Introduction
Bladder cancer is a common malignancy that ranks as
the 9th most frequently diagnosed malignancy worldwide. This type
of cancer is also ranked 13th in terms of mortality rates, with
developing countries having higher mortality rates than developed
countries (1). Non-muscle invasive
bladder cancer (NMIBC) is a subset of bladder cancer that comprises
tumors of stage Ta, T1 and carcinoma in situ (CIS). It is
estimated that NMIBC accounts for 70-75% of all diagnosed cases of
bladder cancer (2,3).
NMIBC is known to have a high recurrence and
progression rate that depends on the tumor risk profile with the
chance of recurrence at 1 year ranging between 15-61% and 31-78% at
5 years. Progression rates also vary significantly, with the 1-year
progression rate into muscle invasive metastatic bladder cancer
(MIBC) ranging from 0.2-17%, and increasing to 0.8-45% at 5 years
(2). Mortality rates associated
with NMIBC are relatively lower than those associated with MIBC,
and increases with higher-risk tumor features. In patients with
low-grade Ta tumors, the 15-year progression-free survival is 95%
with no cancer-specific mortality. This decreases to 61% in Ta
high-grade Ta tumors, with a disease-specific mortality of 26%.
Patients with T1 tumors have a progression-free survival rate of
44%, with a disease-specific mortality reaching 38% (4). When progression to MIBC occurs,
patients are expected to have a poor prognosis, with a 5-year
mortality rate of 50-70% even following radical cystectomy
(5).
Bladder cancer is among the types of cancer
affecting the elderly with the highest treatment costs, with an
estimated economic burden of approximately US $4 billion per year.
This high cost is attributed to the need for lifelong cystoscopic
surveillance and multiple treatment modalities (6). Transurethral resection of bladder
tumor (TURBT) is the initial treatment of choice, where the tumor
is resected using a resectoscope inserted via the urethra. In this
procedure, it is recommended that all visible tumors be resected
along with the underlying detrusor muscle (7). It is known that the risk of upstaging
NMIBC (≥T2) increases up to 49% in the case that the detrusor
muscle is not obtained during resection (8). TURBT is also associated with a high
recurrence rate, with a 5-year recurrence reaching 42% for T1
tumors (9). To mitigate this risk,
clinicians usually recommend a second TURBT and adjuvant therapy.
The instillation of intravesical chemotherapy is recommended by the
European Association of Urologist (EAU) to reduce the recurrence
rate (10). A previous
meta-analysis demonstrated that immediate postoperative
intravesical chemotherapy reduced recurrence to 37% compared to 48%
with TURBT alone (11). Several
agents, such as mitomycin C, epirubicin, doxorubicin and
gemcitabine are used for this purpose. Mitomycin C is the most
commonly studied drug; however, it is currently not available in
Indonesia (12).
Immunotherapy using bacillus Calmette-Guérin (BCG)
is a widely-recognized first line agent for managing high-risk
NMIBC, according to the European Organization for Research and
Treatment of Cancer (EORTC) risk calculator (13,14).
Currently, The EAU Guidelines suggest that BCG following TURBT is
more effective in preventing recurrence than TURBT alone or TURBT
with intravesical chemotherapy (10). Despite its efficacy, BCG is
associated with a greater number of side-effects than chemotherapy
(15).
Given the high economic burden associated with
NMIBC, the careful and evidence-based selection of treatment
modalities is essential for optimizing outcomes and efficiency.
There is a need to compare intravesical chemotherapy agents other
than mitomycin C with first-line BCG immunotherapy in the
Indonesian setting. The present study aimed to investigate the
efficacy and safety of gemcitabine-based intravesical chemotherapy
vs. BCG immunotherapy in order to provide a clearer perspective on
which treatment provides a better prognostic value for patients
with NMIBC.
Data and methods
Eligibility criteria
The present systematic review was conducted
according to the Preferred Reporting Items for Systematic Review
and Meta-analysis (PRISMA) guidelines. The inclusion criteria were
randomized controlled trials (RCTs) and observational studies of
patients diagnosed with NMIBC comparing the efficacy of and safety
between gemcitabine and BCG immunotherapy following TURBT. Only
English-language articles with available full-texts were included.
No date restrictions were applied. Editorials, commentaries, case
reports and review articles were excluded. The selected studies
were then critically appraised for validity, importance and
applicability using the checklist from Oxford's Centre of Evidence
Based Medicine (CEBM) (https://www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools).
Search strategy
A database search was conducted using various
databases, including PubMed, Cochrane, ProQuest and EBSCOhost with
the search strategies detailed in Table S1. Citation searching was
performed in eligible studies and prior systematic reviews to
identify relevant literature not captured through the database
search. An author (FZN) screened both the records and full-text
articles.
Risk of bias assessment and data
extraction
The included studies were assessed using the
Cochrane risk-of-bias tool for randomized control studies (RoB 2.0)
(https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials)
for RCTs or Risk of Bias in the Non-randomized Studies of
Interventions (ROBINS-I) tool for observational studies (https://methods.cochrane.org/bias/risk-bias-non-randomized-studies-interventions).
The following data were extracted: The name of the first author and
year of publication, study design, patient risk group and
classification system, details of intervention and comparison
including dosage and BCG strain, and outcomes related to efficacy
and safety. Efficacy outcomes included, but not limited to.
recurrence, progression, mortality rates and recurrence-free
survival. Safety outcomes included any adverse events (AEs), severe
AEs (defined as grade ≥3 AEs resulting in treatment modification)
and the type of AE. Data extraction and risk of bias assessment
were performed by one of the authors (FZN), as previously described
(16).
Statistical analysis
The extracted data were tabulated and described
narratively. Where feasible (≥2 studies reporting the same
outcome), data were pooled using inverse-variance random-effects
meta-analysis with risk ratios (RR) as the effect measure.
Random-effects weighting was applied due to variations in patient
risk groups and classifications, intervention types and doses, and
follow-up durations. Given inherent differences in study design and
procedures, analyses and syntheses were prioritized for RCTs.
Heterogeneity was assessed using the Chi-squared test (with
P<0.10 indicating statistical heterogeneity) and the I²
statistic, categorized as low (0-25%), moderate (26-50%), high
(51-75%), or very high (>75%). Galbraith plots were produced to
identify outliers and visualize heterogeneity. For primary efficacy
(recurrence and progression) and safety outcomes (any and severe
AEs), analyses were further stratified by patient risk group.
However, stratification by risk group classification system, BCG
dose and strain, and follow-up duration was not possible due to
heterogeneous distributions and limited data. Subgroup analysis by
overall risk of bias was also not conducted, as all included
studies were judged to have moderate-to-high risk of bias. As
<10 articles were included, funnel plots were not generated.
Results
Characteristics of the included
studies
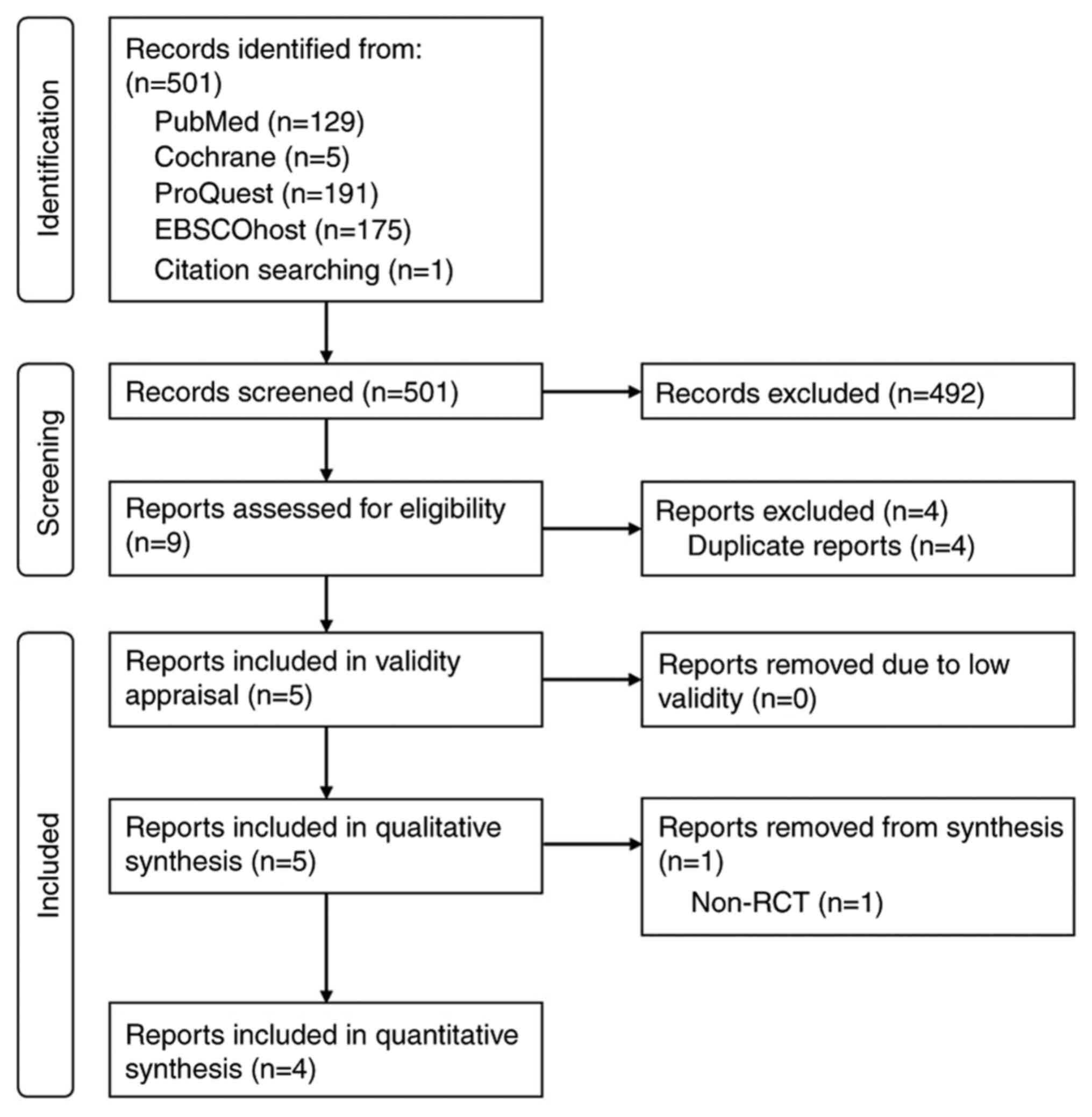
A total of 501 records were retrieved from the
search (Fig. 1). Following
screening, five studies were included: Four RCTs (16-19)
and one prospective cohort study (20). All studies were deemed valid
following critical appraisal using the Oxford's CEBM checklists
(Table SII). Across the five
studies, a total of 447 patients with NMIBC were included, with 224
(50.1%) patients receiving gemcitabine and 223 (49.9%) patients
receiving BCG. All patients in the gemcitabine group received
either a weekly or twice-weekly dose of 2,000 mg gemcitabine in 50
ml normal saline for 6 weeks (induction phase), followed by
maintenance for 12 months in two studies (16,18),
36 months in one study (17) and
an unspecified duration in two studies (19,20).
By contrast, BCG dosing and strain varied by study: Two studies
used the Connaught strain administered weekly for 6 weeks
(induction phase) with a 3-week maintenance schedule (16,18),
two studies used Tice strains administered weekly for 6 weeks
(17,20), and one study did not report a
strain or dosing interval (19).
Patient risk classification was high-risk in two studies (17,18),
low-to-intermediate risk in two studies (16,19),
and mixed in one study (20). The
EAU classification system was used in three studies (16,17,20),
EORTC in one study (18), and was
unspecified in one study (19).
The duration of follow-up ranged from 3 to 60 months (Table I).
 | Table IDetails of studies included in the
qualitative synthesis. |
Table I
Details of studies included in the
qualitative synthesis.
| First author, year
of publication | Study design | Chemotherapeutic
agent | Population
characteristics | Intervention | Outcomes | (Refs.) |
|---|
| Porena, 2009 | RCT | Gemcitabine and
BCG | • 64 High-risk
superficial bladder cancer patients based on EAU Guideline •
Patients randomized into two groups; Gemcitabine group (n=32) with
mean age 70.2±5.5 and BCG group (n=32) mean age, 68.7±10.2
years | • All patients
underwent first TURBT and bladder mapping to determine the presence
of CIS; 4 weeks later the second TURBT was conducted without prior
instillation of chemotherapeutic agents • Gemcitabine group: 14
days after the second TURBT, the patients received 6 weekly
installations of Gemcitabine with a dose of 2,000 mg diluted in 50
ml saline held in bladder for 2 h and received maintenance therapy
at 3,6, 12, 18, 24, 30 and 36 months after TURBT • BCG group: 14
days after the second TURBT, the patients received 6 weekly
installations of Tice-strain BCG with a dose of 5x108
CFU diluted in 50 ml saline held in the bladder for 2 h and
received maintenance therapy at 3, 6, 12, 18, 24, 30 and 36 months
after TURBT • Outcome measured consists of recurrence and
progression rates detected with cystoscopy and TURBT, tolerability
and safety | • Recurrence rate ◦
BCG group: 28,1% ◦ Gemcitabine group: 53,1% ◦ P=0.037 • Mean
recurrence-free survival time ◦ Gemcitabine group: 25.6 months ◦
BCG group: 39.4 months ◦ P=0.042 • Local toxicity (urinary tract
infection, cystitis, dysuria) ◦ BCG group: 12.5% ◦ Gemcitabine
group: 9.4% ◦ P>0.05 • Systemic toxicity (fever) ◦ BCG group:
6.25% ◦ Gemcitabine group: 12.5% ◦ P>0.05 • Rates of persistent
high-risk disease ◦ BCG group: 44.4% ◦ Gemcitabine group: 41.1% ◦
P>0.05 • Rates of regression ◦ BCG group: 55.5% ◦ Gemcitabine
group: 59.2% ◦ P>0.05 | (17) |
| Di Lorenzo,
2010 | RCT | Gemcitabine and
BCG | • 80 High-risk
NMIBC patients based on the EORTC scoring system Patients
randomized into two groups; gemcitabine group (n=40) with mean age
69.3±8.4 and BCG group (n=40) mean age, 71.4±7.9 years • Patients
had a history of treatment failure with BCG | • Gemcitabine
group: 4-6 weeks after the last TURBT 0performed after the failure
of the first treatment with BCG, the patients received twice weekly
intravesical gemcitabine with a dose of 2,000 mg/50 ml for 6 weeks
and maintenance at 2, 6 and 12 months. • BCG group: 4-6 weeks after
the last TURBT performed after the failure of the first treatment
with BCG, the patients received Connaught strain BCG (81 mg/50 ml)
over a 6-week induction course and the maintenance at 3, 6 and 12
months • Outcome measured consist of recurrence and progression
rates detected with cystoscopy and TURBT, and toxicity assessed
with toxicity criteria 3.0 | • Recurrence rate ◦
Gemcitabine group: 52.5%, ◦ BCG group: 87.5% ◦ P=0.002 • Time to
first recurrence ◦ Gemcitabine group: 3.9 months ◦ BCG group 3.1
months ◦ P=0.09 • 2-year recurrence-free survival ◦ Gemcitabine
group: 19% ◦ BCG group: 3% ◦ P<0.008 • Toxicity rate (dysuria,
hematuria, fever, dermatitis, nausea-vomiting) ◦ Gemcitabine group:
37.5% ◦ BCG group: 40% ◦ P>0,05 • Mortality incidence ◦
Gemcitabine group: none ◦ BCG group: 1 ◦ P=0.120 | (18) |
| Bendary, 2011 | RCT | Gemcitabine and
BCG | • 80 Patients
diagnosed with primary stage of Ta-T1 without CIS with mean age
56.2±11.18 years • All patients are randomized into two groups, 40
each, to receive either gemcitabine or BCG. Each group had a
subgroup of Ta and T1 tumors | • Gemcitabine group
received a 6 weekly intravesical instillation after 2 weeks from
resection with a dose of 2,000 mg/50 ml of saline. • BCG group
received a 6 weekly intravesical instillation after 2 weeks from
resection with a dose of 6x108 CU in 50 ml saline. •
Follow-up period range from 3-18 months (mean, 10.8±2.7 months) •
Outcome measured were recurrence rate and progression rate | • Recurrence rate
in Ta patient ◦ BCG group: 26.31% ◦ Gemcitabine group: 22.22% ◦
P=0.92 • Recurrence rate in T1 patient ◦ BCG group: 33.33% ◦
Gemcitabine group: 27.27% ◦ P=0.66 • Overall Recurrence rate ◦ BCG
group: 30% ◦ Gemcitabine group: 25% ◦ P=0.61 • Progression rate ◦
BCG group: 9.5% ◦ Gemcitabine group: 9.1% ◦ P=1.0 • Toxicity rate:
dysuria ◦ BCG group: 35% ◦ Gemcitabine group: 12.5% ◦ P<0.05 •
Toxicity rate: urinary frequency ◦ BCG group: 45% ◦ Gemcitabine
group: 10% ◦ P<0.01 | (19) |
| Gontero, 2013 | RCT | Gemcitabine and
BCG | • 120
Intermediate-risk NMIBC patients based on EAU risk stratification •
Patients are randomized into two groups, Gemcitabine (n=61) and BCG
(nS59) • All patients are BCG naïve and had no prior intravesical
chemotherapy in the last 3 months | • BCG group: 7-15
weeks after previous TURBT, patients received 6 weekly inductions
of Connaught strain BCG 1/3 dose (27 mg) in 50 ml saline and
maintenance of 3 weekly instillations at 3, 6 and 12 months •
Gemcitabine group: 7-15 weeks after previous TURBT, patients
received 6 weekly induction of 2000 mg/50 ml saline of gemcitabine
with maintenance of monthly instillations up to 1 year • Secondary
outcome are recurrence and progression at 1 year and assessment of
toxicity | • 1-year recurrence
rate BCG group: 23.7% ◦ Gemcitabine group: 26.2% ◦ P=0.83 •
Recurrence-free survival ◦ BCG group: 10.4 months ◦ Gemcitabine
group: 10.6 months ◦ P=0.66 • 1-year disease progression rate ◦ BCG
group: 11.6 months ◦ Gemcitabine group: 11.6 months ◦ P=0.5 • Both
local and systemic side effects are more common in BCG group
compared to Gemcitabine group after first induction (56.1% vs
35.7%; P=0.03), but not significant after second induction (40.4
vs. 34.1%; P=0.66) | (16) |
| Prasanna, 2017 | Retrospective
cohort | Gemcitabine and
BCG | • 103 Patients;
gemcitabine group (n=51) and BCG group (n=52) • Patients consisted
of all three different risk group with no significant different in
distribution in each group | • BCG treatment:
initial weekly instillation of Oncotice BCG with a dose of
5x108 CFU with 2 h of retention time for 6 weeks •
Gemcitabine: Weekly instillation of 2,000 mg Gemcitabine for 6
weeks Patients were administered maintenance treatment based on
their recurrence risk profile • Primary outcome is disease-free
survival (DFS) with recurrences confirmed by cystoscopic guided
biopsy and histology • Secondary outcomes include toxicity
examination | • Median follow-up,
15 months • Mean disease-free survival time ◦ BCG group: 19.6
months ◦ Gemcitabine group: Not reached, significantly longer ◦
Unadjusted HR of 0.47 (95% CI, 0.23-0.98, P=0.04) in favour of
gemcitabine • 2-year disease-free survival rate ◦ BCG group: 48.0%
◦ Gemcitabine group: 55.1% ◦ P 0.32 • Adverse event rate ◦ BCG
group: 44% ◦ Gemcitabine group: 7% ◦ P<0.05 | (20) |
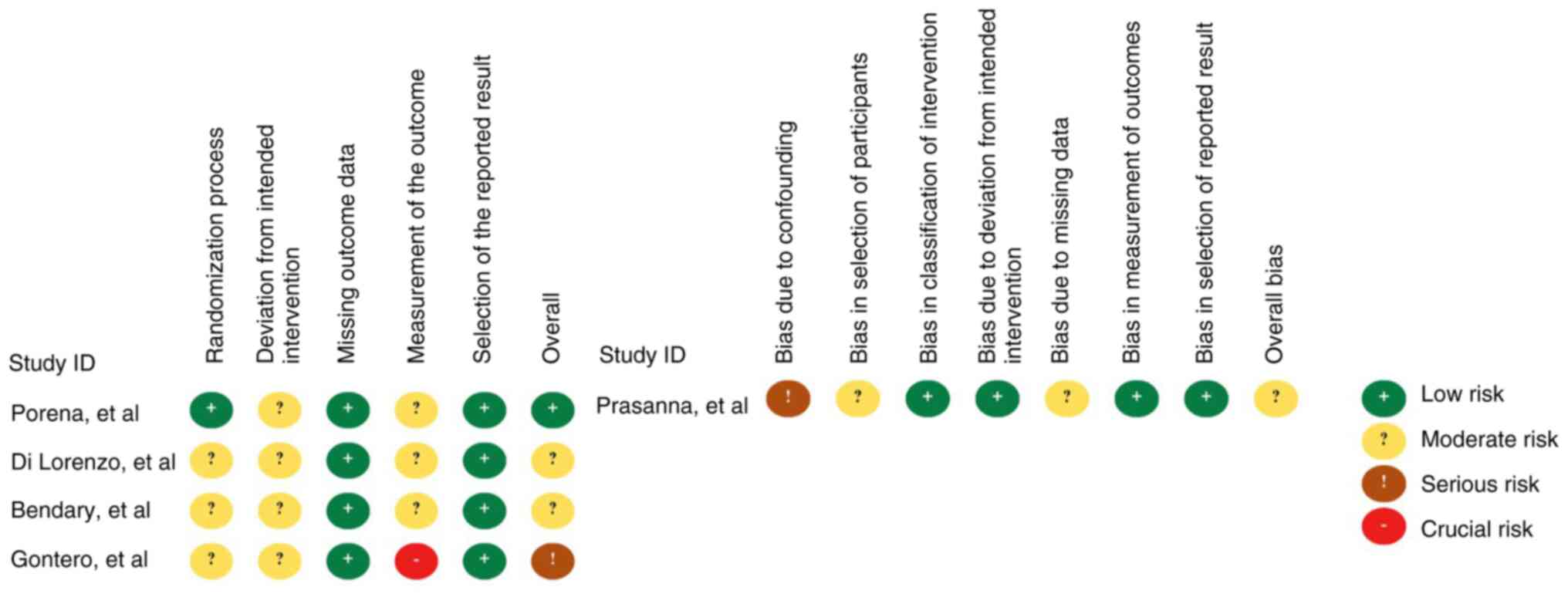
Risk of bias assessment
Risk of bias assessment revealed a moderate overall
risk in four studies (16,18-20)
and low risk in only one study (17). Specifically, all four RCTs had some
concerns regarding the risks of deviation from intended
intervention (16-19),
and three RCTs had some concerns from measurement of the outcome
(16,18,19).
Moreover, the study by Prasanna et al (20) had a moderate risk from selection
and attrition bias and a serious risk from confounding effects
(Fig. 2).
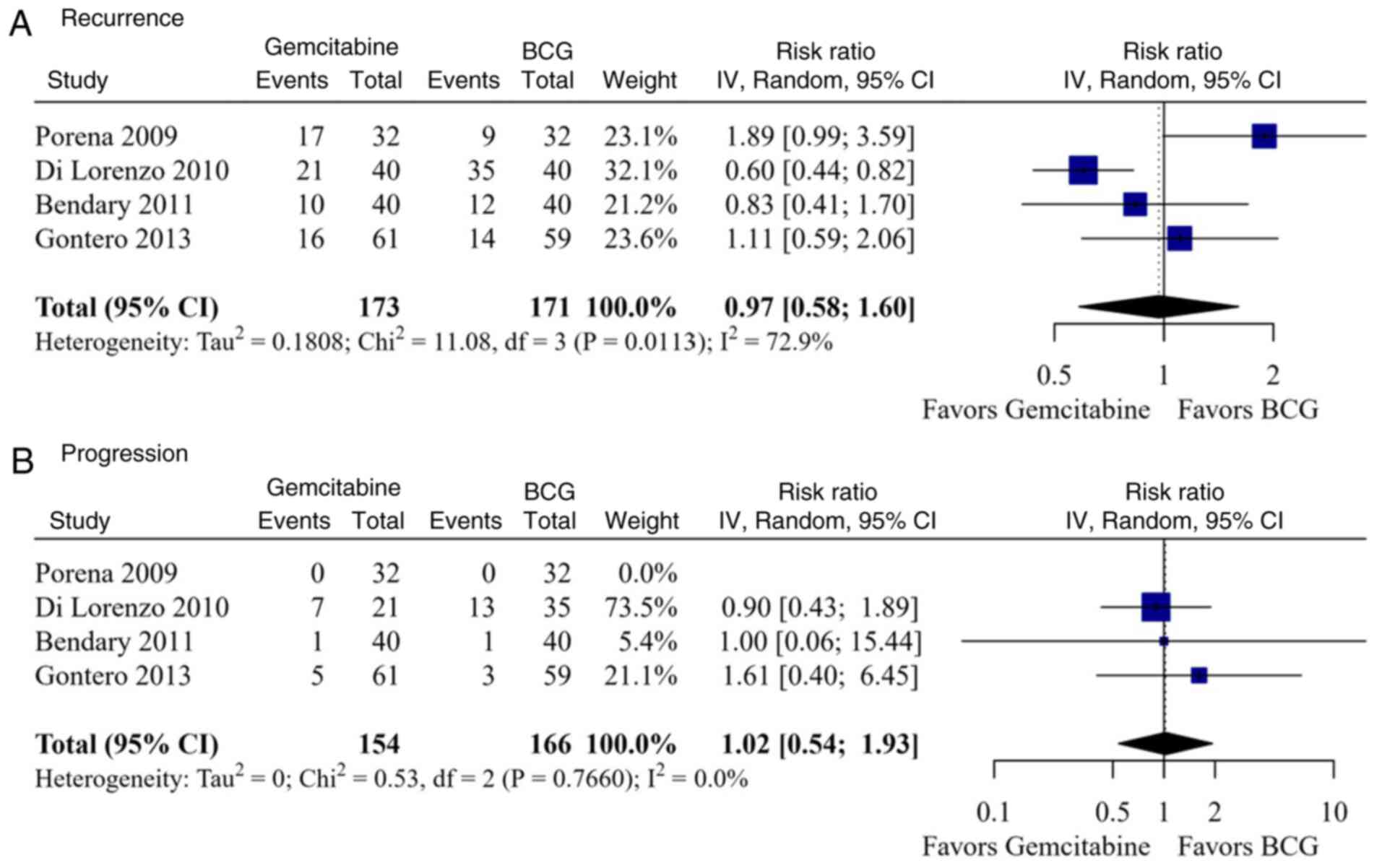
Efficacy outcomes
A total of four studies and 344 patients were
included in the meta-analysis of efficacy outcomes. Intravesical
gemcitabine was non-inferior to gemcitabine in terms of recurrence
[relative risk (RR), 0.97; 95% confidence interval (CI), 0.58-1.60;
high heterogeneity (I2=72.9%, P=0.011); and progression
(RR, 1.02; 95% CI, 0.54-1.93); low heterogeneity
(I2=0.0%, P=0.766; Fig.
3]. Recurrence and progression rates were similar between the
groups both in patients with high-risk NMIBC (RR, 1.03; 95% CI,
0.33-3.15; I2=89.8%, P=0.001; and RR, 0.90; 95% CI,
0.43-1.89) and in those with a low-to-intermediate risk (RR, 0.98;
95% CI, 0.61-1.57; I2=0.0%, P=0.559; and RR, 1.46; 95%
CI, 0.42-5.04; I2=0.0%, P=0.760) (Fig. S1). Galbraith plots did not
identify any outlier, although the wide spread of estimates
suggests apparent heterogeneity (Fig.
S1A and B).
Recurrence-free survival was shorter in the
gemcitabine group in one study (mean, 25.6 vs. 39.4 months,
P=0.042) (17), similar in another
(10.6 vs. 10.4 months, P=0.66) (16), and longer in one study (3.9; 95%
CI, 3.0-7.0 vs. 3.1 months 95% CI, 2.2-6.0; P=0.008) (18). Additionally, Di Lorenzo et
al (18) reported a
significantly higher 2-year recurrence-free survival in patients
with NMIBC receiving gemcitabine compared to those receiving BCG
[hazard ratio (HR), 0.15; 95% CI, 0.10-0.30; P<0.008].
Progression-free survival, as reported in the study by Gontero
et al (16), was similar
between gemcitabine and BCG (both mean 11.6 months, P=0.500).
Mortality was reported by only one study, with one death in the BCG
group and none in the gemcitabine group, although the difference
was not statistically significant (P=0.120) (18). Similarly, Porena et al
(17) reported that the rates of
persistent high-risk disease and regression were similar in both
groups (44.4 vs. 41.1% and 55.5 vs. 52.9%, respectively; both
P-values non-significant) (Table
I).
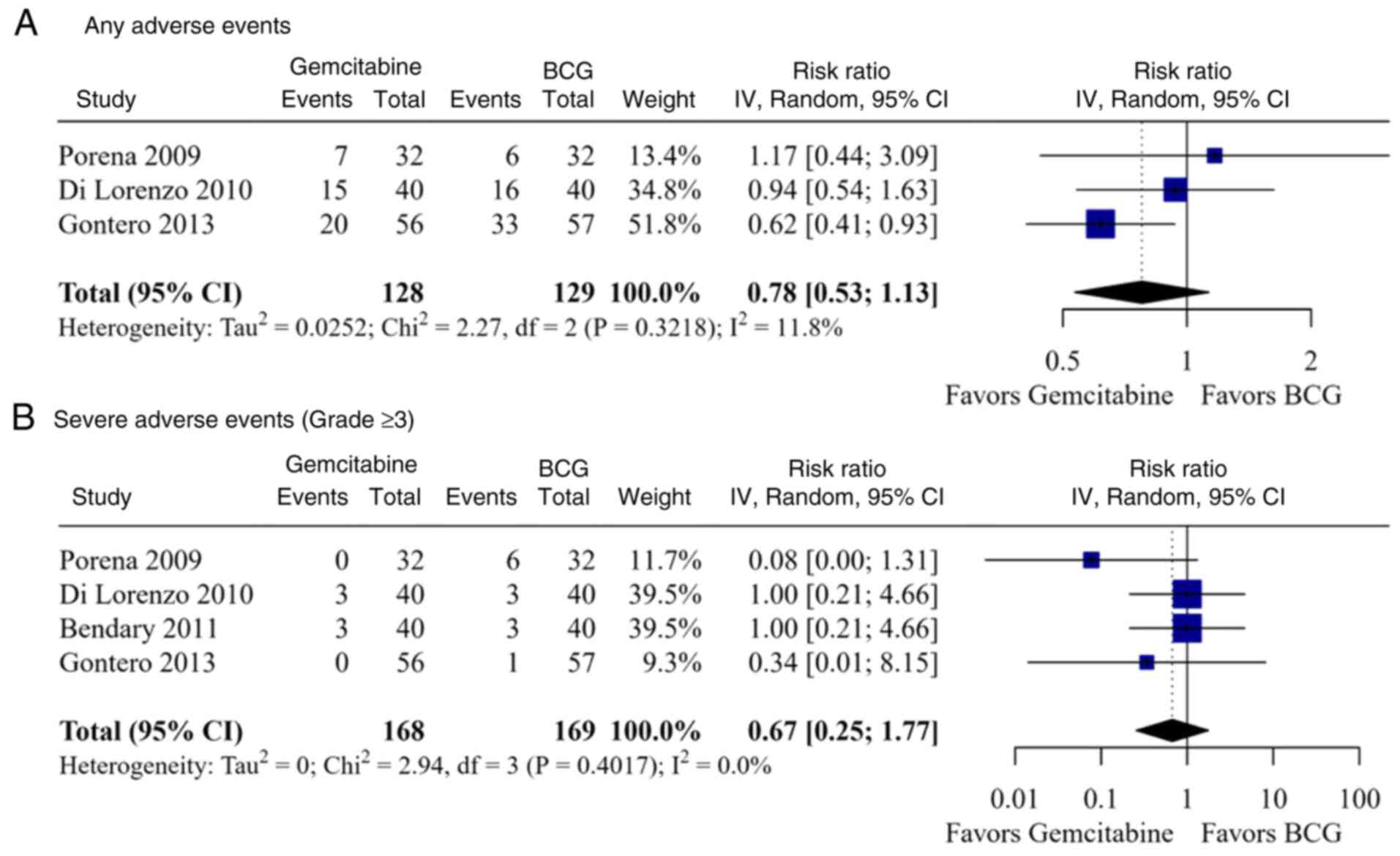
Safety outcomes
A total of four studies comprising a total of 337
patients were included in the meta-analysis of safety outcomes. The
overall risk of AEs was similar between the gemcitabine and BCG
groups (RR, 0.78; 95% CI, 0.53-1.13; I2=11.8%; P=0.322)
(Fig. 4A), as well as among
patients with high-risk disease (RR, 0.99; 95% CI, 0.61-1.60;
I2=0.0%; P=0.702) and low-to-intermediate risk disease
(RR, 0.62; 95% CI, 0.41-0.93) (Table
II). Similarly, the rate of severe AEs, defined as grade ≥3 AEs
requiring treatment modifications, did not differ significantly
between the groups overall (RR, 0.67; 95% CI, 0.25-1.77;
I2=0.0%; P=0.402) (Fig.
4B), or when stratified by patient risk classification
(high-risk: RR, 0.37; 95% CI, 0.03-4.28; I2=58.8%;
P=0.119; low-to-intermediate risk: RR, 0.81; 95% CI, 0.20-3.25;
I2=0.0%; P=0.549) (Table
II). Galbraith plots identified no outlier, although the
limited data may constrain further interpretation (Fig. S1C-H).
 | Table IISummary of meta-analysis results for
efficacy and safety outcomes from the included randomized
controlled trials |
Table II
Summary of meta-analysis results for
efficacy and safety outcomes from the included randomized
controlled trials
| A, Primary
outcomes |
|---|
| | No. of patients;
event/no. | | Heterogeneity |
|---|
| Outcome | No. of studies | Gemcitabine | BCG | RR (95% CI) | I2 | P-value |
|---|
| Efficacy | | | | | | |
| Recurrence | | | | | | |
|
Overall | 4 | 64/173 | 70/171 | 0.97
(0.58-1.60) | 72.9% | 0.011 |
|
High-risk | 2 | 38/72 | 44/72 | 1.03
(0.33-3.15) | 89.8% | 0.001 |
|
Low-to-intermediate
risk | 2 | 26/101 | 26/99 | 0.98
(0.61-1.57) | 0.0% | 0.559 |
| Progression | | | | | | |
|
Overall | 3 | 13/122 | 17/134 | 1.02
(0.54-1.93) | 0.0% | 0.766 |
|
High-risk | 1 | 7/21 | 13/35 | 0.90
(0.43-1.89) | NA | NA |
|
Low-to-intermediate
risk | 2 | 6/101 | 4/99 | 1.46
(0.42-5.04) | 0.0% | 0.760 |
| Safety | | | | | | |
|
Any AE | | | | | | |
|
Overall | 3 | 42/128 | 65/129 | 0.78
(0.53-1.13) | 11.8% | 0.322 |
|
High-risk | 2 | 22/72 | 32/72 | 0.99
(0.61-1.60) | 0.0% | 0.702 |
|
Low-to-intermediate
risk | 1 | 20/56 | 33/57 | 0.62
(0.41-0.93) | NA | NA |
| Severe AE (grade
≥3) | | | | | | |
|
Overall | 4 | 6/168 | 13/169 | 0.67
(0.25-1.77) | 0.0% | 0.402 |
|
High-risk | 2 | 3/72 | 9/72 | 0.37
(0.03-4.28) | 58.8% | 0.119 |
|
Low-to-intermediate
risk | 2 | 3/96 | 4/97 | 0.81
(0.20-3.25) | 0.0% | 0.549 |
| B, Secondary
outcomes |
| Efficacy | | | | | | |
|
Mortality | 1 | 0/21 | 1/35 | 0.55
(0.02-12.92) | NA | NA |
|
Persistence
of high-risk disease | 1 | 13/32 | 14/32 | 0.93
(0.69-1.24) | NA | NA |
|
Regression | 1 | 17/32 | 18/32 | 0.94
(0.75-1.19) | NA | NA |
| Safety | | | | | | |
| Local AEs | | | | | | |
|
Dysuria | 4 | 27/168 | 4/169 | 0.59
(0.39-0.89) | 0.0% | 0.674 |
|
Hematuria | 2 | 2/96 | 14/97 | 0.21
(0.03-1.32) | 32.7% | 0.223 |
|
Dermatitis | 1 | 2/40 | 0/40 | 5.00
(0.25-100.93) | NA | NA |
|
Urinary
frequency | 1 | 4/40 | 18/40 | 0.22
(0.13-0.37) | NA | NA |
|
Bladder
spasm | 1 | 3/56 | 1/57 | 3.05
(0.98-9.54) | NA | NA |
|
Urge
incontinence | 1 | 3/56 | 6/57 | 0.51
(0.26-1.01) | NA | NA |
|
Itching | 1 | 3/56 | 9/57 | 0.34
(0.18-0.64) | NA | NA |
|
Skin
rash | 1 | 1/56 | 1/57 | 1.02
(0.25-4.13) | NA | NA |
| Systemic AEs | | | | | | |
|
Fever | 3 | 1/128 | 15/129 | 0.17
(0.04-0.76) | 0.0% | 0.569 |
|
Nausea and
vomiting | 2 | 5/72 | 0/72 | 5.94
(0.73-48.31) | 0.0% | 0.875 |
|
Asthenia | 1 | 1/32 | 0/32 | 3.00
(0.13-70.96) | NA | NA |
|
Neutropenia
and thrombocytopenia | 1 | 2/40 | 0/40 | 5.00
(0.25-100.93) | NA | NA |
According to the type of AE, patients receiving
gemcitabine had significantly lower risks of dysuria (four studies;
RR, 0.59; 95% CI, 0.39-0.89; I2=0.0%; P=0.674), fever
(three studies; RR, 0.17; 95% CI, 0.04-0.76; I2=0.0%;
P=0.569), urinary frequency (one study; RR, 0.22; 95% CI,
0.13-0.37) and itching (one study; RR, 0.34; 95% CI, 0.18-0.64);
whereas the risk was similar for other local (hematuria: RR, 0.21;
95% CI, 0.03-1.32; I2=32.7%; P=0.223; bladder spasms:
RR, 3.05; 95% CI, 0.98-9.54; dermatitis: RR, 5.00; 95% CI,
0.25-100.93; skin rash: RR, 1.02; 95% CI, 0.25-4.13; and urge
incontinence: RR, 0.51; 95% CI, 0.26-1.01) and systemic (nausea and
vomiting: RR, 5.94; 95% CI, 0.73-48.31; I2=0.0%;
P=0.875; asthenia: RR, 3.00; 95% CI, 0.13-70.96; and neutropenia
and thrombocytopenia) AEs (Table
II).
Discussion
The present systematic review and meta-analysis
included four RCTs comprising 344 patients and one retrospective
cohort study with 103 patients. The earliest RCT was conducted by
Porena et al (17) in 2010
among high-risk superficial bladder cancer patients based on the
EAU risk classification system. BCG was administered as a 6-weekly
instillation of 5x108 CFU of Tice strain BCG retained
intravesically for 2 h (17).
Gemcitabine was administered as a 6-weekly instillation of 2,000
mg, also retained for 2 h. In that study, BCG was associated with a
lower recurrence rate (28.1 vs. 53.1%) and a longer recurrence-free
survival than gemcitabine (17).
These findings are in contrast to those of the other three RCTs
included in the present study.
Di Lorenzo et al (18) compared these treatments in patients
with high-risk NMIBC (per EORTC scoring system) who had previously
failed BCG therapy. In their study, BCG (Connaught strain, 81 mg/50
ml) was administered 4-6 weeks after the first treatment failure,
over a 6-week induction followed by maintenance at 3, 6 and 12
months. The gemcitabine group received 2,000 mg/50 ml over a period
of 6 weeks and similar maintenance at 2, 6 and 12 months (18). In contrast to the study by Porena
et al (17), the study by
Di Lorenzo et al (18)
found lower recurrence rates and a higher 2-year recurrence-free
survival in the gemcitabine group, with no significant differences
in time-to-recurrence or progression. This was the only study to
include BCG-refractory patients, which may explain the divergent
findings from that of Porena et al (17).
In their study, Bendary et al (19) included patients with primary Ta-T1
tumors without CIS and reported no significant differences in
recurrence or progression rates between BCG and gemcitabine. In
that study, BCG was administered at 6x108 CFU (strain
unspecified) and gemcitabine at 2,000 mg in 50 ml saline, both over
6 weekly 2-h instillations (19).
Similarly, Gontero et al (16) found no significant difference in
recurrence, recurrence-free survival, or progression in
intermediate-risk patients with NMIBC. Their BCG regimen used
one-third dose (27 mg) of Connaught strain over a 6-week induction
period. Gemcitabine was administered identically across studies
(2,000 mg/50 ml, 6-week induction) (16).
Prasanna et al (20) conducted a retrospective cohort
study on patients with CIS, pTa and pT1 tumors, stratified into
EAU-defined risk groups. BCG (Oncotice strain, 5x108
CFU) and gemcitabine (2,000 mg) were administered as 6 weekly
instillations. Despite baseline differences in risk-group
distribution, adjusted multivariate analysis revealed no difference
in disease-free survival between the groups (20). All studies agreed that gemcitabine
had a more favorable safety profile, with fewer local and systemic
adverse events, leading to better tolerability and quality of
life.
The variability in the findings across these five
studies is largely attributable to clinical and methodological
heterogeneity. Porena et al (17) included only high-risk patients per
EAU criteria, which included patients with NMIBC with CIS, all T1
tumors without CIS, Ta LG/G2 without CIS with three risk factors,
Ta HG/G3 without CIS and with two risk factors, and T1 G2 without
CIS and with one risk factor (10). Conversely, Gontero et al
(16) focused on intermediate-risk
NMIBC, and accordingly reported no difference in outcomes between
gemcitabine and BCG. Di Lorenzo et al (18) used the EORTC scoring system to
define high risk (recurrence score >9 and/or progression score
>13). Although EORTC risk classification may slightly outperform
EAU in predicting recurrence (c-index 0.64 vs. 0.62; P=0.035), the
key distinguishing feature in the study by Di Lorenzo et al
(18) was the inclusion of
BCG-refractory patients, not the risk model used.
Bendary et al (19) and Prasanna et al (20) included broader and less
well-defined populations. In the study by Bendary et al
(19), the inclusion of all Ta-T1
tumors without CIS spanned a wide risk spectrum but lacked proper
risk stratification. By contrast, Prasanna et al (20) used similarly broad criteria but
stratified patients into EAU risk groups, strengthening the
interpretation of findings. BCG regimens also varied. Although
strain and dose differences existed, a previous meta-analysis of 65
trials involving >12,000 patients concluded that no strain
demonstrated clear superiority (21). Gontero et al (16) used a one-third dose of the
Connaught strain in intermediate-risk patients to reduce toxicity
without sacrificing efficacy. Gemcitabine regimens were consistent
across studies. Only Di Lorenzo et al (18) included patients with prior BCG
failure, and only in this context did gemcitabine demonstrate
superior efficacy.
The findings presented herein align with those of
prior reviews. Shelley et al (22) reported that gemcitabine was
comparable to BCG in intermediate-risk patients, inferior in
high-risk, and superior in BCG-refractory NMIBC. However, the
present systematic review included more representative populations
from Bendary et al (19)
and Prasanna et al (20),
both demonstrating no efficacy difference across wider risk groups.
Similarly, the Cochrane review by Jones et al (23) found gemcitabine less effective in
high-risk NMIBC, comparable in intermediate-risk, and more
effective in BCG-refractory patients. A separate meta-analysis also
revealed no significant difference in recurrence (HR, 1.04; 95% CI,
0.38-2.89) or progression (HR, 0.01; 95% CI, -0.75 to 0.77) between
the two agents (24).
Overall, the findings of the present systematic
review and meta-analysis suggest that intravesical gemcitabine was
non-inferior to BCG immunotherapy in terms of efficacy, while it
resulted in lower rates of dysuria, fever, urinary frequency and
itching. However, intravesical gemcitabine appears particularly
useful for patients with BCG-refractory NMIBC. In this population,
the EAU recommends radical cystectomy (11), although it remains an invasive
procedure with significant morbidity and mortality, and entails
major lifestyle changes (25).
Intravesical gemcitabine may therefore serve as a non-invasive
alternative for NMIBC patients unresponsive to BCG
immunotherapy.
The superiority of gemcitabine over BCG in
BCG-refractory NMIBC may be explained by several factors. BCG, as
with other immunotherapies, relies on an intact immune system to
activate innate immunity and initiate BCG-specific and
tumor-specific T-cell responses. Although the exact mechanisms
remain unclear, BCG failure is considered to result from complex
interactions between host immunity and tumor microenvironment,
leading to immune dysregulation and inadequate immune activation.
Specifically, non-responders have been shown to be associated with
increased levels of CD25+ regulatory T-cells and
tumor-associated macrophage, enrichment of exhausted
CD8+PDL-1(+) T-cells, and reduced levels of
Th2-predominant CD4+ T-cells within the tumor
microenvironment (26). By
contrast, gemcitabine inhibits tumor DNA synthesis, leading to
apoptosis, and is therefore less dependent on host immune function
(23). Its lower risk of adverse
events may be attributed to its immune-selective cytotoxicity and
high plasma clearance, allowing any drug that enters systemic
circulation to be rapidly eliminated (23). Furthermore, BCG is a
live-attenuated strain of Mycobacterium bovis that activates
a strong immune response and induces inflammation following
urothelial invasion (27), which
contributes to a higher incidence of local and systemic
side-effects (27,28).
In conclusion, the present systematic review and
meta-analysis demonstrated that intravesical gemcitabine was
non-inferior to BCG immunotherapy in the treatment of patients with
NMIBC and may serve as a viable option for those unresponsive to
BCG. Although the overall risk of AEs was comparable, gemcitabine
was associated with lower rates of dysuria, fever, urinary
frequency and itching. Further high-quality RCTs with larger sample
sizes, standardized dosing, treatment intervals, and follow-up
durations are warranted to confirm these findings.
Supplementary Material
(A-H) Galbraith plots visualizing
heterogeneity across the meta-analyses of efficacy and safety
outcomes.
Key words used during the database
search.
Validity and applicability assessment
of the experimental studies passed the initial screening.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FZN and ARAHH were involved in the study conception
and design. FZN was involved in the acquisition of data and in the
drafting of the manuscript. FZN, ARAHH, CAM and FR were involved in
the analysis and interpretation of data. ARAHH and CAM critically
revised the manuscript for important intellectual content. FZN and
FR performed the statistical analysis. ARAHH and CAM provided
administrative, technical and material support (data analysis
coaching) and supervised the study as required. All authors have
read and approved the final manuscript. All authors confirm the
authenticity of all the raw data
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Isharwal S and Konety B: Non-muscle
invasive bladder cancer risk stratification. Indian J Urol.
31:289–296. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Guo CC, Lotan Y and Kassouf W: Bladder
cancer. Lancet. 388:2796–2810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Herr HW: Tumor progression and survival of
patients with high grade, noninvasive papillary (TaG3) bladder
tumors: 15-year outcome. J Urol. 163:60–62. 2000.PubMed/NCBI
|
|
5
|
Park JC, Citrin DE, Agarwal PK and Apolo
AB: Multimodal management of Muscle-invasive bladder cancer. Curr
Probl Cancer. 38:80–108. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mossanen M and Gore JL: The burden of
bladder cancer care: Direct and indirect costs. Curr Opin Urol.
24:487–491. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim LHC and Patel MI: Transurethral
resection of bladder tumour (TURBT). Transl Androl Urol.
9:3056–3072. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Herr HW and Donat SM: Quality control in
transurethral resection of bladder tumours. BJU Int. 102:1242–1246.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Martin-Doyle W, Leow JJ, Orsola A, Chang
SL and Bellmunt J: Improving selection criteria for early
cystectomy in high-grade T1 bladder cancer: A meta-analysis of
15,215 patients. J Clin Oncol. 33:643–50. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A and Palou J: others: EAU guidelines on
non-muscle invasive bladder cancer (TaT1 and CIS). Eur Urol,
2021.
|
|
11
|
Sylvester RJ, Oosterlinck W and Van Der
Meijden APM: A single immediate postoperative instillation of
chemotherapy decreases the risk of recurrence in patients with
stage Ta T1 bladder cancer: A meta-analysis of published results of
randomized clinical trials. J Urol. 171:2186–2190. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Maa Y, Djatisoesanto W and Hardjowijoto S:
Profile of bladder transitional cell cancer in Soetomo Hospital
Surabaya. Indones J Urol. 21:1–6. 2014.
|
|
13
|
Porten SP, Leapman MS and Greene KL:
Intravesical chemotherapy in Non-muscle-invasive bladder cancer.
Indian J Urol. 31:297–303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
in nBean J and Sylvester R: Results of
EORTC Genito-Urinary Group phase III trial 30911 [Internet]. 2010
Mar 12 [cited 2025 Aug 22]. Available from: EORTC website.
|
|
15
|
Sylvester RJ, van der MEIJDEN AP and Lamm
DL: Intravesical bacillus Calmette-Guerin reduces the risk of
progression in patients with superficial bladder cancer: A
meta-analysis of the published results of randomized clinical
trials. J Urol. 168:1964–1970. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gontero P, Oderda M, Mehnert A, Gurioli A,
Marson F, Lucca I, Rink M, Schmid M, Kluth LA, Pappagallo G, et al:
The impact of intravesical gemcitabine and 1/3 dose bacillus
Calmette-Guérin instillation therapy on the quality of life in
patients with nonmuscle invasive bladder cancer: Results of a
prospective, randomized, phase II trial. J Urol. 190:857–862.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Porena M, Del Zingaro M, Lazzeri M,
Mearini L, Giannantoni A, Bini V and Costantini E: Bacillus
calmette-guérin versus gemcitabine for intravesical therapy in
high-risk superficial bladder cancer: A randomised prospective
study. Urol Int. 84:23–27. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Di Lorenzo G, Perdonà S, Damiano R,
Faiella A, Cantiello F, Pignata S, Ascierto P, Simeone E, De Sio M
and Autorino R: Gemcitabine versus bacille Calmette-Guérin after
initial bacille Calmette-Guérin failure in non-muscle-invasive
bladder cancer: A multicenter prospective randomized trial. Cancer.
116:1893–1900. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bendary L, Khalil S, Shahin A and Nawar N:
1655 intravesical gemcitabine versus bacillus Calmette-Guerin (BCG)
in treatment of non-muscle invasive bladder cancer: Short term
comparative study. J Urol. 185:e664–e665. 2011.
|
|
20
|
Prasanna T, Craft P, Balasingam G,
Haxhimolla H and Pranavan G: Intravesical gemcitabine versus
intravesical bacillus calmette-guérin for the treatment of
Non-muscle invasive bladder cancer: An evaluation of efficacy and
toxicity. Front Oncol. 7:1–5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Boehm BE, Cornell JE, Wang H, Mukherjee N,
Oppenheimer JS and Svatek RS: Efficacy of bacillus Calmette-guérin
strains for treatment of nonmuscle invasive bladder cancer: A
systematic review and network Meta-Analysis. J Urol. 198:503–510.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shelley MD, Jones G, Cleves A, Wilt TJ,
Mason MD and Kynaston HG: Intravesical gemcitabine therapy for
non-muscle invasive bladder cancer (NMIBC): A systematic review.
BJU Int. 109:496–505. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jones G, Cleves A, Wilt TJ, Mason M,
Kynaston HG and Shelley M: Intravesical gemcitabine for non-muscle
invasive bladder cancer. Cochrane database Syst Rev.
1(CD009294)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lu JL, Xia QD, Lu YH, Liu Z, Zhou P, Hu HL
and Wang SG: Efficacy of intravesical therapies on the prevention
of recurrence and progression of non-muscle-invasive bladder
cancer: A systematic review and network meta-analysis. Cancer Med.
9:7800–7809. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hurle R, Casale P, Morenghi E, Saita A,
Buffi N, Lughezzani G, Colombo P, Contieri R, Frego N, Guazzoni G
and Lazzeri M: Intravesical gemcitabine as bladder-preserving
treatment for BCG unresponsive non-muscle-invasive bladder cancer.
Results from a single-arm, open-label study. BJUI Compass.
1:126–132. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Maroof H, Paramore L and Ali A: Theories
behind bacillus Calmette-Guérin failure in high-risk
non-muscle-invasive bladder cancer and update on current
management. Cancer Pathog Ther. 2:74–80. 2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jiang S and Redelman-Sidi G: BCG in
bladder cancer immunotherapy. Cancers (Basel).
14(3073)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Akbulut I, Ödemiş İ and Atalay S: Analysis
of local and systemic side effects of bacillus Calmette-Guérin
immunotherapy in bladder cancer: A retrospective study in Türkiye.
PeerJ. 13(e18870)2025.PubMed/NCBI View Article : Google Scholar
|