Introduction
The reduced bioactivity of arterial
endothelial-derived nitric oxide (NO) has been demonstrated to be
involved in the pathogenesis of atherosclerosis, which leads to
hypertension (1). Persistent
hypertension is acutely harmful to highly vascularized organs, in
particular, the brain. Endothelial dysfunction with the gradual
loss of NO bioavailability in the brain is accelerated by
progressive atherosclerosis, which impairs general cognitive
function, but also decreases the specific cognitive domains, such
as memory, visual or spatial perception and reaction time to
stimuli.
An epidemiological link between hypertension induced
by NO-deficiency and Alzheimer's disease has been established in
preclinical models (2).
Reportedly, hypertension exacerbates the Alzheimer-like pathology
in amyloid protein mouse models (2), suggesting that impaired NO
bioavailability contributes to the pathophysiology of Alzheimer's
disease and that cognitive impairment appears to be dependent on
hypertension-associated NO-deficiency.
The link between cognitive impairment and low NO
bioavailability is further exacerbated by an increase in natural
endothelial nitric oxide synthase (eNOS) inhibitors in pathological
conditions such as hypertension, resulting in a decrease in the
production of NO in the brain (3).
However, the reversal of this condition by dietary NO donors has
been shown. A series of studies have reported that almost 1,000
healthy seniors who had a daily helping of NO-potent foods had a
slower rate of cognitive decline (4). Brain maps have revealed a higher
cerebral blood flow to areas of the brain involved with working or
short-term memory following the consumption NO-potent natural foods
(5).
A growing number of preclinical and human studies
have suggested that aged-garlic extract (AGE) possesses
anti-hyperlipidemic, anti-platelet, anti-oxidative,
anti-inflammatory, anti-atherosclerotic and cardio-brain protective
activity, which are all likely mediated by reversing endothelial
dysfunction with concomitant increase in restoring cNOS and NO
bioavailability (6).
AGE appears to attenuate the progression of
atherosclerotic and, independently, stimulate eNOS expression in
endothelial cells, without triggering inducible NO synthase (iNOS),
a potentially inflammatory response (7).
AGE may stimulate eNOS in advance of reversing
atherosclerotic plaques, which, in turn, increases NO
bioavailability. Therefore, AGE may restore NO bioavailability by
targeting multiple levels, as follows: i) Attenuating plaque
formation, thereby, reversing endothelial dysfunction, resulting in
eNOS expression; ii) directly stimulating eNOS, independent of
plaque regression; iii) inhibiting or not augmenting a
NO-associated inflammatory response associated with progressive
atherosclerotic lesions, i.e., AGE selectively upregulating eNOS,
not iNOS.
Previous research has demonstrated an
anti-hypertensive effect on both systolic and diastolic lowering
with daily AGE consumption (6).
The antihypertensive properties of AGE are likely the result of
restoring endothelial function and stimulating eNOS, which in turn
promotes vasodilation and thus, reduces blood pressure (7).
Nitric oxide generated by L-arginine metabolism by
eNOS is a critical regulator of vascular function. Recent research
has also shown that NO can regulate intracellular heme allocation
in a concentration-dependent manner, impacting redox-sensitive
proteins involved in immunity and cellular metabolism (8).
As previously mentioned, AGE may be unique among
orally active ‘NO boosters’ since it may target both the lesion by
slowing it's progression and independently triggering eNOS in the
endothelium despite the damage incurred by the advancing atheroma.
Furthermore, it has been demonstrated in vivo that AGE
selectively upregulates eNOS without eliciting a potentially
inflammatory iNOS response (8).
A growing number of studies have demonstrated the
beneficial effects of AGE on plaque progression, including
increases in NO bioavailability, plaque stability, lumen diameter
and fibrous cap thickness with a corresponding decrease in
cholesterol crystalline, oxidized low-density lipoprotein, arterial
stiffness, platelet activation among other nitric oxide-mediated
outcomes (7-10).
Arterial stiffness is an increasingly used metric to assess
cardiovascular risk (11-15).
A growing body of evidence suggests that garlic may
improve cognitive health by increasing NO bioavailability (6,16-18).
Increases in NO bioavailability have been shown in multiple studies
to enhance cerebral blood flow, particularly to those areas in the
brain associated with working memory. A previous preclinical study
suggested that garlic may improve spatial memory (18).
Materials and methods
Study design and study
participants
The present study was a single-center, randomized,
placebo-controlled, double-blind comparison of AGE (2,400 mg)
provided by Wakunaga of America Co. Ltd. compared to matching
placebo capsules for 12 weeks on endothelial function and brain
function. A total of 80 eligible subjects were enrolled using at a
1:1 ratio. Subjects who met the eligibility criteria were randomly
assigned to receive AGE or the placebo (cellulose based). Subjects
provided written informed consent after the nature and scope of the
investigation had been explained. The present study was approved by
the Institutional Review Board (IRB) of the Lundquist Institute.
All patients signed an informed consent prior to any study
procedures being informed. The study obtained ethics approval from
the WCG Institutional Review Board, study no. 132877, which acted
as the central IRB.
The inclusion criteria included an age between 30-75
years, patients with pre-hypertension, 121-140 systolic blood
pressure and 81-90 mmHg diastolic blood pressure or hypertension.
The exclusion criteria included any contraindication to AGE
including: Any unstable psychiatric, medical, or substance abuse
disorder that by the principal investigator precluded the subject's
participation in the study or was likely to affect the subject's
ability to complete the study, a history of malignancy within the
past 5 years (other than skin cancer) or any evidence of active
cancer requiring cancer chemotherapy, prior known myocardial
infarction, stroke or life-threatening arrhythmia within the prior
6 months; weight in excess of 350 pounds, NYHA Class II-IV heart
failure, as well as any known hypersensitivity to drugs.
Baseline information regarding risk factors for
atherosclerotic cardiovascular disease (systemic hypertension,
cigarette smoking, a sedentary lifestyle, a family history of
premature atherosclerosis, current medications, menopausal and
hormone replacement status in women, measures of obesity and chest
pain questionnaire) were collected. Baseline systemic and coronary
vascular function were obtained. Following randomization,
participants returned at 6-week intervals to assess compliance with
medication, testing and to receive an additional supply of
medicine. At 12 weeks follow-up, endothelial function, inflammatory
biomarkers, and repeat cognitive testing were performed.
Upon randomization into the two groups, the
participants in the control and AGE group each received four
capsules daily. Both the investigators and participants were
blinded and medications were dispensed by a research pharmacy. Each
group was randomly assigned to one of the two treatment categories
in a double-blinded manner. Randomization occurred according to a
computer-generated randomization code.
Study endpoints
Upon assessing cognition, the Montreal Cognitive
Assessment (MOCA) tool was utilized. This is a well-validated tool,
used repeatedly to assess cognitive function and changes over time.
MOCA was used at week 0, and again at week 6 and week 12 (following
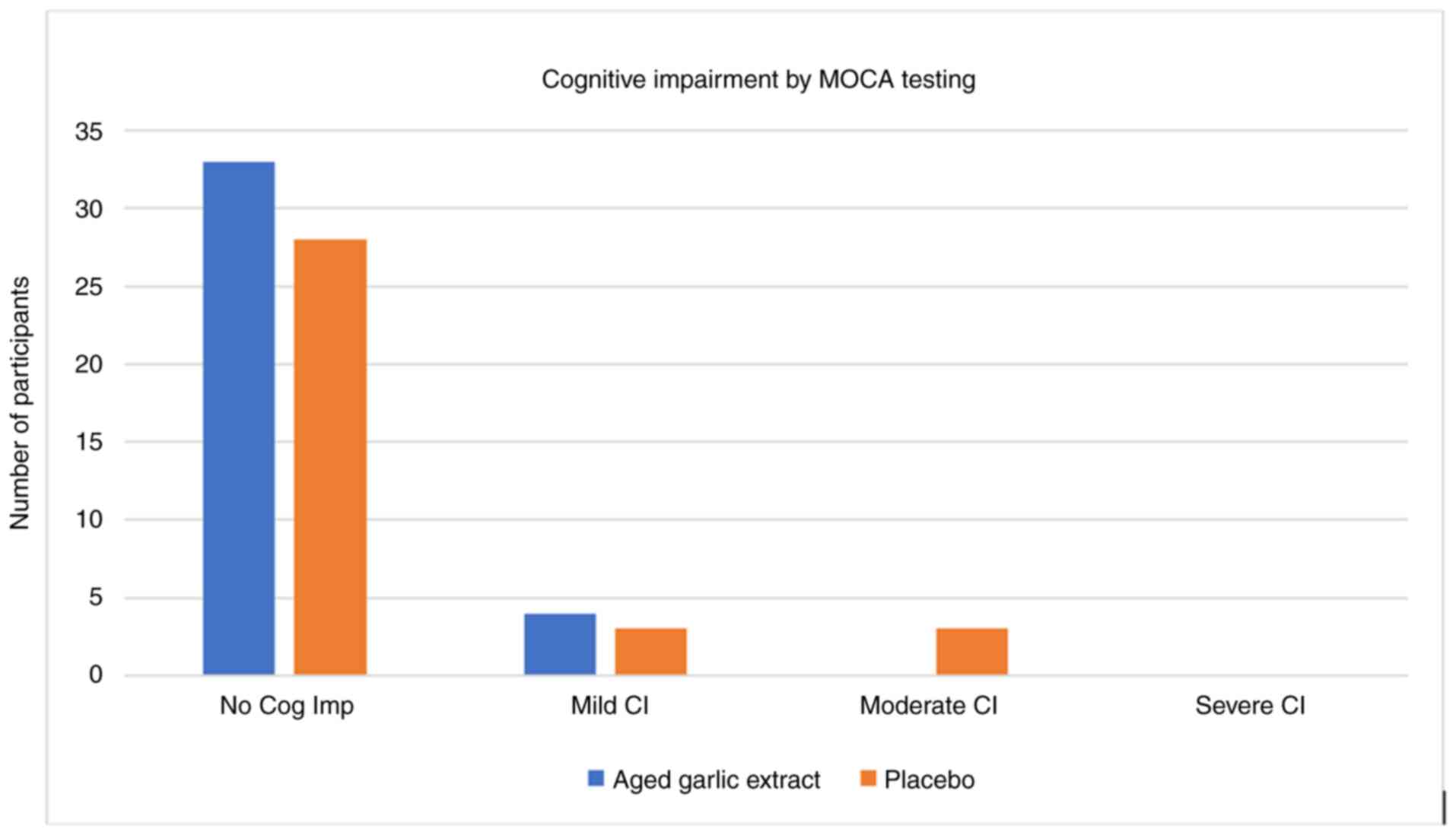
randomization into either the placebo or AGE groups) (Fig. 1).
The domains of this test include: Visuospatial;
naming; digit list; letter list; serial 7 subtraction; repeat;
fluency; abstraction; delayed recall; orientation. The results were
categorized as follows: 18-25 points, mild cognitive impairment;
10-17 points, moderate cognitive impairment; and <10 points,
severe cognitive impairment.
Different tests will provide different insights. Of
course, these types of tests can be influenced by a number of
factors, including the time of day, amount of sleep, sedentary
behavior vs. physically active, medical condition, emotional state
and stress among other lifestyle choices, in particular dietary
choices, which is no different than a blood pressure reading. Thus,
all tests (baseline, 6 and 12 weeks) were administered under the
same conditions, including the same room, by the same investigator
and same time of day.
Statistical analysis
Data were analyzed using intention-to-treat
principles, with the study subjects analyzed by treatment group
assigned regardless of study drug adherence. A sensitivity analysis
was performed for those adhering to interventions for >80% of
the duration of the study. Differences in baseline characteristics
between groups were analyzed using independent t-tests for normally
distributed continuous traits and the Chi-squared test for
categorical traits. Outcomes were analyzed between the AGE and
placebo group using one-way ANOVA. Least-squares means with 95% CIs
and between-group differences were reported. A descriptive
sub-analysis is presented with triglyceride levels categorized by
<150, 150-199 and >200 mgdl. All analyses were performed
using SAS for Windows, version 9.4 (SAS Institute). All statistical
tests report two-sided P-values. A P-value <0.05 was considered
significant to indicate a statistically significant difference.
Results
A total of 80 subjects were enrolled in a
prospective, randomized, placebo-controlled clinical trial, with 72
completing both baseline and the 12-week follow-up visit. The study
was conducted according to the CONSORT (Consolidate Standards of
Reporting Trials) guidelines and statement. The mean age of the
participants was 53.0±12.3 years with 45 (63%) subjects being
female. The baseline characteristics, stratified by arm (AGE group,
n=37; and the placebo group, n=35) of the study participants were
well balanced (Table I).
 | Table IBaseline characteristics of the
subjects in the aged garlic extract trial (n=72). |
Table I
Baseline characteristics of the
subjects in the aged garlic extract trial (n=72).
| | All subjects (n=72)
Mean or count (Std or %) | Aged garlic extract
(n=37) Mean or count (Std or %) | Placebo (n=35) Mean
or count (Std or %) | P-valuea,b |
|---|
| Age, years | 53.0 (±12.3) | 51.2 (±12.4) | 55.0 (±12.1) | 0.194 |
| Body mass index | 31.6 (±6.9) | 31.9 (±6.3) | 31.2 (±7.6) | 0.642 |
| Sex | | | | 0.301 |
|
Female | 45 (63%) | 21 (57%) | 24 (69%) | |
|
Male | 27 (38%) | 16 (43%) | 11 (31%) | |
| Heart rate | 71.7 (±8.6) | 72.0 (±8.1) | 71.4 (±9.3) | 0.771 |
| Systolic blood
pressure | 130.8 (±10.5) | 129.8 (±10.1) | 131.9 (±11.0) | 0.407 |
| Diastolic blood
pressure | 82.0 (±7.0) | 82.9 (±7.2) | 81.0 (±6.7) | 0.238 |
| Diabetes
mellitus | | | | 0.345 |
|
Yes | 19 (26%) | 8 (22%) | 11 (31%) | |
|
No | 43 (74%) | 29 (78%) | 24 (69%) | |
| Hypertension | | | | 0.231 |
|
Yes | 20 (28%) | 8 (22%) | 12 (34%) | |
|
No | 52 (72%) | 29 (78%) | 23 (66%) | |
| Hyperlipidemia | | | | 0.360 |
|
Yes | 29 (40%) | 13 (35%) | 16 (46%) | |
|
No | 43 (60%) | 24 (65%) | 19 (54%) | |
| Past smoking | | | | 0.660 |
|
Yes | 11 (15%) | 5 (15%) | 6 (13%) | |
|
No | 61 (85%) | 32 (85%) | 29 (87%) | |
| Total
cholesterol | 182.8 (±50.4) | 189.7 (±51.0) | 175.6 (±49.5) | 0.246 |
| HDL-C | 53.3 (±15.7) | 54.4 (±17.5) | 52.1 (±13.7) | 0.549 |
| LDL-C | 107.3 (±43.6) | 112.3 (±46.0) | 101.9 (±40.9) | 0.320 |
| Triglycerides | 122.9 (±56.1) | 128.6 (±52.6) | 116.8 (±59.7) | 0.381 |
There was a significant effect of AGE on
triglyceride levels. The triglyceride levels in the placebo group
at baseline were 116.8±59.7 mg/dl vs. 124.9±74.0 mg/dl at the study
endpoint, whereas in the AGE group, baseline values were 128.6±52.6
mg/dl, which decreased to 117.6±46.7 mg/dl. The AGE group exhibited
a mean decrease of 14.7±46.4 vs. an increase in the placebo group
of 8.6±41.8, for a between-group difference of 23.4±44.2 mg/dl
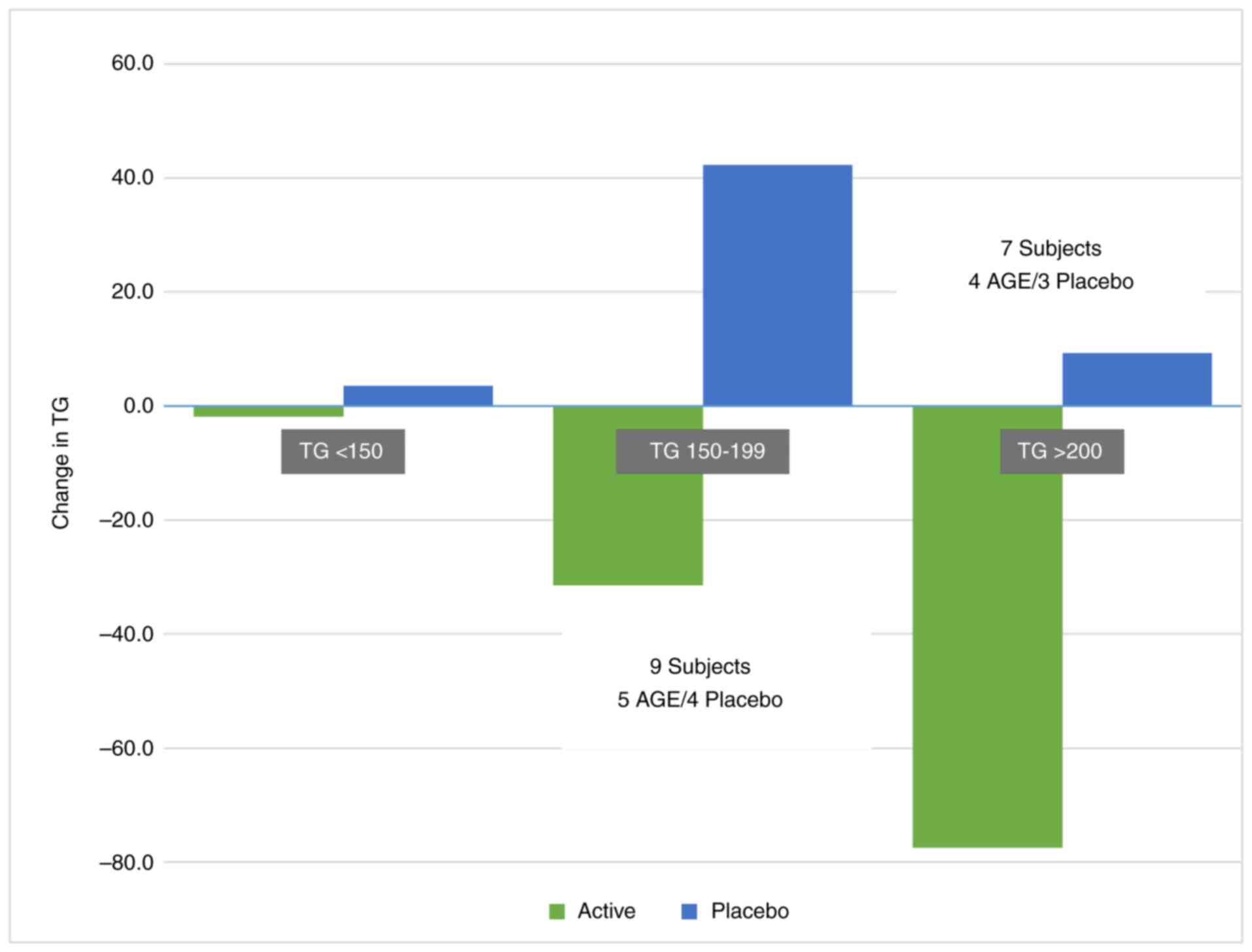
(P=0.032) (Fig. 2).
In those with baseline triglyceride levels of ≤150
mg/dl [AGE group, n=27 (92.2±26.8 mg/dl) and the placebo group,
n=25 (119.7±64.4 mg/dl)], the AGE group exhibited a decrease of
-1.9±37.8 mg/dl vs. an increase in the placebo group of 3.6±23.3
mg/dl. In those with baseline triglyceride levels of 150-200 mg/dl
[AGE group, n=5 (166.2±12.8 mg/dl) and the placebo group, n=4
(172.3±15.5 mg/dl)], the AGE group exhibited a decrease of
-31.4±26.3 mg/dl vs. increase in the placebo group.
For assessing cognition, the MOCA tool was utilized.
At the conclusion of the trial, there were more patients with no
cognitive impairment in the AGE arm (92 vs. 82%), similar numbers
in the mild cognition arm (8 vs. 9%) and the AGE group had no
patients with moderate cognitive impairment (0 vs. 9% for tge
placebo). Of note, more patients had no cognitive impairment and 0
patients had moderate cognitive impairment in the AGE group. From
visit 1 to the 12-week visit, MOCA increased by 3 points in the AGE
group from 27 to 30, while it did not increase in the placebo group
(28 to 28) (Fig. 1). A breakdown
by scores of the test is presented in Table II.
 | Table IIFinal visit: Achieving maximum score
per variable of the Montreal Cognitive Assessment (MOCA) tool. |
Table II
Final visit: Achieving maximum score
per variable of the Montreal Cognitive Assessment (MOCA) tool.
| Variable | Active (n=37), count
(%) | Placebo (n=35), count
(%) |
|---|
| Visuospatial
(1-5) | 28(76) | 18(51) |
| Naming (1-3) | 33(89) | 32(91) |
| DigitList (0-2) | 31(84) | 27(77) |
| LetterList
(0-1) | 37(100) | 34(97) |
| Serial7Subtraction
(0-3) | 31(84) | 30(86) |
| Repeat (0-2) | 35(95) | 32(91) |
| Fluency (0-1) | 33(89) | 27(77) |
| Abstraction
(0-2) | 37(100) | 32(91) |
| DelayedRecall
(0-5) | 26(70) | 20(57) |
| Orientation
(1-6) | 36(97) | 33(94) |
| MOCA total
score | IQR 30 | IQR 28 |
| (1-30) | (28,30) | (26,30) |
Discussion
Garlic (Allium sativum L.) is deemed to have
a variety of therapeutic applications, including platelet
aggregation inhibition, the reduction of cholesterol and the
control of blood pressure. These therapeutic actions of garlic
parallel NO physiological effects (18). The present study demonstrated that
two potential actions of NO, brachial artery reactivity and brain
function, which are improved with use of AGE, substantiating
earlier research on garlic with brain function and garlic with
endothelial function. The present study demonstrated that AGE led
to an improvement in cognitive function, as demonstrated by
improvements in MOCA, as well as independent improvements in
triglyceride levels. In the present small study, AGE improved
cognition and triglycerides, consistent with previous research
(1). AGE imparts benefits through
multiple mechanisms. It has previously been demonstrated that AGE
leads to improvements in lipids, inflammation and triglycerides,
all of which have been shown to impact cardiovascular outcomes,
including flow mediated dilation and lipid values, similar to the
present study (1,19-23).
AGE improved cognitive function in the present short-term (12-week)
study. However, longer studies, including studies performed on
individuals with more baseline cognitive impairment, are
warranted.
The limitations of the present study are the small
sample size, short duration (12 weeks), the use of a single
cognitive tool and the lack of biomarker assessment for NO
directly. Given the known benefits of AGE on hypertension, the
present study opted to only include those patients with
pre-hypertension. However, generalizability is limited due to the
inclusion of mainly pre-hypertensive subjects. Further larger and
longer studies are warranted to fully assess the physiological
changes that explain cognitive improvements with AGE.
Acknowledgements
The present study is part of a clinical trial
(ClinicalTrials.Gov-NCT01534910).
Funding
Funding: The present study was funded by Wakunaga Pharmaceutical
Co., Ltd.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MJB was the principal investigator. MB, HH, NK and
MM assisted with the recruitment of the participants. KA, DR, TE,
AG and MD assisted with the testing of the participants. AK
performed the statistical analysis. All authors worked on final
version and have read and approved the final manuscript. MB and AK
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (IRB) of the Lundquist Institute. All patients signed
an informed consent prior to any study procedures being informed.
The study obtained ethics approval from the WCG Institutional
Review Board, study no. 1328797, which acted as the central
IRB.
Patient consent for publication
Not applicable.
Competing interests
Financial support was received from Wakunaga
Pharmaceutical Co., Ltd.
References
|
1
|
Beverly JK and Budoff MJ: Atherosclerosis:
Pathophysiology of insulin resistance, hyperglycemia,
hyperlipidemia, and inflammation. J Diabetes. 12:102–104.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Perrotta M, Lembo G and Carnevale D:
Hypertension and dementia: Epidemiological and experimental
evidence revealing a detrimental relationship. Int J Mol Sci.
17(347)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nakhaee S, Azadi R, Salehinia H, Moodi M,
Zarban A, Sharifi F, Khorashadeizadeh M and Farrokhfall K: The role
of nitric oxide, insulin resistance, and vitamin D in cognitive
function of older adults. Sci Rep. 14(30020)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Barnes LL, Dhana K, Liu X, Carie VJ,
Ventrelle J, Johnson K, Hollings CS, Bishop L, Laranjo N, Stubbs
BJ, et al: Trial of the MIND diet for prevention of cognitive
decline in older persons. N Engl J Med. 389:602–611.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Regan C, Heiland EG, Ekblom O, Tarassova
Ö, Kjellenberg K, Larsen FJ, Walltott H, Fernström M, Nyberg G,
Ekblom MM and Helgadóttir B: Acute effects of nitrate and breakfast
on working memory, cerebral blood flow, arterial stiffness, and
psychological factors in adolescents: Study protocol for a
randomised crossover trial. PLoS One. 18(e0285581)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Varshney R and Budoff MJ: Garlic and heart
disease. J Nutr. 146:416S–421S. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zeb I, Ahmadi N, Flores F and Budoff MJ:
Randomized trial evaluating the effect of aged garlic extract with
supplements versus placebo on adipose tissue surrogates for
coronary atherosclerosis progression. Coron Artery Dis. 29:325–328.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Biswas P, Palazzo J, Schlanger S, Jayaram
DT, Islam S, Page RC and Stuehr DJ: Visualizing mitochondrial heme
flow through GAPDH in living cells and its regulation by NO. Redox
Biol. 71(103120)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hutchins E, Shaikh K, Kinninger A,
Cherukuri L, Birudaraju D, Mao SS, Nakanishi R, Almeida S,
Jayawardena E, Shekar C, et al: Aged garlic extract reduces left
ventricular myocardial mass in patients with diabetes: A
prospective randomized controlled double-blind study. Exp Ther Med.
19:1468–1471. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Almeida SO, Honoris L, Defranco A, Port S,
Li D, Nasir K, Kronmal R, Barr RG and Budoff M: Reliability of CAC
scoring on nongated compared with gated cardiac CT scans from MESA.
JACC Cardiovasc Imaging. 13:177–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Itano S, Yano Y, Nagasu H, Tomiyama H,
Kanegae H, Makino H, Higashi Y, Kobayashi Y, Sogawa Y, Satoh M, et
al: Association of arterial stiffness with kidney function among
adults without chronic kidney disease. Am J Hypertens.
33:1003–1010. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Osawa K, Nakanishi R, Miyoshi T, Rahmani
S, Ceponiene I, Nezarat N, Kanisawa M, Qi H, Jayawardena E, Kim N,
et al: Correlation of arterial stiffness with left atrial volume
index and left ventricular mass index in young adults: evaluation
by coronary computed tomography angiography. Heart Lung Circ.
28:932–938. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Matsumoto S, Nakanishi R, Luo Y, Kim M,
Alani A, Nezarat N, Dailing C and Budoff MJ: The relationship
between cardio-ankle vascular index and subclinical atherosclerosis
evaluated by cardiac computed tomographic angiography. Clin
Cardiol. 40:549–553. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Namekata T, Suzuki K, Ishizuka N and
Shirai K: Establishing baseline criteria of cardio-ankle vascular
index as a new indicator of arteriosclerosis: A cross-sectional
study. BMC Cardiovasc Disord. 11(51)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu H, Cui H, Han W, Ye L, Qiu W, Yang H,
Zhang C, Guo X and Mao G: A cutoff point for arterial stiffness
using the cardio-ankle vascular index based on carotid
arteriosclerosis. Hypertens Res. 36:334–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abd Alamir M, Goyfman M, Johnson D, Liu Y,
Dabbous F, Chaus A and Budoff M: The relationship between
endothelial function and aortic valve calcification: Multi-ethnic
study of atherosclerosis. Atherosclerosis. 280:155–165.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Larijani VN, Ahmadi N, Zeb I, Khan F,
Flores F and Budoff M: Beneficial effects of aged garlic extract
and coenzyme Q10 on vascular elasticity and endothelial function:
The FAITH randomized clinical trial. Nutrition. 29:71–75.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Morihara N, Sumioka I, Moriguchi T, Uda N
and Kyo E: Aged garlic extract enhances production of nitric oxide.
Life Sci. 71:509–517. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahmadi N, Hajsadeghi F, Nabavi V, Olango
G, Molla M, Budoff M, Vaidya N, Quintana J, Pynoos R, Hauser P and
Yehuda R: The long-term clinical outcome of posttraumatic stress
disorder with impaired coronary distensibility. Psychosom Med.
80:294–300. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shaikh K, Cherukuri L, Birudaraju
Nakanishi R, Almeida S, Jayawardena E, Shekar C, Flores F, Hamal S,
Sheikh S, et al: Aged garlic extract reduces low attenuation plaque
in coronary arteries of patients with diabetes in a prospective
randomized double-blind study. J Am Coll Cardiol.
73(1645)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Musunuru K, Nasir K, Pandey S, Campbell
CC, Carvalho JAM, Meneghello R, Budoff MJ, Blumenthal RS and Santos
RD: A synergistic relationship of elevated low-density lipoprotein
cholesterol levels and systolic blood pressure with coronary artery
calcification. Atherosclerosis. 200:368–373. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Budoff MJ, Oudiz RJ, Zalace CP, Bakhsheshi
H, Goldberg SL, French WJ, Rami TG and Brundage BH: Intravenous
three-dimensional coronary angiography using contrast enhanced
electron beam computed tomography. Am J Cardiol. 83:840–845.
1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shaikh K, Kinninger A, Cherukuri L,
Birudaraju D, Nakanishi R, Almeida S, Jayawardena E, Shekar C,
Flores F, Hamal S, et al: Aged garlic extract reduces low
attenuation plaque in coronary arteries of patients with diabetes:
A randomized, double-blind, placebo-controlled study. Exp Ther Med.
19:1457–1461. 2020.PubMed/NCBI View Article : Google Scholar
|