Introduction
Helicobacter pylori (H. pylori) is a
Gram-negative bacteria that normally inhabits the human stomach
(1). It is known that H.
pylori augments the risk of developing gastric diseases, such
as gastric ulcers, gastric cancers and gastric mucosa-assisted
lymphoid tissue lymphoma by infecting the gastric mucosa epithelial
cells (2). It has been reported
that approximately half of the population worldwide is infected
with H. pylori, with a particularly high prevalence rate in
African regions (3). The
prevalence rate of H. pylori is 30-40% in developed
countries and ≥80% in developing countries, suggesting that
socioeconomic conditions are markedly associated with H.
pylori infection (4). H.
pylori infection occurs during childhood via oral transmission
as an asymptomatic infection (5).
It may be challenging to demonstrate the
colonization of H. pylori in the oral cavity (6). It has been reported that H.
pylori DNA can be detected in inflamed dental pulp and
subgingival dental plaque in the oral cavity, as well as in the
gastric mucosa (6-8).
A recent meta-analysis revealed that gastric H. pylori
infection is more frequently found in patients with current oral
H. pylori infection than in patients without oral H.
pylori infection (9).
Additionally, it has been reported that treatment for oral H.
pylori infection is effective for the successful eradication of
gastric H. pylori (10),
indicating that there is a significant relationship between oral
H. pylori and gastric H. pylori infection. Therefore,
the oral cavity may be an important reservoir for gastric H.
pylori. It is speculated that poor oral hygiene and periodontal
inflammation may be linked to the prevalence of oral H.
pylori and gastric H. pylori infection. However, the
association between the prevalence of oral H. pylori and
oral health has not yet been fully investigated in Japanese older
adults. Therefore, the aim of the present study was to investigate
the association between the presence of oral H. pylori DNA
and dental hygiene condition in older adults.
Patients and methods
Study participants
The present study targeted patients aged ≥65 years
who visited the Oral Health Department at Hiroshima University
Hospital from April, 2021 to February, 2023. Patients with
immunodeficiency (i.e., post-operative inpatients, cancer patients
receiving chemotherapy and patients with immune deficiency
disorders) were excluded (n=0). Finally, 98 older patients (28
males, 70 females; median age, 75 years; range, 65-91 years) were
included in the present study. The present cross-sectional study
was a part of a general research project on the association between
oral microbiome and oral health approved by the Ethics Committee of
Hiroshima University (Approval no. E-1115). Patients who agreed to
participate in this study signed an informed consent form. Clinical
variables (i.e., participants' age, gender, lifestyle-related
diseases, number of remaining teeth and denture use) were obtained
from medical records of the participants. Periodontal pocket depth
and bleeding on probing (BOP) were investigated at six sites
(mesio-buccal, mid-buccal, disto-buccal, disto-lingual, mid-lingual
and mesio-lingual sites) for each tooth. The accumulation of dental
plaque was evaluated using a modified O'Leary Plaque Control Record
by assessing six surfaces (mesio-buccal, mid-buccal, disto-buccal,
disto-lingual, mid-lingual and mesio-lingual surfaces) of each
tooth, as previously described (11).
Oral sample collection method and DNA
extraction
Swab samples were collected from the tongue surface
using a sterile disposable Orcellex® Brush (Rovers
Medical Devices). After the collected samples were dissolved in
cell lysis buffer, DNA was extracted and purified using a PureLink™
Microbiome DNA Purification kit (Invitrogen; Thermo Fisher
Scientific Inc.) in accordance with the manufacturer's
instructions.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed in the Thermal Cycler
Dice® Real Time System III (Takara Bio, Inc.) using
THUNDERBIRD SYBR qPCR mix (Toyobo Co., Ltd.). The amplification
cycle consisted of 95˚C for 2 min, followed by 50 cycles of 95˚C
for 1 min, 55˚C for 1 min and 72˚C for 1 min, and 72˚C for 2 min. A
primer pair targeting the H. pylori ureA gene was used in
the present study, as it has high specificity for detecting H.
pylori DNA, as described in a previous study (8). The sequence of primers for H.
pylori was as follows: forward, 5'-ATGAAACTCACCCCAAAAGA-3' and
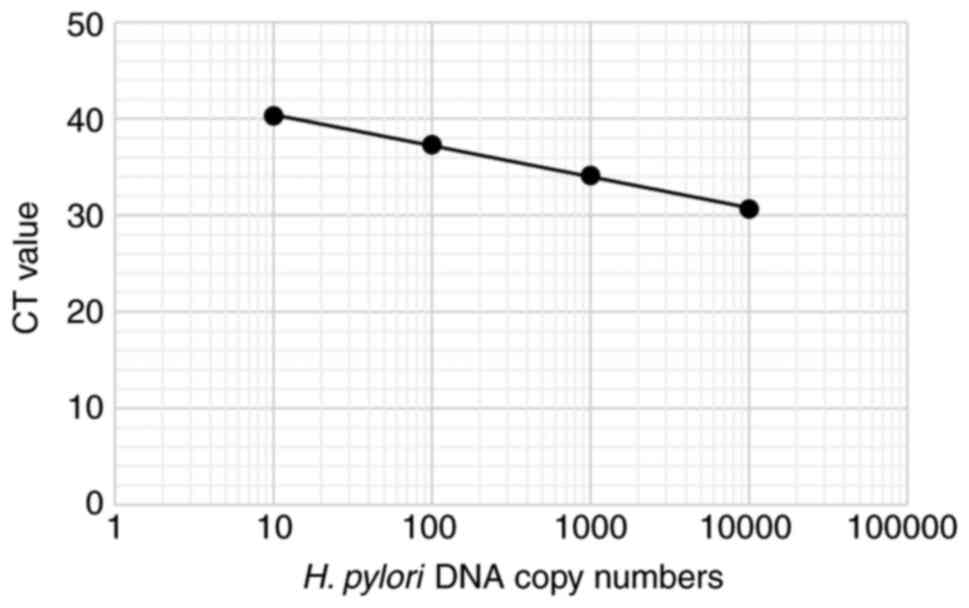
reverse, 5'-TTCACTTCAAAGAAATGGAAGTGTGA-3' (8). A standard curve for H. pylori
was generated using 10-fold serially diluted samples of the
AMPLIRUN® HELICOBACTER PYLORI DNA CONTROL (10,000-20,000
H. pylori DNA copies/µl; Vircell) (Fig. 1). A no-template control was used as
the negative control in qPCR analysis.
Statistical analysis
Statistical analysis was conducted using SPSS
software, version 24.0 (IBM Corp.). Logistic regression analysis
was performed to examine the association between H. pylori
as a dependent variable and clinical variables as independent
variables. Clinical parameters with a P-value of <0.2 through
univariate analysis were used as independent variables for logistic
regression analysis. Continuous variables are expressed as the
median and interquartile range (IQR). P-values <0.05 were
considered to indicate statistically significant differences.
Results
Association between oral H. pylori and
clinical variables
The association between the presence of oral H.
pylori DNA and clinical variables is presented in Table I. Among the 98 participants, 7
participants (7.1%) were positive for oral H. pylori DNA.
Participants in their 70s exhibited a greater positive rate of oral
H. pylori DNA (12.5%) than the other age groups.
Additionally, female participants exhibited a higher positive rate
of oral H. pylori DNA (8.6%) than male participants (3.6%).
There was no significant association between the presence of oral
H. pylori DNA and clinical variables such as age, sex,
lifestyle-related diseases, number of remaining teeth, or denture
use.
 | Table IClinical characteristics of the study
participants and their association with the presence of oral H.
pylori DNA. |
Table I
Clinical characteristics of the study
participants and their association with the presence of oral H.
pylori DNA.
| Clinical variables
(n) | H.
pylori-negative (n=91) | H.
pylori-positive (n=7) | P-value |
|---|
| Age, median
(IQR) | 75 (11.0) | 75 (5.0) | 0.86a |
| Age group, n
(%) | | | |
|
65-69 | 20 (22%) | 0 (0%) | 0.3b |
|
70-79 | 42 (46.2%) | 6 (85.7%) | |
|
80-89 | 27 (29.7%) | 1 (14.3%) | |
|
90-99 | 2 (2.2%) | 0 (0%) | |
| Sex, n (%) | | | |
|
Male | 27 (29.7%) | 1 (14.3%) | 0.67b |
|
Female | 64 (70.3%) | 6 (85.7%) | |
| Hypertension, n
(%) | | | |
|
Yes | 22 (24.2%) | 3 (42.9%) | 0.37b |
|
No | 69 (75.8%) | 4 (57.1%) | |
| Diabetes, n
(%) | | | |
|
Yes | 14 (15.4%) | 0 (0%) | 0.59b |
|
No | 77 (84.6%) | 7 (100%) | |
| Dyslipidemia, n
(%) | | | |
|
Yes | 20 (22%) | 2 (28.6%) | 0.65b |
|
No | 71 (78%) | 5 (71.4%) | |
| Number of remaining
teeth, median (IQR) | 24 (7.0) | 27 (8.0) | 0.27a |
| Denture user, n
(%) | | | |
|
Yes | 43 (47.3 %) | 2 (28.6%) | 0.45b |
|
No | 48 (52.7%) | 5 (71.4%) | |
Association between oral H. pylori and
the oral health condition
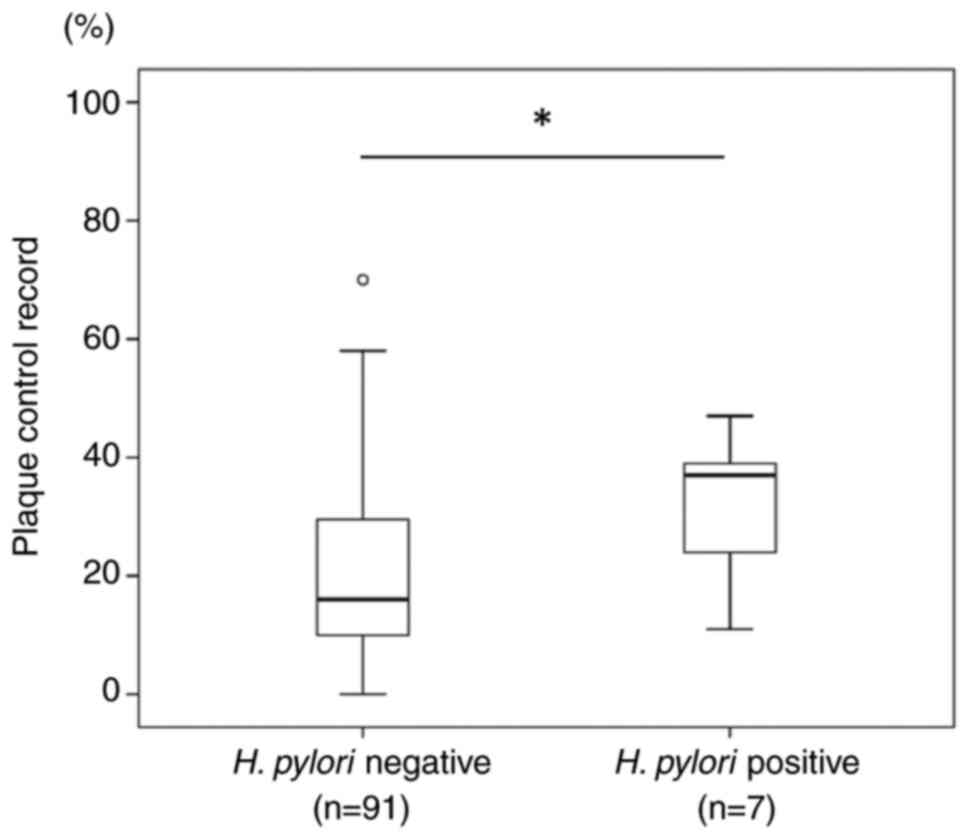
The participants who were positive for oral H.
pylori exhibited higher median plaque control record scores
(37%) than those who were negative for oral H. pylori (16%)
(Fig. 2). A significant difference
in plaque control record scores was found between the oral H.
pylori-negative and -positive participants (P=0.04). As regards
the association between oral H. pylori and clinical
periodontal conditions, the oral H. pylori-positive
participants exhibited a higher rate of ≥4 mm deep periodontal
pockets with BOP (85.7%) than the participants who were negative
for oral H. pylori (49.5%) (Table II). Additionally, the oral H.
pylori-positive participants exhibited a higher rate of ≥6 mm
deep periodontal pockets with BOP (28.6%) than the participants who
were negative (15.4%) (Table II).
However, no significant association between oral H. pylori
and deep periodontal pockets with BOP was found. These results
indicate that oral H. pylori-positive participants may have
poorer dental hygiene than oral H. pylori-negative
participants.
 | Table IIPeriodontal condition of the study
participants and its association with the presence of oral H.
pylori DNA. |
Table II
Periodontal condition of the study
participants and its association with the presence of oral H.
pylori DNA.
| Clinical
periodontal variables | H.
pylori-negative (n=91) | H.
pylori-positive (n=7) | P-value |
|---|
| Probing pocket
depth, n (%) | | | |
|
<4
mm | 18 (19.8%) | 0 (0%) | 0.6a |
|
≥4 mm and
<6 mm | 44 (48.4%) | 4 (57.1%) | |
|
≥6 mm | 29 (31.9%) | 3 (42.9%) | |
| BOP (%) (IQR) | 5.0 (10.0) | 3.0 (12.0) | 0.78b |
| ≥4 mm periodontal
pocket with BOP, n (%) | | | |
|
Yes | 45 (49.5%) | 6 (85.7%) | 0.11a |
|
No | 46 (50.5%) | 1 (14.3%) | |
| ≥6 mm periodontal
pocket with BOP, n (%) | | | |
|
Yes | 14 (15.4%) | 2 (28.6%) | 0.32a |
|
No | 77 (84.6%) | 5 (71.4%) | |
Logistic regression analysis with oral
H. pylori as the dependent variable
Logistic regression analysis was conducted as
independent variables in univariate analysis (i.e., variables with
a P-value <0.2) and with oral H. pylori as the dependent
variable. The results of logistic regression analysis are presented
in Table III. There was no
significant association between oral H. pylori and ≥4 mm
deep periodontal pockets with BOP or plaque control record
score.
 | Table IIILogistic regression analysis with
oral H. pylori as the dependent variable. |
Table III
Logistic regression analysis with
oral H. pylori as the dependent variable.
| Clinical
variables | Odds ratio | 95% confidence
interval | P-value |
|---|
| ≥4 mm periodontal
pocket with BOP | 5.92 | 0.66-53.2 | 0.11 |
| Plaque control
record | 1.05 | 0.99-1.1 | 0.08 |
Correlation between oral H. pylori DNA
copy numbers and plaque control record scores
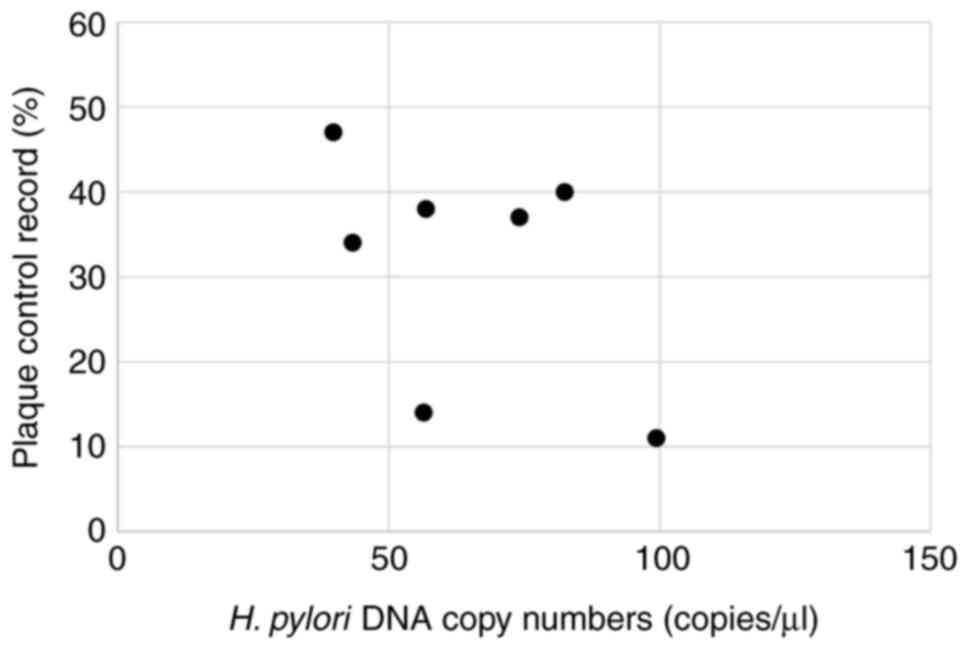
The present study then calculated the H.
pylori copy numbers per each 1 µl DNA sample from the
participants who were positive for oral H. pylori. The
median H. pylori copy number was 56.8 (IQR, 39.2) copies/µl.
The scatter plot illustrates correlation between oral H.
pylori DNA copy numbers and plaque control record scores
(Fig. 3). There was no significant
correlation between the oral H. pylori copy numbers and
plaque control record scores, as demonstrated in Table IV.
 | Table IVCorrelation between oral H.
pylori DNA copy numbers and plaque control record scores. |
Table IV
Correlation between oral H.
pylori DNA copy numbers and plaque control record scores.
| | Oral H.
pylori DNA copy numbers |
|---|
| Variables | Spearman's rank
correlation coefficient | P-value |
|---|
| Plaque control
record scores | -0.36 | 0.43 |
Discussion
The prevalence rate of oral H. pylori
infection varies widely from 1 to 87% in the population, including
among children, young, middle-aged and older individuals, as
previously reported since 2016 (7,12-21).
The majority of studies investigated the positive rate of oral
H. pylori DNA using PCR. Oral samples included dental
plaque, dental pulp, saliva and swabs from the tongue dorsum. It is
speculated that variation in the age and regional characteristics
of the participants, and sample collection method may have affected
the positive rate of oral H. pylori. Additionally,
periodontal inflammation may be implicated in the high prevalence
rate of oral H. pylori as oral H. pylori was more
frequently detected in individuals with periodontitis than in those
without periodontitis (22,23).
Furthermore, the detection sensitivity of PCR and the specificity
of PCR primers may have varied in each study.
It is considered that the chronic inflammation of
periodontal tissues is involved in persistent infection with oral
H. pylori. In the present study, the oral H.
pylori-positive participants exhibited a higher percentage of
≥4 mm periodontal pockets with BOP than the oral H.
pylori-negative participants. However, there was no significant
association between oral H. pylori and deep periodontal
pockets with BOP (i.e., active periodontitis). A number of
participants had mild periodontal inflammation as the participants
regularly received supportive periodontal therapy. Therefore,
relatively mild periodontal inflammation may have been associated
with a low positive rate of oral H. pylori in the present
study. Therefore, additional studies are required to clarify the
association between oral H. pylori and moderate to severe
periodontitis.
Previous studies have reported that oral H.
pylori can be detected in subgingival dental plaque (14,19,21).
Thus, H. pylori, a component of the subgingival microbiome,
may play a pathogenic role in the periodontium, as well as a
carcinogenic role in the stomach. However, the biological
mechanisms by which H. pylori is involved in periodontal
inflammation have not yet been elucidated. H. pylori
contributes to the induction of inflammatory cytokines, such as
IL-17, which can facilitate chronic periodontal inflammation
(24,25). Porphyromonas gingivalis
(P. gingivalis), a Gram-negative oral anaerobe, is a major
periodontopathic bacteria (26).
P. gingivalis was detected in the oral cavity of ~50% of
middle-aged and older Japanese adults (27). P. gingivalis was more
frequently found in H. pylori-positive dental plaque than in
H. pylori-negative dental plaque (18). These results indicate that H.
pylori may be involved in the acceleration of periodontal
inflammation by inducing inflammatory cytokines. However, the
potential role of H. pylori in periodontitis has not yet
been elucidated. Additionally, it remains unclear whether H.
pylori enhances cytokine induction in cooperation with P.
gingivalis in periodontal tissues.
In the present study, the level of dental plaque
accumulation was significantly higher in the oral H.
pylori-positive participants than in the oral H.
pylori-negative participants, suggesting that participants with
oral H. pylori exhibit poor oral hygiene. Additionally,
H. pylori may be detected more abundantly in dental plaque
than on the tongue dorsum. Although the present study did not find
a significant association between oral H. pylori DNA and
plaque control record score in the logistic regression analysis, a
significant association may be found using a larger study
population with dental plaque sampling. These findings highlight
the importance of daily oral hygiene practice and regular
professional oral care to prevent oral H. pylori infection
in older adults. However, the detection of oral H. pylori
DNA is not necessarily associated with H. pylori
colonization (i.e., active H. pylori infection) in the oral
cavity. Additionally, it remains unknown whether the prevention of
oral H. pylori infection contributes to the reduction of
gastric H. pylori infection. Further research is required to
investigate active oral H. pylori infection and its
associations with gastric H. pylori infection.
Tongue coating samples are composed of food debris,
oral bacteria, epithelial cells and blood cells. Tongue bacterial
populations may be associated with oral health conditions. In a
previous study, the authors detected periodontopathic bacteria DNA
using swab samples collected from the tongue surface (27). Another group also reported that
H. pylori DNA was abundantly detected from tongue coating
samples (7). Therefore, the
present study aimed to collect oral samples from the tongue
surface. However, the presence of H. pylori DNA in
periodontal pockets could not be determined in the present study.
Therefore, further research to detect H. pylori DNA using
subgingival dental plaque is required to clarify the association
between H. pylori and periodontitis.
The present cross-sectional study had some
limitations. First, the present study did not investigate the
presence of H. pylori DNA in periodontal pockets. Therefore,
it is necessary to prove the presence of H. pylori using
subgingival dental plaque in future studies. Second, it remains
unclear whether active H. pylori infection is associated
with the oral hygiene condition as H. pylori colonization
could not be examined in the present study. Third, the present
study did not investigate gastric H. pylori infection or the
history of eradication treatments for H. pylori infection
(i.e., gastric lavage). Therefore, associations between oral H.
pylori and gastric H. pylori remain unknown. Fourth, the
presence of oral H. pylori in young individuals with healthy
periodontal tissue remains unknown. Fifth, it was impossible to
match H. pylori-positive and -negative cases by adjusting
confounding factors such as age, sex and health conditions due to
the small number of H. pylori-positive participants.
Finally, the present study was performed at a single hospital.
In conclusion, the presence of oral H. pylori
DNA may be associated with poor dental hygiene and periodontal
inflammation in older adults. It is critical to maintain good oral
health by practicing daily oral care to reduce the risk of oral
H. pylori infection.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Hiroshima University
(Grant no. 0G220).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
HY performed the experiments and analyzed the data.
HS conceived the study, performed the experiments, analyzed the
data, and wrote and reviewed the manuscript. HM, NH, HK, YK and YN
performed the experiments. TT and MS interpreted the data and
supervised the study. KO analyzed and interpreted the data and
reviewed the manuscript. HS and KO confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and informed consent
statement
The Ethics Committee of Hiroshima University
approved the study (No. E-1115). All patients agreed to participate
in this study and signed the informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marshall BJ and Warren JR: Unidentified
curved bacilli in the stomach of patients with gastritis and peptic
ulceration. Lancet. 1:1311–1315. 1984.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kusters JG, van Vliet AH and Kuipers EJ:
Pathogenesis of Helicobacter pylori infection. Clin
Microbiol Rev. 19:449–490. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hooi JKY, Lai WY, Ng WK, Suen MMY,
Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu
JCY, et al: Global Prevalence of Helicobacter pylori
Infection: Systematic review and meta-analysis. Gastroenterology.
153:420–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mnichil Z, Nibret E, Hailegebriel T,
Demelash M and Mekonnen D: Prevalence and associated risk factors
of Helicobacter pylori infection in East Africa: A
systematic review and meta-analysis. Braz J Microbiol. 55:51–64.
2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Go MF: Review article: Natural history and
epidemiology of Helicobacter pylori infection. Aliment
Pharmacol Ther. 16:3–15. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mao X, Jakubovics NS, Bächle M, Buchalla
W, Hiller KA, Maisch T, Hellwig E, Kirschneck C, Gessner A,
Al-Ahmad A and Cieplik F: Colonization of Helicobacter
pylori in the oral cavity-an endless controversy? Crit Rev
Microbiol. 47:612–629. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nagata R, Ohsumi T, Takenaka S and Noiri
Y: Current Prevalence of Oral Helicobacter pylori among
Japanese adults determined using a nested polymerase Chain reaction
assay. Pathogens. 10(10)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ogaya Y, Nomura R, Watanabe Y and Nakano
K: Detection of Helicobacter pylori DNA in inflamed dental
pulp specimens from Japanese children and adolescents. J Med
Microbiol. 64:117–123. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Anand PS, Kamath KP, Gandhi AP, Shamim MA,
Padhi BK and Das S: Dental plaque as an Extra-gastric reservoir of
Helicobacter pylori: A systematic review and meta-analysis.
Arch Oral Biol. 170(106126)2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang XM, Yee KC, Hazeki-Taylor N, Li J, Fu
HY, Huang ML and Zhang GY: Oral Helicobacter pylori, its
relationship to successful eradication of gastric H. pylori
and saliva culture confirmation. J Physiol Pharmacol. 65:559–566.
2014.PubMed/NCBI
|
|
11
|
O'Leary TJ, Drake RB and Naylor JE: The
plaque control record. J Periodontol. 43(38)1972.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tirapattanun A, Namwat W, Kanthawong S,
Wongboot W, Wongwajana S, Wongphutorn P and Chomvarin C: Detection
of Helicobacter pylori and virulence-associated genes in
saliva samples of asymptomatic persons in northeast thailand.
Southeast Asian J Trop Med Public Health. 47:1246–1256.
2016.PubMed/NCBI
|
|
13
|
Aksit Bıcak D, Akyuz S, Kıratlı B, Usta M,
Urganci N, Alev B, Yarat A and Sahin F: The investigation of
Helicobacter pylori in the dental biofilm and saliva samples
of children with dyspeptic complaints. BMC Oral Health.
17(67)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Medina ML, Medina MG and Merino LA:
Correlation between virulence markers of Helicobacter pylori
in the oral cavity and gastric biopsies. Arq Gastroenterol.
54:217–221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nomura R, Ogaya Y, Matayoshi S, Morita Y
and Nakano K: Molecular and clinical analyses of Helicobacter
pylori colonization in inflamed dental pulp. BMC Oral Health.
18(64)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wongphutorn P, Chomvarin C, Sripa B,
Namwat W and Faksri K: Detection and genotyping of Helicobacter
pylori in saliva versus stool samples from asymptomatic
individuals in Northeastern Thailand reveals intra-host
tissue-specific H. pylori subtypes. BMC Microbiol.
18(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Iwai K, Watanabe I, Yamamoto T, Kuriyama
N, Matsui D, Nomura R, Ogaya Y, Oseko F, Adachi K, Takizawa S, et
al: Association between Helicobacter pylori infection and
dental pulp reservoirs in Japanese adults. BMC Oral Health.
19(267)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kadota T, Hamada M, Nomura R, Ogaya Y,
Okawa R, Uzawa N and Nakano K: Distribution of Helicobacter
pylori and periodontopathic bacterial species in the oral
cavity. Biomedicines. 8(161)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Moosavian M, Kushki E, Navidifar T,
Hajiani E and Mandegari M: Is there a real relationship between the
presence of Helicobacter pylori in dental plaque and gastric
infection? A genotyping and restriction fragment length
polymorphism study on patient specimens with dyspepsia in southwest
iran. Int J Microbiol. 2023(1212009)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ogaya Y, Kadota T, Hamada M, Nomura R and
Nakano K: Characterization of the unique oral microbiome of
children harboring Helicobacter pylori in the oral cavity. J
Oral Microbiol. 16(2339158)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wongsuwanlert M, Teanpaisan R, Pahumunto
N, Kaewdech A, Ruangsri P and Sunpaweravong S: Prevalence and
virulence factors of Helicobacter pylori isolated from oral
cavity of non-disease, gastritis, and gastric cancer patients. J
Dent Sci. 19:1036–1043. 2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Umeda M, Kobayashi H, Takeuchi Y, Hayashi
J, Morotome-Hayashi Y, Yano K, Aoki A, Ohkusa T and Ishikawa I:
High prevalence of Helicobacter pylori detected by PCR in
the oral cavities of periodontitis patients. J Periodontol.
74:129–134. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gebara EC, Pannuti C, Faria CM, Chehter L,
Mayer MP and Lima LA: Prevalence of Helicobacter pylori
detected by polymerase chain reaction in the oral cavity of
periodontitis patients. Oral Microbiol Immunol. 19:277–280.
2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tang Z, Jin L and Yang Y: The dual role of
IL-17 in periodontitis regulating immunity and bone homeostasis.
Front Immunol. 16(1578635)2025.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khamri W, Walker MM, Clark P, Atherton JC,
Thursz MR, Bamford KB, Lechler RI and Lombardi G: Helicobacter
pylori stimulates dendritic cells to induce interleukin-17
expression from CD4+ T lymphocytes. Infect Immun. 78:845–853.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Holt SC, Kesavalu L, Walker S and Genco
CA: Virulence factors of Porphyromonas gingivalis. Periodontol
2000. 20:168–238. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shigeishi H, Oka I, Su CY, Hamada N,
Nakamura M, Nishimura R, Sugiyama M and Ohta K: Prevalence of oral
Epstein-Barr virus and Porphyromonas gingivalis and their
association with periodontal inflamed surface area: A
cross-sectional study. Medicine (Baltimore).
101(e31282)2022.PubMed/NCBI View Article : Google Scholar
|