Introduction
Cheese production has long relied on animal-derived
rennet, a complex of proteolytic enzymes primarily chymosin
extracted from the stomach lining of young ruminants. However,
growing global demand for cheese, coupled with ethical, religious,
dietary and environmental concerns, has accelerated the search for
sustainable alternatives to animal rennet. Vegetarianism, the rise
of vegan products and issues regarding animal welfare have
intensified the need for microbial milk-clotting enzymes (MCEs),
which provide a scalable and animal-free solution for coagulating
milk during cheese manufacture (1). Among microbial sources, filamentous
fungi have attracted increasing attention due to their high enzyme
productivity, the ease of cultivation under diverse conditions, and
the ability to secrete extracellular enzymes with milk-clotting
potential (2,3).
The genus Penicillium, well-known for its
enzymatic versatility, includes several species with the capacity
to produce proteases that exhibit milk-clotting activity. However,
a number of microbial proteases are often accompanied by high
levels of non-specific proteolytic activity, which can adversely
affect cheese texture and flavor due to excessive casein hydrolysis
(4). Therefore, an ideal
milk-clotting enzyme should possess a high milk-clotting activity
(MCA)-to-proteolytic activity (PA) ratio, ensuring specificity
toward κ-casein the key substrate responsible for casein micelle
destabilization and curd formation. In this context, exploring
novel fungal strains capable of producing enzymes with a favorable
MCA/PA profile is a critical step toward developing viable
alternatives to animal rennet (5).
Recent advances made in enzyme biotechnology have
enabled the identification, production and biochemical
characterization of fungal MCEs with industrial potential.
Solid-state fermentation (SSF), in particular, has emerged as an
efficient technique for fungal enzyme production due to its cost
effectiveness, high product yield, and alignment with sustainable
waste management practices. Agricultural residues such as wheat
bran and rice husks serve as inexpensive substrates for fungal
growth, rendering SSF a preferred method for enzyme production in
resource-limited settings (6). The
optimization of SSF parameters, including pH, temperature, inoculum
size, metal ion supplementation and fermentation time is essential
to maximize enzyme yields and ensure functional integrity (7).
In the present study, an extracellular MCE produced
by Penicillium purpurescens was optimized under SSF
conditions using wheat bran as a substrate. The effects of
processing variables, such as incubation temperature, medium pH,
calcium ion supplementation and inoculum size were systematically
evaluated to identify the optimal conditions for MCE production.
The enzyme was subsequently purified through acetone precipitation
and ion-exchange chromatography, and its molecular mass was
estimated via SDS-PAGE. Kinetic characterization, including the
determination of the Michaelis-Menten constant (Km),
provided insight into substrate affinity and enzymatic efficiency,
which are crucial for evaluating its industrial applicability.
The enzyme exhibited a molecular mass of 29 kDa,
high specificity toward κ-casein, and optimal activity in the
temperature range of 45-55˚C across a broad pH spectrum (5.0-10.0),
which are desirable traits for industrial cheese-making
applications. Furthermore, the enzyme demonstrated notable thermal
and pH stability, rendering it suitable for various dairy
processing conditions. The influence of metal ions on enzyme
activity revealed significant a enhancement in of Ca2+
and Mg2+, which are commonly present in milk and known
to stabilize casein micelles, while heavy metals, such as
Hg2+ and Cu2+ were inhibited; these findings
are findings consistent with those of previous research on
microbial MCEs (8,9).
Compared to conventional calf rennet, the enzyme
derived from Penicillium purpurescens demonstrates
comparable clotting performance with a lower proteolytic
degradation of milk proteins, indicating its potential as a
functional rennet substitute. The food industry is increasingly
moving towards greener, animal-free and sustainable processes, and
fungal MCEs provide a promising platform for innovation in this
domain. Moreover, fungal enzymes have been deemed to be generally
recognized as safe (GRAS) by regulatory bodies such as the FDA,
further supporting their adoption in dairy processing (FAO/WHO,
2021) (10).
It was thus hypothesized that Penicillium
purpurescens can produce a MCE with a favorable MCA/PA ratio
under optimized solid-state fermentation conditions. The present
study aimed not only to characterize the biochemical and functional
properties of a novel MCE from Penicillium purpurescens, but
also to lay the groundwork for its application in commercial cheese
production. Although the genus Penicillium includes several
species known for their proteolytic and clotting activities, there
are limited or no detailed reports on the milk-clotting potential
of P. purpurescens, rendering the present study a novel
contribution to the search for effective microbial coagulants.
However, further is required to focus on recombinant expression
systems, enzyme immobilization techniques and pilot-scale cheese
trials to establish its efficacy across diverse cheese types and
processing conditions.
Materials and methods
Microorganisms and culture
conditions
All the fungal strains used in the present study
were obtained from the laboratory of the Department Microbial
Chemistry at the National Research Centre in Cairo, Egypt. The
strains were maintained by sub-culturing them on slants of solid
Czapek Dox medium containing: glucose, 30 g/l; NaNO, 2 g/l;
KH2 PO4, 1 g/l;
MgSO4·7H2O, 0.5 g/l; KCl, 0.5 g/l and agar,
20 g/l (BDH Chemicals Limited) at 28˚C and then stored at 4˚C. The
inoculum size was adjusted by manually counting the spores.
Screening of different organisms for
MCE production
All organisms were cultivated in liquid Czapek Dox
medium (BDH Chemical, Ltd.), containing (g/l): glucose, 30; NaNO,
2; KH2PO4, 1; MgSO4.7
H2O, 0.5; KCl, 0.5 and following incubation at 28˚C for
5 days, the cultures were centrifuged at 5,000 x g, for 10 min at
room temperature to obtain cell-free supernatants as crude enzyme
extracts. These extracts were assayed for milk-clotting activity
(MCA) by mixing 0.5 ml enzyme with 4.5 ml pre-warmed (35˚C)
reconstituted skim milk containing CaCl2, and the
clotting time was recorded visually. The activity was expressed in
arbitrary units relative to the time required for visible
coagulation. To assess non-specific proteolysis, proteolytic
activity (PA) was determined by incubating enzyme with azocasein,
(Merck KGaA) (or casein), followed by trichloroacetic acid (TCA;
El-Gomhouria Company), precipitation and measurement of soluble
peptides spectrophotometrically. The ratio of MCA to PA was then
calculated for each organism to evaluate enzyme suitability for
cheesemaking. Organisms with high MCA and high MCA/PA ratio were
considered the best candidates and selected for further
purification and characterization, as shown Table I.
 | Table IScreening of different organisms for
MCE production. |
Table I
Screening of different organisms for
MCE production.
| Tested strains | Milk-clotting
activity MCA/ml (mean ± SD) |
|---|
| Scopulariopsis
brevicaulis - natural potato | - |
| Penicillium
javanicum - exo- dox | - |
| Aspergillus
flavus - dox | - |
| Aspergillus
flavus - natural potato | - |
| Penicillium
purpurescens | 190±3.4 |
| Penicillium
oxalicum - exo | - |
| Penicillium
oxalicum - endo | - |
| Aspergillus
ustus - dox | - |
| Aspergillus
ustus - potato | - |
| Aspergillus
phoenix - exo | - |
| Aspergillus
phoenix - endo | - |
| Erytherma
sp. - exo- dox | - |
| Penicillium
oxalicum - endo | - |
| Curvularia sp.
DHE5 | 60.8±2.1 |
| Aspergillus
Fumigatus | - |
| Aspergillus
tamari DHE10 homogenate | - |
| Streptomyces
aurecens | - |
| Penicillium
politans | - |
| Aspergillus
oryzae static - dox | - |
| Aspergillus
oryzae static - PDA | - |
| Aspergillus
oryzae shaking - dox | - |
| Aspergillus
oryzae shaking - PDA | 102±3.4 |
| Noh2 | 45±1.2 |
| New Aspergillus
sp. - endo | - |
Chemicals and reagents
All chemicals and analytical-grade reagents used in
the present study were procured from Merck KGaA. Skimmed milk
powder (for MCA assays) was obtained from a local commercial
supplier (El-Nasr Co. for Dairy Products). Wheat bran substrate was
provided by ARMA Food Industries.
Optimization of fermentation
conditions. Evaluation of different culture media for MCE
production
The effects of various culture media on MCE
production were investigated using both submerged fermentation
(SmF) and SSF under static and shaking conditions. A total of 10
liquid media were evaluated, including natural potato broth,
Sabouraud dextrose broth, Czapek Dox broth, modified Czapek Dox
with 5% sucrose and peptone, modified Czapek Dox with 5% sucrose
and NaNO3, starch nitrate broth, malt extract broth,
yeast extract peptone dextrose (YPD) broth, synthetic potato
dextrose agar (PDA) broth and a specific medium (Merck KGaA)
(11). For each medium, 50 ml were
inoculated with 1 ml spore suspension (7x106 spores/ml)
and incubated at 28˚C for 7 days under static and shaking (180 rpm)
conditions. Additionally, eight agro-industrial residues, namely
wheat bran, sugarcane bagasse, banana peel, guava seeds, corn
stover, pomegranate peel, tangerine peel and sawdust (purchased
from the local Arma Food Industry, Egyptian Company for solid waste
recycling) were screened as solid substrates for MCE production in
SSF. For SSF experiments, 5 g of each substrate were moistened with
5 ml distilled water, sterilized (121˚C for 15 min) and then
inoculated with 1 ml of the same spore suspension. The mixtures
were homogenized and incubated at 28˚C for 7 days. For enzyme
extraction, the content of each flask was mixed with 100 ml of 0.05
M extraction buffer (Na-citrate, citric acid, pH 6.0) (1:10, w/v).
The culture was mechanically agitated at 180 rpm in a shaking
incubator (Benchmark Scientific Inc.) at 28˚C for 30 min. The
mixture was then filtered to separate the mycelia from the medium.
The supernatant (crude-enzyme extract) developed was utilized for
MCE activity and determination of protein content, the method
described by Nema et al (12). Through this optimization process,
the ideal cultivation conditions for Penicillium
purpurescens were determined to be as follows: A moisture
content maintained between 60-70%, an aeration level of 75%,
shaking at 180 rpm, a temperature range of 28-30˚C, an initial pH
of 6.0-6.5 and an incubation period of 5 days.
Initial pH. The effect of incubation pH on
MCE production was examined in the pH range of 3.0 to 10. The media
pH was adjusted using either dilute HCl or dilute NaOH
(El-Gomhouria Company) prior to autoclaving the bran (13).
Incubation temperature. Temperature was
optimized for the growth and production of MCE by incubating the
flasks containing the inoculated bran with pH 6, at 20, 25, 30, 35,
40 and 45˚C for 7 days (13).
Fermentation time. The MCE activity was
monitored at 24-h intervals for up to 240 h. MCE production at
optimum pH 6 and temperature 28˚C was followed. At the end of each
time point, the extent of MCE production was determined as
described by Vishwanatha et al (13).
Inoculum size. A total of 5 g of wheat bran
was moistened with 5 ml distilled water (pH 6) and autoclaved. The
substrate was inoculated with different volumes of the same spore
suspension (0.25, 0.5, 1, 2, 3, 4 and 5 ml). The flasks were
incubated at 25˚C for 5 days.
Analysis of the effect of the
CaCl2 concentration on MCE
Different volumes of the substrate (milk +
CaCl2) were tested (0.125, 0.25, 0.5, 0.75, 1, 1.5, 2,
2.5 and 3 ml) against the same volume of the enzyme.
Determination of MCA
The MCA was determined using the method previously
described by Kumar et al (14), based on the appearance of the first
discontinuous particles and expressed in Soxhlet units (SU). The
enzyme solution was incubated at 35˚C for 10 min before being added
to skim milk (10% wt/vol, containing 10 mM CaCl2 (Merck
KGaA), which had been pre-incubated at the same temperature for 5
min. The reaction was terminated once discontinuous particles
formed. The MCA was calculated using the following formula:
SU=(2,400 x VS x N)/(T x VE), where VS represents the skim milk
volume (ml), N is the MCE dilution factor, T is the milk-clotting
time (seconds) and VE is the volume of the MCE (ml) used in the
assay.
Determination of PA
PA was determined using N,N-dimethyl casein
(DMC) (Merck KGaA) as the substrate, following the method described
in the study by Fan et al (15). In brief, 45 µl enzyme solution were
combined with 45 µl substrate solution (10 mg/ml DMC in 20 mM
potassium phosphate buffer, pH 5.8) and incubated at 35˚C for 30
min. The reaction was terminated by the addition of 350 µl of 100
mg/ml trichloroacetic acid (TCA) (El-Gomhouria Company), followed
by incubation on ice for 20 min to precipitate undigested proteins.
The samples were centrifuged at 2,000 x g for 15 min at 22˚C, and
the absorbance of the supernatant was measured at 280 nm (UNICO
advanced UV-VISIBLE spectrophotometer). A blank was prepared for
each sample by adding TCA before the enzyme to correct for
non-enzymatic hydrolysis. A total of one unit of PA [measured in
proteolytic units (PU)] was defined as the amount of enzyme that
releases 1 µg tyrosine per minute at 35˚C, using a tyrosine
extinction coefficient (ε) of ml/µg for quantification:
MCE purification
The crude enzyme solution was fractioned by acetone
and the active fraction with high MCA was further purified by
passing through a column (1.5x40 cm) of DEAE-cellulose (Pharmacia
Fine Chemicals AB) pre-equilibrated with 0.02 M sodium phosphate
buffer at pH 6. The elution of protein was then carried out by the
batch-wise addition of 40 ml portions of increasing molarities
(0.0-0.4 M) of NaCl in 0.02 M phosphate buffer at pH 6. Fractions
of 5 ml each were collected at room temperature (25˚C) at a flow
rate of ~20 ml/h. The eluted fractions from the DEAE-cellulose
column were dialyzed against cold distilled water to remove excess
NaCl. The resulting purified enzyme solution was aliquoted into
multiple test tubes, each containing 2 ml, and stored at -20˚C for
later use. Under these storage conditions, the enzyme remained
stable for >1 month. However, subjecting the enzyme to more than
three freeze-thaw cycles resulted in approximately a 21% loss of
activity. The enzyme remains active in the refrigerator for up to 1
week (16).
Determination of MCE purity and
concentration
The molecular weight of the enzyme was determined
using sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) following the method described by Laemmli (17). A 12% acrylamide gel was prepared,
and protein bands were visualized by staining with Coomassie Blue
R-250 (Merck KGaA) at room temperature for 1 h, followed by
de-staining until clear background was obtained. Zymography was
performed in two steps: First, native-PAGE was conducted using a
12% polyacrylamide gel without SDS, as described by Snoek-van
Beurden and Von den Hoff (18)
with minor modifications. Samples were loaded without prior
boiling.
Effect of pH and temperature on
Penicillium purpurescens MCE
To evaluate the effect of pH on the MCA of
Penicillium purpurescens, the enzyme activity was assayed at
different pH values ranging from 3.0 to 8.0. Substrate buffer
solutions were prepared using: 0.05 M citrate buffer for pH
3.0-6.0, and 0.05 M phosphate buffer for pH 6.5-8.0. Each reaction
mixture adjusted to the desired pH. The optimal pH was defined as
the pH exhibiting the highest MCA. To assess the influence of
temperature on MCA, enzyme assays were performed at temperatures
ranging from 20 to 80˚C.
Effect of metal ions on Penicillium
purpurescens MCE
The Penicillium purpurescens MCE was
dissolved in solutions containing 10 or 50 µM Na+,
Ca2+, Mg2+, Hg2+ and
Cu2+, respectively, and incubated for 30 min at 40˚C.
The changes in MCA were measured, with the MCA of the untreated
control (no metal ions) set as 100%.
Kinetic measurement for Penicillium
purpurescens MCE
The kinetic parameters of fungal MCE were determined
using a modified version of the method described by He et al
(19). Casein solutions at varying
concentrations (0.1 and 3.0 g/l) were used as substrates to measure
the PA following the protocol described in the study by Kumar et
al (14). The Michaelis-Menten
constant (Km) and maximal velocity (Vmax) were
derived from a Lineweaver-Burk double-reciprocal plot, as
established by Lineweaver and Burk (20). This analysis provided insight into
the catalytic efficiency and substrate affinity of the enzyme.
Statistical analysis
All experiments were performed in triplicate. All
reported values are expressed as the mean ± standard deviation
(SD). Statistical analysis was conducted using SPSS 17.0 (IBM
Corp.). Comparisons between two groups were performed using a
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
Screening of different organisms for
MCE production
The MCA of various fungal and bacterial strains,
measured in MCA/ml is presented in Table I. Among the tested strains, only a
few exhibited detectable MCA, while the majority did not exhibit
any activity. Penicillium purpurescens demonstrated the
highest MCA (190±3.4 MCA/ml), suggesting its potential as a robust
MCA. Aspergillus oryzae under shaking conditions in PDA
medium (Materials and methods) also exhibited notable activity
(102±3.4 MCA/ml), indicating that cultivation conditions
significantly influence enzyme production (9,21).
Curvularia lunata DHE5 and Noh2 displayed moderate activity
(60.8±2.1 and 45±1.2 MCA/ml, respectively), which may warrant
further optimization for industrial applications. The absence of
MCA in the majority of strains, including Scopulariopsis
brevicaulis, Penicillium javanicum and various
Aspergillus and Penicillium species, suggests that
MCEs are strain-specific and not universally produced (22). The variability in MCA between
different growth conditions (static vs. shaking, dox vs. PDA)
highlights the importance of optimizing fermentation parameters for
maximal enzyme yield (23).
Effect of different media on MCE
production using SSF and SmF (shaking and static conditions)
The results obtained (Table II) revealed variations in MCE
production by Penicillium purpurescens across different
media, aligning with findings from other fungal studies in Table I. For example, Aspergillus
oryzae exhibited high MCE activity in wheat bran-based SSF
(24), comparable to the optimal
performance observed in Penicillium purpurescens under
similar conditions. Likewise, Thermomucor indicae-seudaticae
N31 was investigated using submerged fermentation with 4% wheat
bran in a 0.3% saline solution, incubated at 45˚C and 150 rpm for
72 h (25), Furthermore, in
solid-state fermentation of Mucor racemosus, molasses and
casein were identified as significant carbon and nitrogen sources
(26). Further emphasized is the
role of substrate composition in fungal enzyme production,
demonstrating that lignocellulosic substrates significantly
influence MCE yields in SSF. The lack of clotting activity in
Sabouraud and natural potato media for Penicillium
purpurescens is consistent with reports on Neurospora
intermedia, which also displayed low enzyme production in
similar nutrient-limited substrates (27). These comparisons underscore the
critical influence of media composition on fungal MCE production,
with SSF often proving superior due to better fungal growth and
enzyme induction, as demonstrated by Penicillium
purpurescens and other fungi such as Aspergillus
oryzae.
 | Table IIEffect of different media on
Penicillium purpurescens MCE production using SSF and SmF
(shaking (180 rpm) and static). |
Table II
Effect of different media on
Penicillium purpurescens MCE production using SSF and SmF
(shaking (180 rpm) and static).
| A, SmF |
|---|
| | Milk-clotting
activity MCA/ml | |
|---|
| Medium | Shaking (mean ±
SD) | Static (mean ±
SD) | P-value |
|---|
| Synthetic potato
dextrose broth | 0.0 | 0.0 | - |
| Modified dox
(NaNO3+5% sucrose) | 290±3 | 266±0.7 | 0.079 |
| Yeast extract
peptone dextrose | 0.0 | 213.3±2.5 | 0.0001a |
| Malt broth | 290±5.1 | 80±1.8 | 0.005a |
| Czapex dox | 133.3±3.1 | 100± | 0.075 |
| Starch nitrate | 100±2 | 260±1.9 | 0.0001a |
| Specific
medium | 0.0 | 290±4.5 | 0.0001a |
| Modified dox (5%
sucrose + yeast + peptone) | - | - | - |
| Natural potato | - | - | - |
| Sabouraud | - | - | - |
| B, SSF |
| Agricultural
waste | Shaking (mean ±
SD) | Static (mean ±
SD) | P-value |
| Wheat bran | 320±1.3 | 106±2.5 | 0.0001a |
| Sugar cane
baggase | 0.0 | 0.0 | |
| Banana peel | 0.0 | 0.0 | |
| Guava seeds | 94±2.1 | 21±1.98 | 0.005a |
| Rough corn
stover | 0.0 | 0.0 | |
| Pomegranate
peel | 0.0 | 0.0 | |
| Tangerine peel | 0.0 | 0.0 | |
| Saw dust | 0.0 | 0.0 | |
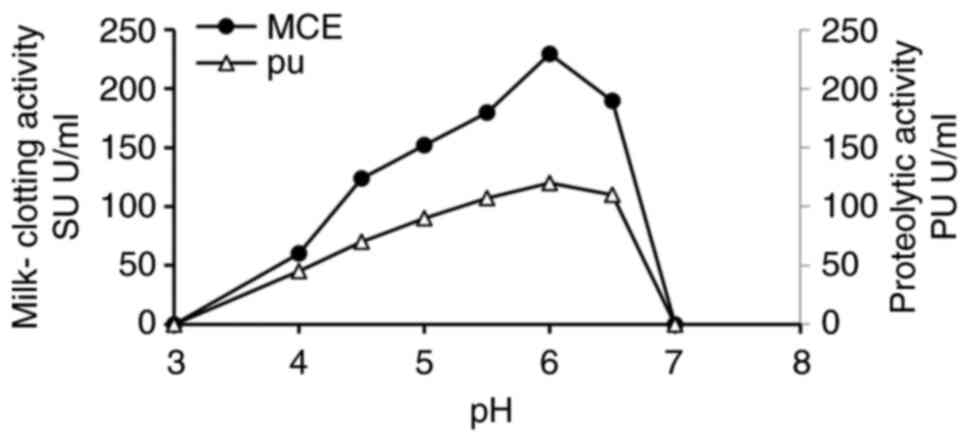
Effect of pH on Penicillium
purpurescens MCE
As illustrated in Fig.
1, the pH of the culture medium significantly influenced both
MCE production and PA in Penicillium purpurescens, with
optimal activity observed at near-neutral pH (6.0-6.5). No activity
was detected at highly acidic (pH 2-3) or alkaline (pH 7)
conditions, while a progressive increase was noted from pH 4 to 6,
peaking at pH 6 (188 MCE units, 120 PU). This trend is in
accordance with findings in other fungi, such as Aspergillus
oryzae, which exhibited maximal MCE activity at pH
5.5-6.0(24), and Mucor
racemosus, which exhibited optimal production at pH 4.8
(3,26). Similarly, Neurospora
intermedia demonstrated reduced enzyme yields at extreme pH
levels, emphasizing the importance of near-neutral conditions for
fungal protease stability and secretion (27). The sharp decline in Penicillium
purpurescens activity at pH 7 suggests enzyme denaturation or
impaired fungal metabolism under alkaline conditions, a phenomenon
also observed in Bacillus spp (28), which are known to produce enzymes
that remain stable across a broad pH range (6.0-10.0).
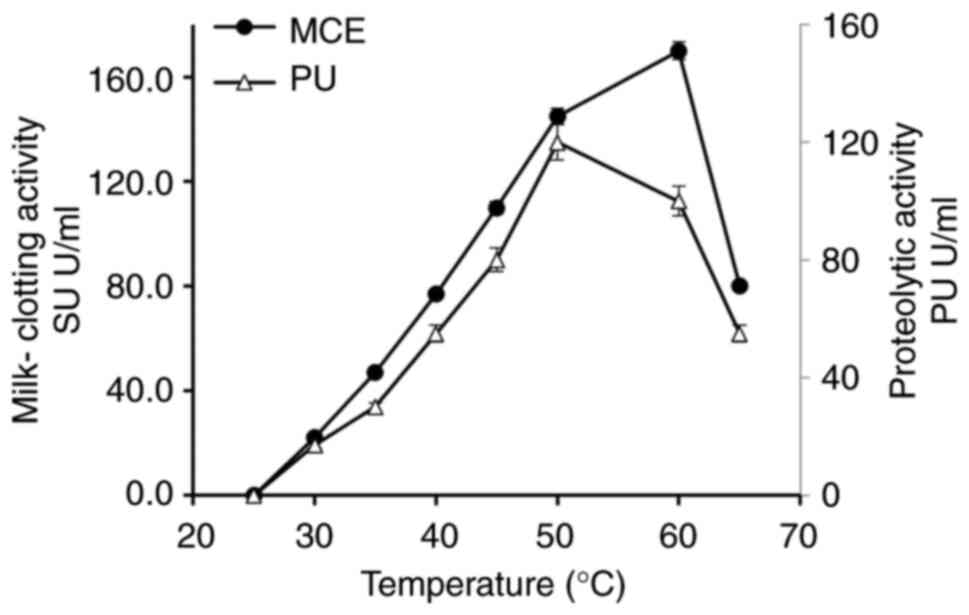
Effect of temperature on Penicillium
purpurescens MCE production
The initial temperature significantly influences the
production of MCE and PA in Penicillium purpurescens. As
demonstrated in Fig. 2, the MCE
and PA increased with temperature, peaking at 50˚C (145 U/ml MCE,
120 U/ml PU), followed by a sharp decline beyond 60˚C due to enzyme
denaturation. Similar trends have been observed in other fungi,
such as Aspergillus oryzae and Rhizopus miehei, which
exhibit optimal MCE production at 30-40˚C and 45-55˚C, respectively
(24,29). Aspergillus niger has also
been shown to exhibit a reduced PA >50˚C, suggesting that
thermal stability is common among fungal enzymes. These findings
highlight the importance of optimizing temperature for maximal
enzyme yield and stability in microbial MCE production.
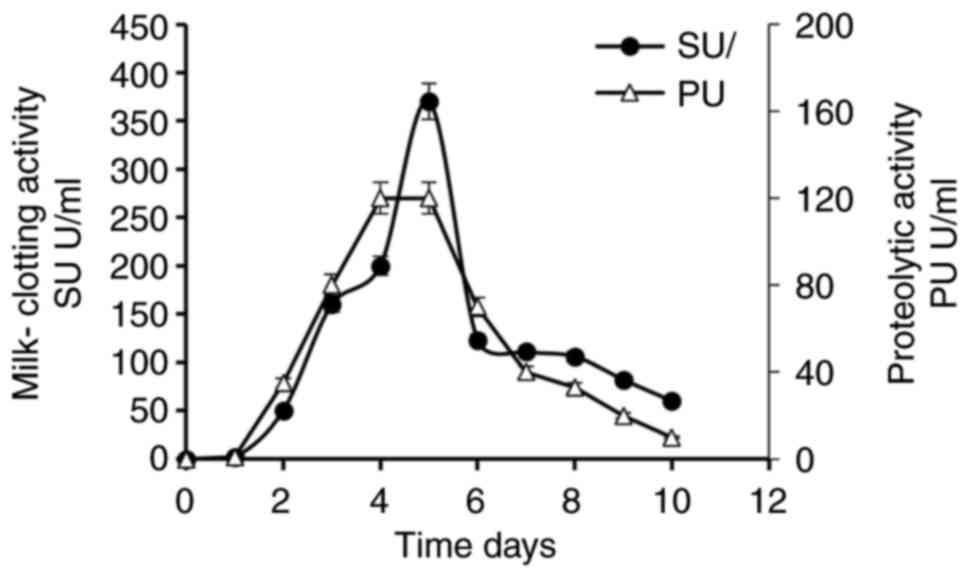
Fermentation time
The effect of fermentation time on Penicillium
purpurescens MCE production and proteolytic activity follows a
trend where enzyme activity initially increases with time before
declining due to proteolytic degradation or nutrient depletion. As
demonstrated in Fig. 3, optimal
MCE production often occurs between 4-5 days, coinciding with peak
fungal biomass and secondary metabolite secretion (30). Beyond this period, prolonged
fermentation leads to reduced enzyme stability and increased
proteolytic degradation, as observed in Penicillium
purpurescens, where MCE activity decreased after day 4. Similar
patterns have been observed in other fungi, such as Aspergillus
oryzae and Rhizomucor miehei, where maximal MCE yield
was achieved within 3-5 days before the activity diminishes
(24,31). In the present study, the MCE
demonstrated a ratio of 320, which is higher than the reported
ratios for commercial counterparts and moderately close to that of
calf rennet (~433), suggesting promising industrial potential, As
shown in Table III,
Aspergillus niger exhibits peak protease secretion at 72-96
h, after which activity declines due to autolysis. In contrast, the
enzyme from Thermomucor indicae-seudaticae N31 was
successfully used in the production of high-quality Prato cheese,
maintaining stability without changes over 60 days (32). These findings highlight the
importance of optimizing fermentation duration to balance enzyme
yield and stability, which may vary across species.
 | Table IIIComparative MCA/PA ratios of fungal
and animal rennets. |
Table III
Comparative MCA/PA ratios of fungal
and animal rennets.
| Source | MCA (SU/mg) | PA (U/mg) | MCA/PA Ratio | (Refs.) |
|---|
| Penicillium
purpurescens | 620 | 2.0 | 320 | Present study |
| Rhizomucor
miehei | 450 | 2.2 | ~204 | (32) |
| Mucor
pusillus | 520 | 3.8 | ~137 | (25) |
| Calf rennet | 650 | 1.5 | ~433 | (46) |
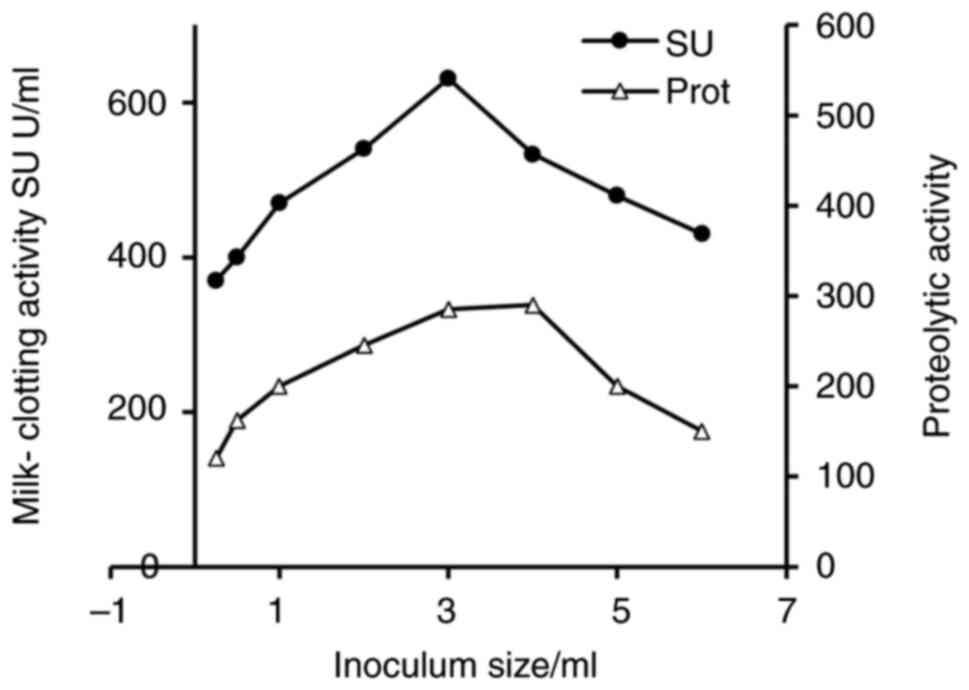
Effect of inoculum size on Penicillium
purpurescens MCE production
As illustrated in Fig.
4, the inoculum size significantly influenced the production of
MCE and PA in Penicillium purpurescens. Research has shown
that increasing the inoculum size from 0.25 to 3 ml enhances both
enzyme activity (from 370 to 631 SU) and proteolytic activity (from
120 to 285 U/ml), likely due to improved fungal biomass and
nutrient utilization (33).
However, beyond 3 ml, a decline in MCE activity (533 SU at 4 ml)
and PA (290 U/ml), occurs, possibly due to nutrient depletion or
metabolic stress. Similar trends have been observed in other fungi,
such as Aspergillus oryzae, and Aspergillus clavatus,
where optimal inoculum size (1-2x106 spores/ml)
maximizes protease production, while higher densities reduce yields
due to oxygen limitation (24,33).
Likewise, Rhizopus microsporus exhibits peak MCE activity at
5% (v/v) inoculum, with declines at higher concentrations (34,35).
These findings suggest that inoculum optimization is critical for
balancing microbial growth and enzyme synthesis across fungal
species.
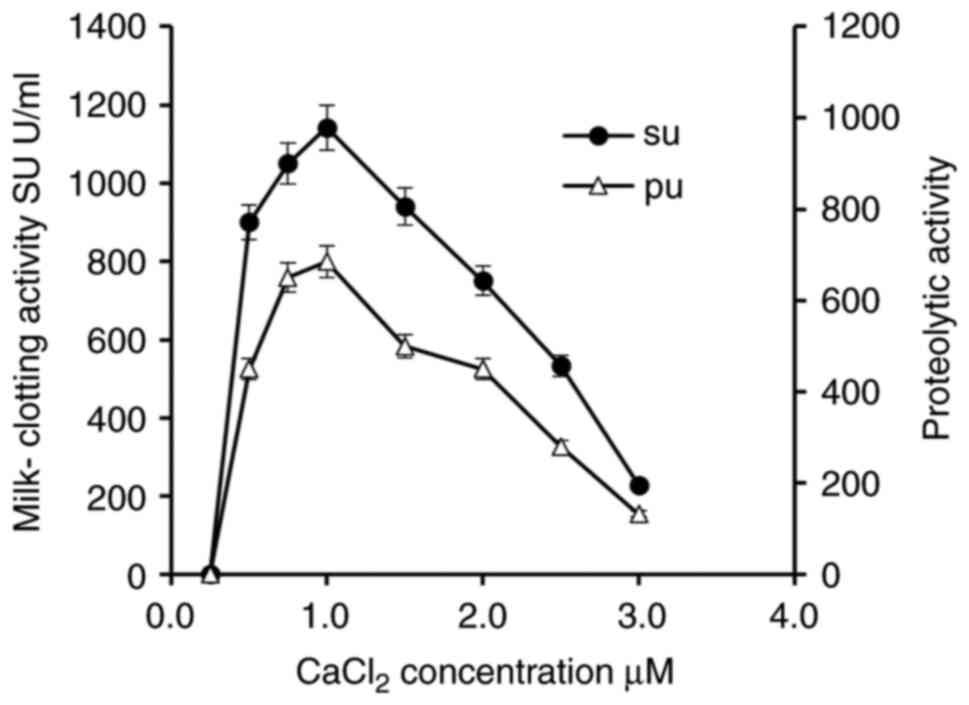
Effect of CaCl2 on
Penicillium purpurescens MCE
As demonstrated in Fig.
5, MCA peaked at 1% CaCl2 (1,140 U/ml), while PA
reached its maximum level (685 U/ml) at the same concentration.
Higher CaCl2 levels (>1%) lead to a decline in both
MCA and PA, suggesting enzyme inhibition or destabilization at an
elevated ionic strength. Similar trends have been observed in other
fungi, such as Bacillus velezensis DB219, where optimal MCE
production occurred at 0.5-1% CaCl2 beyond which
activity decreased due to possible metal ion interference with
enzyme stability (36).
Rhizopus miehei also exhibited reduced proteolytic activity
at CaCl2 concentrations above 1.5%, attributed to
conformational changes in the enzyme (37). These findings highlight the
critical role of Ca2+ in modulating fungal MCE activity,
with species-specific optimal thresholds. The decline in
Penicillium purpurescens MCE beyond 1% CaCl2
aligns with previous research on Neurospora crassa, where
excessive Ca2+ disrupted secretion pathways (38) underscoring the delicate balance
between cation stimulation and inhibition in fungal enzyme
systems.
MCE preparation and purification
As illustrated in Fig.
6, the purification of the MCE from Penicillium
purpurescens involves fractionation steps that enhance specific
activity, as evidenced by the data exhibiting increased activity in
fractions 7-9, with peak specific activities of 286. 296 and 320
U/mg U/mg, respectively. These fractions likely contain the
purified enzyme, as indicated by their high specific activities
compared to earlier fractions with negligible activity. Similar
purification trends have been observed in other fungal species,
such as Aspergillus oryzae and Rhizomucor miehei,
also produce proteases with milk-clotting properties (24,37).
For instance, Rhizomucor miehei yields a highly active
aspartic protease (rennin-like enzyme) after purification, with
specific activities comparable to those of P. purpurescens
(37). However, some fractions
(e.g., 10-12) exhibit variability, possibly due to contamination or
partial denaturation. Further purification steps, such as
ion-exchange or gel-filtration chromatography, could improve
homogeneity, as demonstrated in Aspergillus niger protease
purification (23). These data
suggest that Penicillium purpurescens is a promising source
of milk-clotting enzymes, with purification efficiency comparable
to other industrially relevant fungi.
Molecular weight of Penicillium
purpurescens MCE
The MCE from Penicillium purpurescens has a
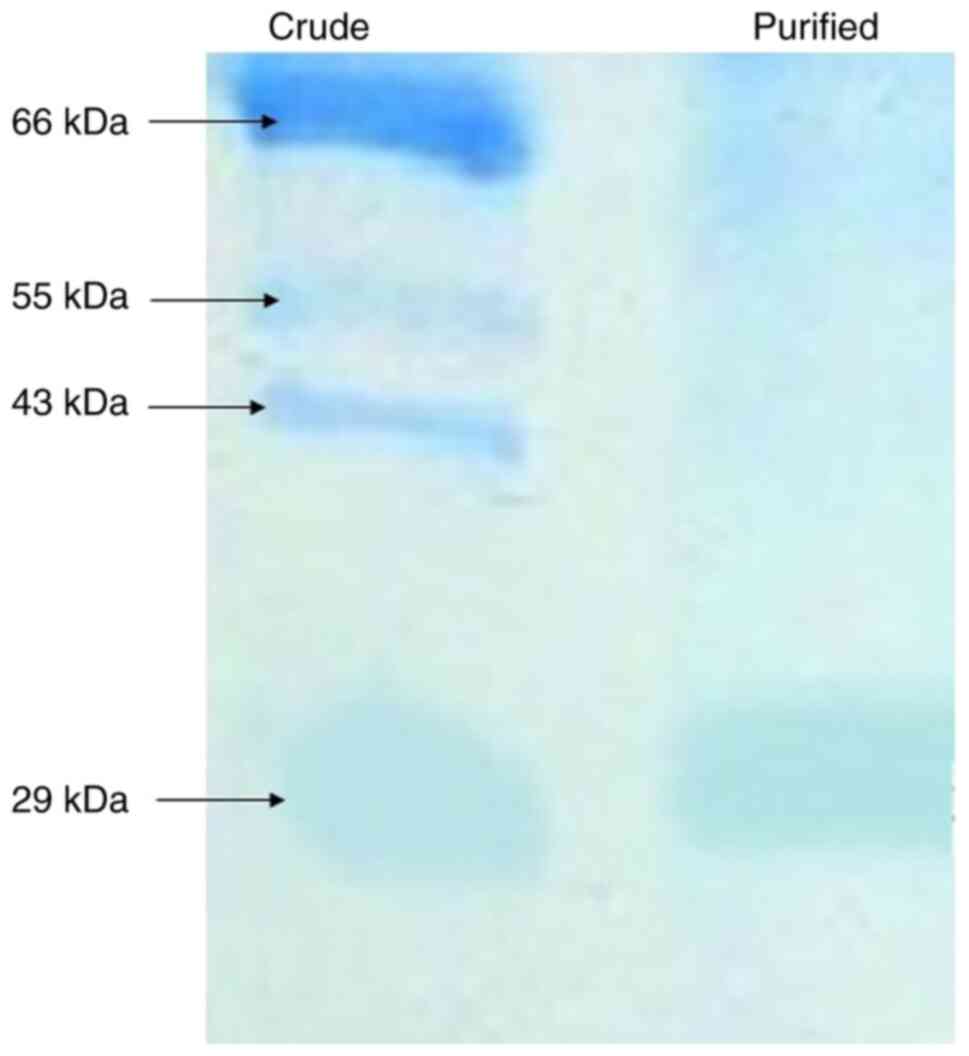
molecular weight of ~29 kDa (Fig.
7), which is comparable to other fungal aspartic proteases used
in cheese production. For instance, Rhizomucor miehei
produces a 38.6-kDa aspartic protease (rennet) (37), while Aspergillus oryzae
secretes a 43-kDa MCE (39).
Similarly, Endothia parasitica yields a 33.8 kDa protease
(40). The lower molecular weight
of Penicillium purpurescens enzyme suggests structural
differences that may influence substrate specificity and thermal
stability. These fungal enzymes are preferred over animal rennet
due to their cost-effectiveness and suitability for vegetarian
cheese production. Further comparative studies on their biochemical
properties could optimize their industrial applications.
Effect of pH and temperature on cheese
quality
The MCA of the enzyme produced by Penicillium
purpurescens exhibited a distinct dependence on pH, with
optimal activity observed at pH 6, as shown in Fig. 1, where the enzyme reached a maximum
activity of 336 MCE/ml. This result suggests that the enzyme
functions best under mildly acidic to neutral conditions, aligning
with observations from other microbial MCEs. For instance, similar
pH optima have been reported for enzymes derived from Mucor
pusillus and Rhizomucor miehei, which also exhibit peak
MCA around pH 5.5-6.5 (37,41).
The activity was significantly lower at both extreme acidic (pH 3,
120 MCE/ml) and alkaline (pH 10, 200 MCE/ml) conditions, indicating
a narrow optimal pH range for maximum proteolytic efficiency.
Fungal milk-clotting enzymes, as all enzymes, are sensitive to pH;
deviations from their optimal pH range can lead to decreased
stability and a reduced ability to bind to their substrate, milk
proteins. This phenomenon underscores the critical need to control
pH during dairy processing to ensure optimal enzyme performance,
which directly impacts the efficiency and quality of products such
as cheese (42).
The MCE derived from Penicillium purpurescens
exhibited a pronounced temperature-dependent activity profile.
Enzyme activity increased steadily from 30˚C, reaching its maximum
at 50˚C with an activity of 360 U/ml, which indicates this
temperature as optimal for catalytic function, as shown in Fig. 2. A sharp decline in activity was
observed at 55˚C, suggesting the onset of thermal denaturation;
however, the enzyme retained partial activity at 60˚C. Beyond this
temperature, activity decreased markedly, with complete
inactivation occurring at 90˚C. This thermal behavior is consistent
with MCEs from other fungal species, such as Rhizomucor
miehei and Mucor pusillus, which generally show optimal
activity within the 45-55˚C range and lose enzymatic function at
elevated temperatures due to structural instability (37,41).
These observations underscore the critical need for precise
temperature control when employing fungal MCEs such as MCE from
Penicillium purpurescens in industrial processes such as
cheese manufacturing.
Effect of metal ions on Penicillium
purpurescens MCE activity
As demonstrated in Table IV, a marked influence of various
metal ions was observed on the MCE activity of Penicillium
purpurescens. The data revealed that Ca2+ (50 µM)
enhanced enzyme activity by 150%, suggesting a potential
stabilizing or activating role, while Mg2+ (50 µM) also
promoted activity (120%), albeit to a lesser extent. By contrast,
Cu2+ (10 µM) and Hg2+ (10 µM) markedly
inhibited MCE function, reducing activity to 40 and 10%,
respectively, likely due to metal-induced protein denaturation or
active-site interference (43).
The negligible effect of Na+ (10 µM; 95% activity)
implies that monovalent ions may have minimal impact on this enzyme
system. These findings align with recent studies highlighting the
dual role of metal ions as either cofactors or inhibitors in fungal
MCE kinetics (44).
 | Table IVEffect of metal ions on
Penicillium purpurescens MCE activity. |
Table IV
Effect of metal ions on
Penicillium purpurescens MCE activity.
| Metal ion | Concentration
(µM) | Relative activity
(%) |
|---|
|
Ca2+ | 10 | 110 |
|
Ca2+ | 50 | 150 |
|
Mg2+ | 50 | 120 |
|
Cu2+ | 10 | 40 |
|
Hg2+ | 10 | 10 |
| Na+ | 10 | 95 |
Kinetic parameters of Penicillium
purpurescens MCE
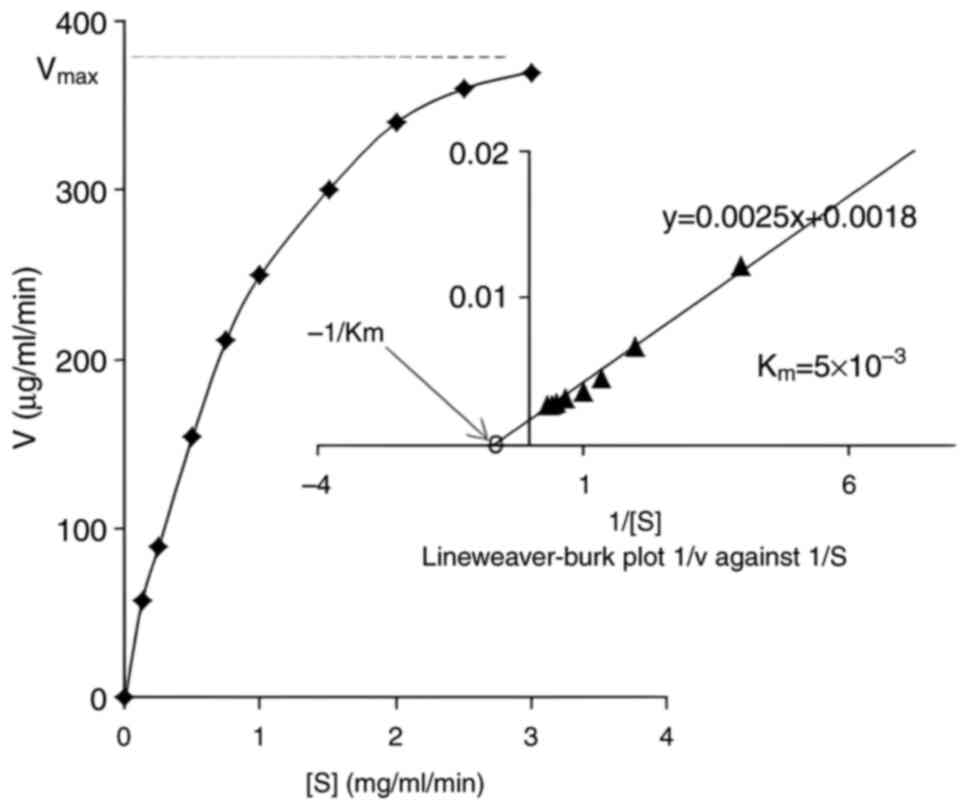
The Michaelis constant (Km) of the MCE
from Penicillium purpurescens was determined to be
5x10-2 M (Fig. 8),
indicating its moderate affinity for the substrate compared to
other fungal proteases. For instance, Aspergillus oryzae
produces a milk-clotting enzyme with a lower Km
(2.5x10-2 M), suggesting higher substrate affinity,
while Rhizomucor miehei exhibits a markedly lower
Km (3x10-3 M), reflecting superior catalytic
efficiency in cheese-making (37,44).
By contrast, Endothia parasitica has a higher Km
(12x10-1 M), indicating weaker substrate binding
(40). The Km value of
Penicillium purpurescens positions it as an intermediate
among fungal coagulants, potentially offering balanced proteolytic
activity for dairy applications.
In the authors' laboratory, the solid-state
fermentation process described herein demonstrated good
reproducibility, as repeated batches produced under identical
conditions consistently yielded comparable enzyme activity and
quantity. This reproducibility at the laboratory scale supports the
robustness of the optimized process. However, the scalability of
the method has not yet been assessed due to some limitations we
faced, including the lack of a pilot at the current time, a lack of
funds, and the absence of an experienced team to deal with a pilot
and its maintenance. Future research is required to focus on
pilot-scale trials to evaluate the feasibility of large-scale
enzyme production. Scaling up SSF may present challenges, including
maintaining uniform moisture distribution, temperature control and
adequate aeration in larger bioreactors, which need to be addressed
to ensure consistent enzyme quality and yield in industrial
applications (45).
Based on the results presented herein, the MCE from
Penicillium purpurescens produces high-quality cheese curds
with optimal characteristics, including curd yield, firmness and
sensory properties (Table V).
Further comparative studies are required to optimize its use in
industrial processes. Collectively, Penicillium purpurescens
demonstrates strong potential as a microbial rennet source, with
performance comparable to established fungal producers. Further
optimization and industrial-scale validation could enhance its
applicability in cheese manufacturing.
 | Table VPhysicochemical and sensory
properties of curd formed by Penicillium purpurescens
milk-clotting enzyme. |
Table V
Physicochemical and sensory
properties of curd formed by Penicillium purpurescens
milk-clotting enzyme.
| Parameter | Observation |
|---|
| Curd yield (%) | 37% fresh
weight |
| Dry matter (%) | 45-50% |
| Firmness | High,
non-fragile, |
| Coagulation
time | 1-5 min |
| pH at
coagulation | 6.0-6.5 |
| Bitterness | None |
| Odor | Clean, milky |
| Syneresis | Low to
moderate |
The present study had certain limitations which
should be mentioned. The activity of the enzyme against α- and
β-casein fractions could not be assessed due to logistical
challenges beyond the authors' control, including delays in
chemical imports.
In conclusion, the present study highlights
Penicillium purpurescens as a highly promising fungal strain
for MCE production, demonstrating superior clotting efficiency
compared to other screened strains. Optimal MCE production was
achieved under SSF with wheat bran, near-neutral pH (6.0-6.5), and
moderate temperatures (50˚C), with fermentation kinetics peaking at
4-5 days. The enzyme exhibited enhanced activity at 1%
CaCs2, but declined at higher concentrations, suggesting
ionic strength sensitivity. Purification yielded fractions with
high specific activity (up to 320 U/mg), and the molecular weight
of the enzyme (29 kDa) and kinetic properties
(Km=5x10-2 M) position it as a competitive
alternative to commercial fungal rennets such as those and
Aspergillus oryzae. These findings underscore Penicillium
purpurescens as a viable candidate for industrial cheese
production, though further scale-up and stability studies are
needed to confirm its commercial applicability. Future research is
warranted.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
THA, LAM, EMA and DHEG performed the screened for
MCE in different strains, enzyme production form selected strain,
enzyme characterization, purification and biochemical enzyme
kinetics. SF performed the electrophoresis, and assisted in the
drafting of the manuscript. THA wrote the manuscript. THA and LAM
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu X, Wu Y, Guan R, Jia G, Ma Y and Zhang
Y: Advances in research on calf rennet substitutes and their
effects on cheese quality. Food Res Int. 149(110704)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shahryari Z and Niknezhad SV: Production
of industrial enzymes by filamentous fungi. In: Current
developments in biotechnology and bioengineering. Elsevier,
pp293-323, 2023.
|
|
3
|
Qasim F, Diercks-Horn S, Herlevi LM and
Fernandez-Lahore HM: Production of a fungal aspartic protease via
solid-state fermentation using a rotating drum bioreactor. J Chem
Technol Biotechnol. 100:273–285. 2025.
|
|
4
|
Claverie-MartÌn F and Vega-Hernàndez MC:
Aspartic proteases used in cheese making. In: Industrial Enzymes:
Structure, Function and Applications. Springer, pp207-219,
2007.
|
|
5
|
Li L, Zheng Z, Zhao X, Wu F, Zhang J and
Yang Z: Production, purification and characterization of a milk
clotting enzyme from Bacillus methanolicus LB-1. Food Sci
Biotechnol. 28:1107–1116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
He D and Cui C: Fermentation of organic
wastes for feed protein production: Focus on agricultural residues
and industrial by-products tied to agriculture. Fermentation.
11(528)2025.
|
|
7
|
Kabir MF, Ovi AQ and Ju LK: Real-time pH
and temperature monitoring in solid-state fermentation reveals
culture physiology and optimizes enzyme harvesting for tailored
applications. Microb Cell Fact. 24(188)2025.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hang F, Liu P, Wang Q, Han J, Wu Z, Gao C,
Liu Z, Zhang H and Chen W: High milk-clotting activity expressed by
the newly isolated Paenibacillus spp. Strain BD3526.
Molecules. 21(73)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nicosia FD, Puglisi I, Pino A, Caggia C
and Randazzo CL: Plant milk-clotting enzymes for cheesemaking.
Foods. 11(871)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Benford D, Boobis A, Cantrill R, Cressey
P, Dessipri E, Kabadi SV, Jeurissen S, Mueller U and Barlow S:
Contributions of the joint FAO/WHO expert committee on food
additives to international food safety: Celebrating the 100th
meeting of the committee. Regul Toxicol Pharmacol.
160(105833)2025.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hashem AM: Optimization of milk-clotting
enzyme productivity by Penicillium oxalicum. Biores Technol.
70:203–207. 1999.
|
|
12
|
Nema A, Patnala SH, Mandari V, Kota S and
Devarai SK: Production and optimization of lipase using
Aspergillus niger MTCC 872 by solid-state fermentation.
Bulletin of the National Research Centre. 43:1–8. 2019.
|
|
13
|
Vishwanatha KS, Appu Rao AG and Singh SA:
Production and characterization of a milk-clotting enzyme from
Aspergillus oryzae MTCC 5341. Appl Microbiol Biotechnol.
85:1849–1859. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kumar A, Grover S, Sharma J and Batish VK:
Chymosin and other milk coagulants: Sources and biotechnological
interventions. Crit Rev Biotechnol. 30:243–258. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fan T, Wang J, Yuan W, Zhong Q, Shi Y and
Cong R: Purification and characterization of hatching enzyme from
brine shrimp Artemia salina. Acta Biochim Biophys Sin
(Shanghai). 42:165–171. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
El-Bendary MA, Moharam ME and Ali TH:
Purification and characterization of milk clotting enzyme produced
by Bacillus sphaericus. J Appl Sci Res. 3:695–699. 2007.
|
|
17
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Snoek-van Beurden PA and Von den Hoff JW:
Zymographic techniques for the analysis of matrix
metalloproteinases and their inhibitors. Biotechniques. 38:73–83.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
He X, Yan YL, DeLaurier A and Postlethwait
JH: Observation of miRNA gene expression in zebrafish embryos by in
situ hybridization to microRNA primary transcripts. Zebrafish.
8:1–8. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lineweaver H and Burk D: The determination
of enzyme dissociation constants. J Am Chem Soc. 56:658–666.
1934.
|
|
21
|
Guleria S, Walia A, Chauhan A and Shirkot
CK: Optimization of milk-clotting enzyme production by Bacillus
amyloliquefaciens SP1 isolated from apple rhizosphere.
Bioresour Bioprocess. 3(30)2016.
|
|
22
|
Jacob M, Jaros D and Rohm H: Recent
advances in milk clotting enzymes. Int J Dairy Technol. 64:14–33.
2011.
|
|
23
|
Ahamed A, Singh A and Ward OP: Chymostatin
can combine with pepstatin to eliminate extracellular protease
activity in cultures of Aspergillus niger NRRL-3. J Ind
Microbiol Biotechnol. 34:165–169. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shata HM: Extraction of milk-clotting
enzyme produced by solid state fermentation of Aspergillus
oryzae. Pol J Microbiol. 54:241–247. 2005.PubMed/NCBI
|
|
25
|
Merheb-Dini C, Gomes E, Boscolo M and da
Silva R: Production and characterization of a milk-clotting
protease in the crude enzymatic extract from the newly isolated
Thermomucor indicae-seudaticae N31:(Milk-clotting protease
from the newly isolated Thermomucor indicae-seudaticae N31).
Food Chem. 120:87–93. 2010.
|
|
26
|
Qasim F, Diercks-Horn S, Gerlach D,
Schneider A and Fernandez-Lahore HM: Production of a novel
milk-clotting enzyme from solid-substrate Mucor spp. culture. J
Food Sci. 87:4348–4362. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kaya B, Wijayarathna ERKB, Yüceer YK,
Agnihotri S, Taherzadeh MJ and Sar T: The use of cheese whey powder
in the cultivation of protein-rich filamentous fungal biomass for
sustainable food production. Front Sustain Food Syst.
8(1386519)2024.
|
|
28
|
Balachandran C, Vishali A, Nagendran NA,
Baskar K, Hashem A and Abd Allah EF: Optimization of protease
production from Bacillus halodurans under solid state
fermentation using agrowastes. Saudi J Biol Sci. 28:4263–4269.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
El-Bendary MA, Moharam ME and Ali TH:
Efficient immobilization of milk clotting enzyme produced by
Bacillus sphaericus. Pol J Food Nutr Sci. 59:67–72.
2009.
|
|
30
|
Bensmail S, Mechakra A and Fazouane-Naimi
F: Optimization of milk-clotting protease production by a local
isolate of Aspergillus niger ffb1 in solid-state
fermentation. J Microbiol Biotech Food Sci. 4:467–472. 2015.
|
|
31
|
Yang J, Teplyakov A and Quail JW: Crystal
structure of the aspartic proteinase from Rhizomucor miehei
at 2.15 A resolution. J Mol Biol. 268:449–459. 1997.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Merheb-Dini C, Garcia GAC, Penna ALB,
Gomes E and Da Silva R: Use of a new milk-clotting protease from
Thermomucor indicae-seudaticae N31 as coagulant and changes
during ripening of Prato cheese. Food Chem. 130:859–865. 2012.
|
|
33
|
Krishna C and Nokes SE: Influence of
inoculum size on phytase production and growth in solid-state
fermentation by Aspergillus niger. Transactions of the ASAE.
44:1031–1036. 2001.
|
|
34
|
Hajji M, Rebai A, Gharsallah N and Nasri
M: Optimization of alkaline protease production by Aspergillus
clavatus ES1 in Mirabilis jalapa tuber powder using statistical
experimental design. Appl Microbiol Biotechnol. 79:915–923.
2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun Q, Wang XP, Yan QJ, Chen W and Jiang
ZQ: Purification and characterization of a chymosin from
Rhizopus microsporus var. rhizopodiformis. Appl
Biochem Biotechnol. 174:174–185. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang Y, Hu J, Wang J, Liu C, Liu X, Sun
J, Song X and Wu Y: Purification and characteristics of a novel
milk-clotting metalloprotease from Bacillus velezensis
DB219. J Dairy Sci. 106:6688–6700. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Preetha S and Boopathy R: Purification and
characterization of a milk clotting protease from Rhizomucor
miehei. World J Microb Biotechnol. 13:573–578. 1997.
|
|
38
|
Duan Y, Katrolia P, Zhong A and Kopparapu
NK: Production, purification and characterization of a novel
antithrombotic and anticoagulant serine protease from food grade
microorganism Neurospora crassa. Prep Biochem Biotechnol.
52:1008–1018. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Takyu Y, Asamura T, Okamoto A, Maeda H,
Takeuchi M, Kusumoto KI, Katase T, Ishida H, Tanaka M and Yamagata
Y: A novel milk-clotting enzyme from Aspergillus oryzae and
A. luchuensis is an aspartic endopeptidase PepE presumed to
be a vacuolar enzyme. Biosci Biotechnol Biochem. 86:413–422.
2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hagemeyer K, Fawwal I and Whitaker JR:
Purification of protease from the Fungus Endothia
parasitica. J Dairy Sci. 51:1916–1922. 1968.
|
|
41
|
Arima K, Yu J, Iwasaki S and Tamura G:
Milk-clotting enzyme from microorganisms: V. Purification and
crystallization of Mucor rennin from Mucor pusillus
var. Lindt. Appl Microbiol. 16:1727–1733. 1968.
|
|
42
|
Nájera AI, De Renobales M and Barron LJR:
Effects of pH, temperature, CaCl2 and enzyme concentrations on the
rennet-clotting properties of milk: A multifactorial study. Food
Chem. 80:345–352. 2003.
|
|
43
|
Wang X, Kang Y, Gao L, Zhao Y, Gao Y, Yang
G and Li S: The effect of salt reduction on the microbial community
structure and metabolite composition of cheddar cheese. Foods.
13(4184)2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mane A and McSweeney PLH: Proteolysis in
Irish farmhouse Camembert cheese during ripening. J Food Biochem.
44(e13101)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang Y, Aziz T, Hu G, Liu J, Min Z,
Zhennai Y, Alharbi M, Alshammari A and Alasmari AF: Production,
purification, and characterization of a novel milk-clotting
metalloproteinase produced by Bacillus velezensis YH-1
isolated from traditional Jiuqu. LWT. 184(115080)2023.
|
|
46
|
Mamo A and Balasubramanian N: Calf rennet
production and its performance optimization. J Appl Nat Sci.
10:247–252. 2018.
|















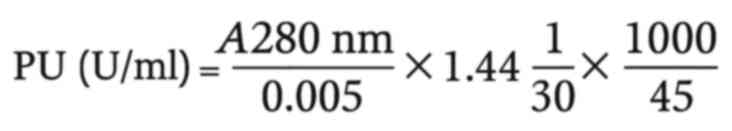
![Elution profile of milk-clotting
enzyme from Penicillium purpurescens purified by acetone
fractionation, then DEAE-cellulose ion-exchange chromatography.
Protein content was monitored at 280 nm, and milk-clotting activity
was assayed in collected fractions. Active MCE fractions were
observed at (NaCl concentration range] 0.05 to 0.1 mM), indicating
successful separation from non-active proteins.](/article_images/wasj/7/6/wasj-07-06-00399-g06.jpg)