount of energy to
prevent the interaction surface between the two molecules from
sterically influencing each other (13). This energy requirement explains why
the binding energy of manotriose with BCL-2 and MCL-1 is less
negative than of the existing inhibitors because more energy is
needed to maintain the number of van der Waals bonds between the
ligand and the protein.
Hydrogen bonds is an attractive interaction
involving a hydrogen atom in a molecule with another atom that has
a high affinity for electrons (electronegativity). The difference
in electronegativity causes this bond to have a higher strength
than normal dipole-dipole interaction but less than covalent bonds,
with a bond strength of 10-30 kJ/mol (14). Hydrogen bonds had the second
highest number after van der Waals bonds between mannotriose and
BCL-2 and MCL-1 in the present study, each involving 3 amino acids.
This bond plays a role in stabilizing the complex formed, so that
the bond affinity is still negative, which means that manotriose
can bind to both proteins.
In conclusion, the results of the present in
vitro study demonstrated that the HMF mannotriose concentration
was higher than that in the ME and was more potent towards the HeLa
cancer cell line, with higher selectivity, as well relative to the
C2C12 cell line. However, it was found that the apoptotic indices
of HMF at both 0.5xIC50 and 1xIC50 were lower
than those of the corresponding ME group, although the differences
were not statistically significant. The results of in silico
analysis revealed that mannotriose from S. sennicomposti
GMY01 could bind to BCL-2 and MCL-1. Therefore, it can be concluded
that the ME and HMF from S. sennicomposti GMY01, which was
suspected to contain mannotriose, have the potential to induce
apoptosis in cancer cells, presumably through the mechanism of
inhibition of the anti-apoptotic proteins, BCL-2 and MCL-1.
However, the present study had certain limitations, particularly in
the testing of pure mannotriose compounds isolated from S.
sennicompostii GMY01. The isolation process for these compounds
is protracted and requires a substantial quantity of samples.
Future studies are thus required to focus on optimizing the
production of mannotriose from S. sennicomposti GMY01 and
evaluating its potential as an anticancer agent.
Acknowledgements
The authors would like to thank Miss Mosa Rini Nurul
Hidayati at the Cell Culture Laboratory and Farid Abdullah at the
Clinical Pathology Laboratory, Faculty of Medicine, Public Health,
and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia for
technical assistance with the anticancer assay.
Funding
Funding: The present study was funded by the Indonesian Ministry
of Research, Technology and Higher Education, Basic Research
Funding Scheme (grant no. 2128/UN.1.P.III/DIT-LIT/PT/2021).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MP, ED, WRP and M conceived and designed the study.
MP, ED, JW and M were involved in data acquisition. MP and ED were
involved in data analysis and interpretation. MP, ED and M were
involved in the drafting of the manuscript. MP, ED, WRP and M
critically revised the manuscript. MP performed the statistical
analysis. M was involved in the provision of funding. ED, JW and M
provided administrative, technical and material support. ED, WRP
and M supervised the study. All authors confirm the authenticity of
all the raw data All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri: 2021 update. Int J
Gynecol Obstet. 155 (Suppl 1):S28–S44. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Plati J, Bucur O and Khosravi-Far R:
Apoptotic cell signaling in cancer progression and therapy. Integr
Biol (Camb). 3:279–296. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Widada J, Damayanti E, Herdini C,
Wijayanti N, Hosoyama A, Yamazoe A, Suzuki-Minakuchi C, Hariwiyanto
B, Mubarika S, Dinoto A, et al: Draft genome sequence of the
Marine-derived, anticancer Compound-producing bacterium
Streptomyces sp. Strain GMY01. Microbiol Resour Announc.
12(e0136620)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Widada J, Damayanti E, Mustofa M, Dinoto
A, Febriansah R and Hertiani T: Marine-derived Streptomyces
sennicomposti GMY01 with Anti-plasmodial and anticancer
activities: Genome analysis, in vitro bioassay, metabolite
profiling, and molecular docking. Microorganisms.
11(1930)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Al-Mohaya M, Mesut B, Kurt AS and Çelik
YS: In Silico Approaches which are used in pharmacy. J Appl Pharma
Sci. 14:225–239. 2024.
|
|
9
|
Damayanti E, Nisa K, Handayani S, Dewi RT,
Febriansah R, Mustofa , Dinoto A and Widada J: Cytotoxicity
and molecular mechanism of Marine-derived Streptomyces sp.
GMY01 on human lung cancer cell line A549. J Appl Pharm Sci.
11:46–55. 2020.
|
|
10
|
Valdés AF, Martínez JM, Lizama RS, Gaitén
YG, Rodríguez DA and Payrol JA: In vitro antimalarial activity and
cytotoxicity of some selected Cuban medicinal plants. Rev Inst Med
Trop Sao Paulo. 52:197–201. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Febriansyah R, Hertiani T, Widada J, Taher
M, Damayanti E and Mustofa M: Isolation of active compounds from
Streptomyces sennicomposti GMY01 and cytotoxic activity on
breast cancer cells line. Heliyon. 10(e24195)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Aronson JK: Concentration-effect and
dose-response relations in clinical pharmacology. Br J Clin
Pharmacol. 63:255–257. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baburao G and Ragupathy G: Weak molecular
interactions of 1:1 Nitrile-Lewis base complexes: A theoretical
perspective on nature of chemical bonding. Computational Theoret
Chem. 1244(115072)2025.
|
|
14
|
Chimarro-Contreras A, Lopez-Revelo Y,
Cardenas-Gamboa J and Terencio T: Insights into the effect of
charges on hydrogen bonds. Int J Mol Sci. 25(1613)2004.PubMed/NCBI View Article : Google Scholar
|