Introduction
Breast cancer stands as the most prevalent
malignancy affecting women, exhibiting the highest incidence rates
within developed nations. While notable advances in screening and
therapy have improved the survival outcomes of patients, the
complexity of breast cancer biology continues to challenge clinical
management. Breast cancer is a heterogeneous disease, and both
inter-tumor and intra-tumor variability affect prognosis and the
therapeutic response. This heterogeneity, although critical, also
underscores the need to individualize therapy and monitor for
diverse treatment outcomes, including adverse effects (1,2).
A crucial, yet often overlooked complication of
systemic therapy is the development of secondary malignancies
(3,4). Chemotherapy-induced tumor progression
and secondary genitourinary malignancies have become more
clinically relevant as survival rates improve (5). Among the agents used in adjuvant and
neoadjuvant regimens, cyclophosphamide, an alkylating agent, has
long been a cornerstone in breast cancer treatment protocols, such
as cyclophosphamide, methotrexate and 5-fluorouracil (CMF) and
fluorouracil, epirubicin and cyclophosphamide (FEC) (6,7).
Cyclophosphamide has been demonstrated in certain trials to
increase the breast cancer pathological complete response rate,
whereas other investigations have found no differences (8).
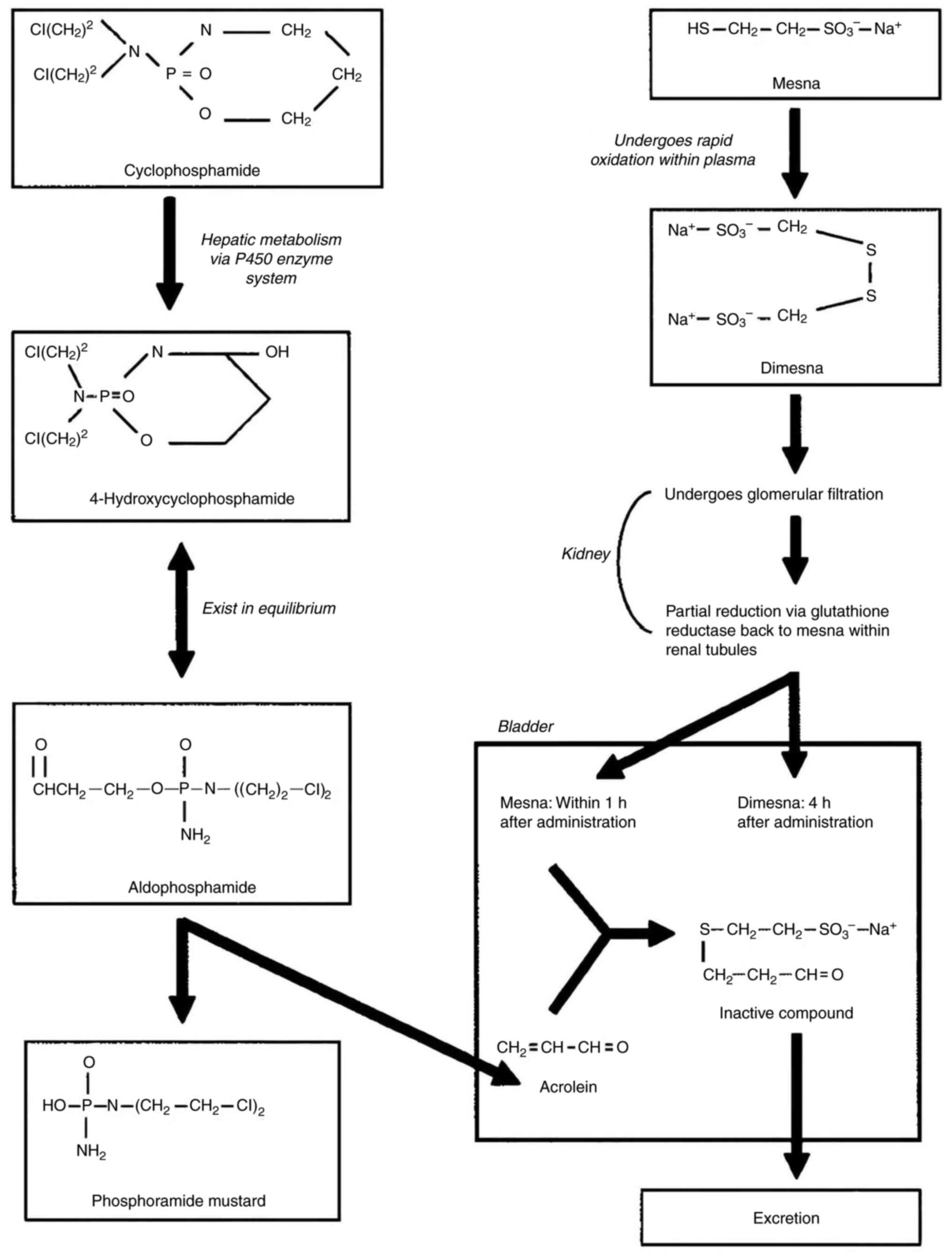
Cyclophosphamide functions as a prodrug metabolized
in the liver into active alkylating agents and inactive byproducts,
such as acrolein. Acrolein is known to irritate the urothelium and
has been implicated in the generation of reactive oxygen species,
which compromise the antioxidant defense of the bladder and
contribute to urothelial damage (9,10).
The first reported case of bladder transitional cell carcinoma
linked to cyclophosphamide was published in 1971 (5,11,12).
The cumulative dose and duration of exposure are key risk factors
for developing such malignancies. This highlights the importance of
long-term urological surveillance for patients treated with
cyclophosphamide.
The present study describes the case of a patient
with iatrogenic muscle-invasive urothelial carcinoma occurring 7
years following adjuvant cyclophosphamide-based chemotherapy for
breast cancer; the patient was without any other known risk
factors. The case described herein underscores the need for
heightened clinical awareness of secondary bladder malignancies in
long-term breast cancer survivors.
Case report
A 54-year-old woman was diagnosed with breast cancer
in 2015. The histopathological examination revealed invasive
lobular carcinoma grade I with metastases in six lymph nodes. It
should be noted that the initial microscopic evaluation of this
patient is not retrievable due to the lack of proper documentation
beforehand, although the formal report and description on the
findings remain available in the offline medical records system. A
mastectomy was performed in the authors' center (Haji Adam Malik
General Hospital, Medan, Indonesia), followed by chemoradiation
(six rounds of chemotherapy and 27 rounds of radiation) which was
completed in 2016 at Haji Adam Malik General Hospital, Medan,
Indonesia. Cyclophosphamide at a dose of 600 mg/m2 and
docetaxel (Brexel) at a dose of 75 mg/m2 per cycle were
administered as part of the chemotherapeutic regimen. Based on a
body surface area (1.6 m2), the estimated total
cumulative dose of cyclophosphamide was ~5,760 mg. During
chemotherapy, the patient did not suffer from any side-effects,
such as or hemorrhagic cystitis; however, the patient experienced
dysuria. The patient received hormonal therapy, such as tamoxifen
20 mg daily between 2015 and 2017, followed by exemestane 25 mg
daily until 2020, as part of standard endocrine management for
hormone receptor-positive breast cancer.
In June 2022, the patient experienced both hematuria
and dysuria and reported back to the Haii Adam Malik General
Hospital, Medan, Indonesia. Vital signs and clinical examination
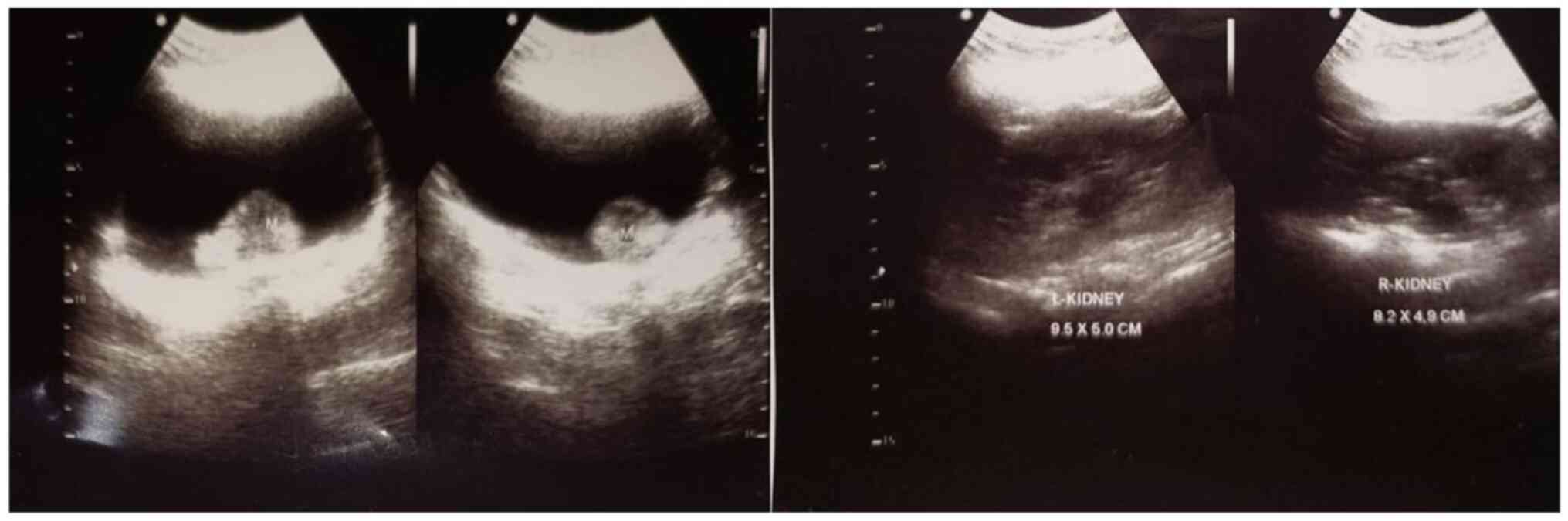
were normal. An ultrasonography revealed tumors in the bladder
(Fig. 1). The patient underwent a
cystoscopy in September 2022; a bladder tumor was discovered on the
inferior wall of the bladder and was completely removed by
transurethral resection. From the histopathological analysis shown
in Figs. 2 and 3, the majority of the tumor cells
observed in the fragmented tissue from the base of the bladder were
organized in an infiltrative, solid growth pattern. All procedures
were performed in the Department of Surgical Pathology, Haji Adam
Malik General Hospital, Medan, Indonesia following a standardized
protocol. Tissue samples were paraffin-embedded and sectioned at a
thickness of 4-5 µm, then fixed in 10% neutral buffered formalin at
room temperature for 24 h. Staining was carried out using
hematoxylin and eosin reagents (MilliporeSigma) under standardized
conditions according to the manufacturer's instructions, including
maintaining reagent concentrations according to the manufacturer's
specifications, staining at room temperature (20-25˚C), and using
consistent incubation times (hematoxylin, 5-7 min; eosin, 1-2 min)
to ensure uniform staining and reproducible microscopic results.
Microscopic evaluation was performed using a light microscope
(Olympus BX53, Olympus Corporation). This protocol reflects the
routine, validated staining and analysis procedures established in
our laboratory.
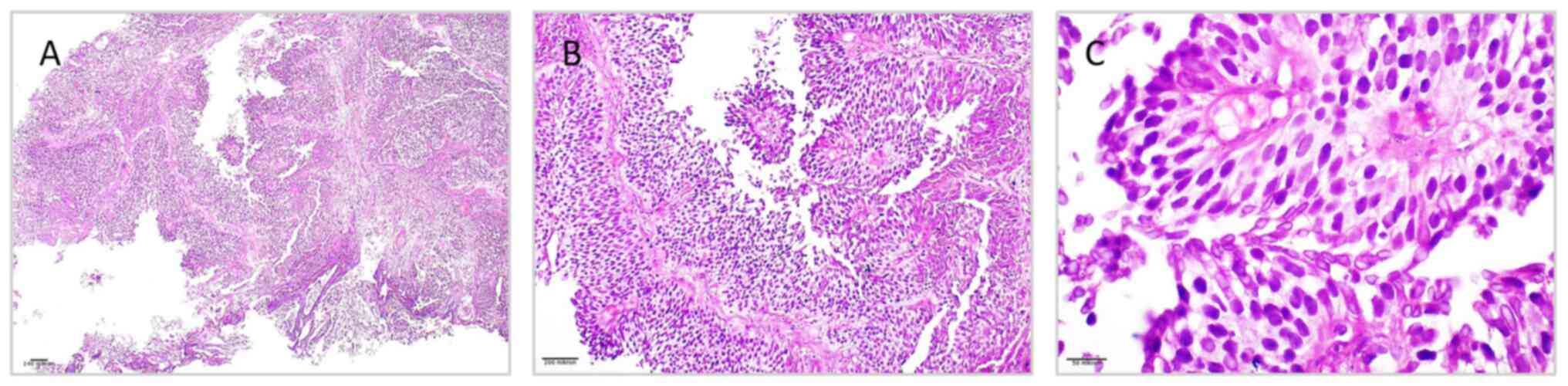
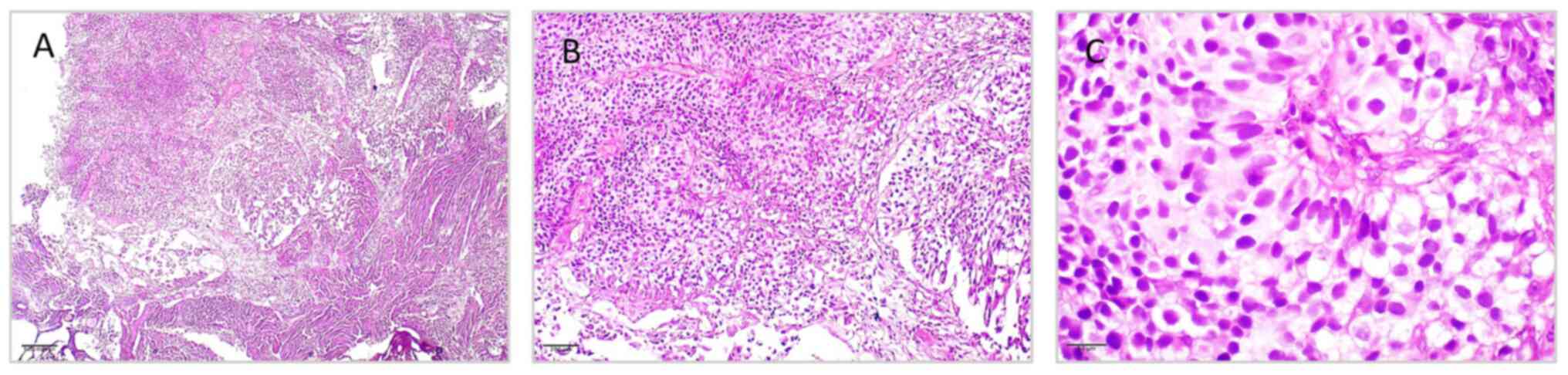 | Figure 3Histopathological features of
low-grade papillary urothelial carcinoma from tumor mass of the
urinary bladder, illustrating (A) delicate papillary architecture
with fibrovascular cores lined by multiple layers of urothelial
cells, without evidence of stromal invasion (H&E staining;
magnification, x40); (B) mild nuclear pleomorphism, preserved
polarity and orderly cell arrangement (H&E staining;
magnification, x100); (C) tumor cells with uniform round-to-oval
nuclei, fine chromatin, inconspicuous nucleoli, and low mitotic
activity, consistent with low-grade morphology (H&E staining;
magnification, x400). H&E, hematoxylin and eosin. |
With their large irregular nuclei, numerous nucleoli
and thick chromatin, the tumor cells exhibited notable
pleomorphism. Throughout the sample, numerous mitotic figures,
including unusual forms, were observed. Tumor cells infiltrated the
muscularis propria, indicating muscle invasion. There were
interstitial bleeding and necrotic areas. The final diagnosis was
that of low-grade muscle-invasive urothelial carcinoma (MIBC). A
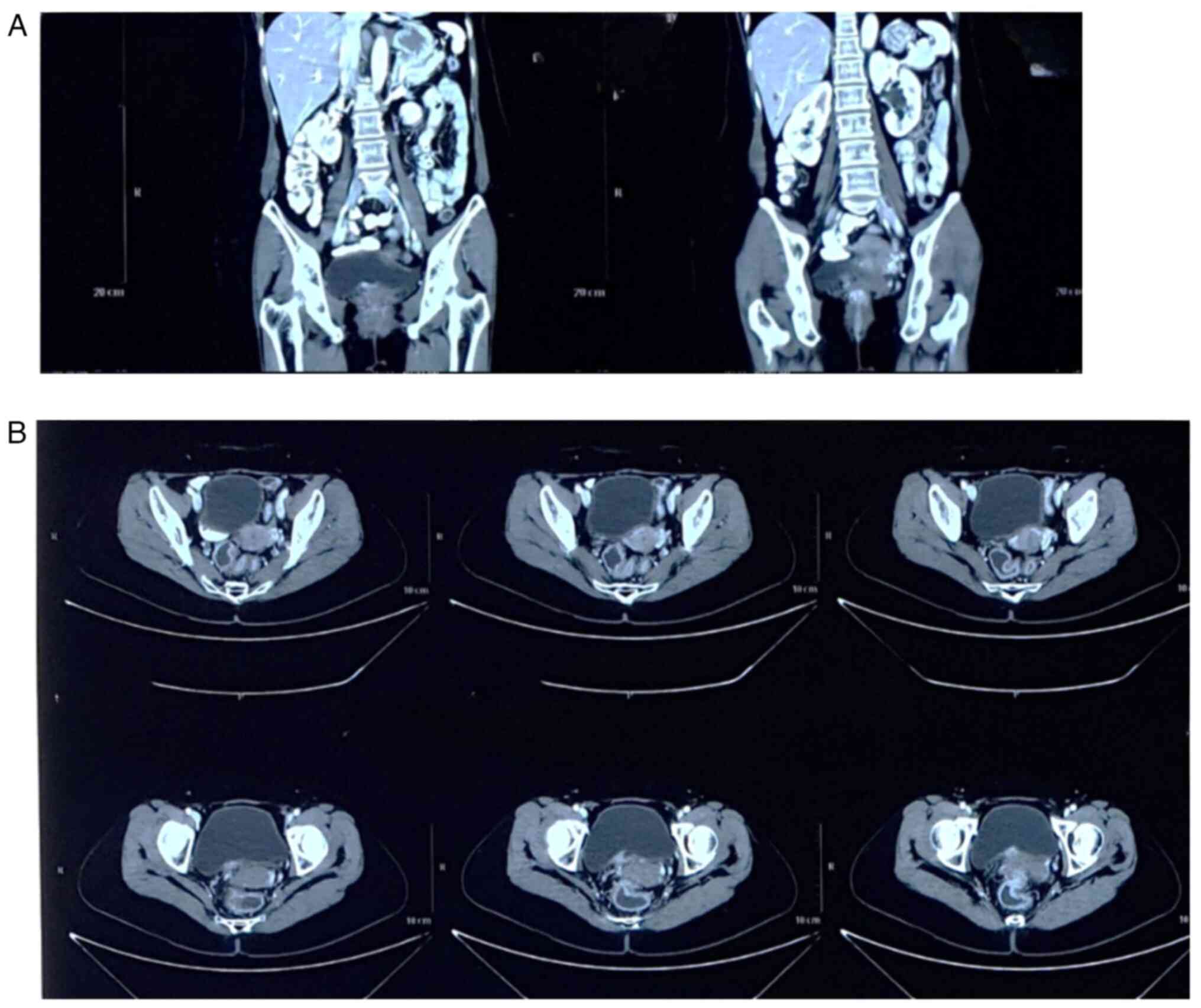
contrast computed tomography scan of the abdomen indicated focal
inferior bladder thickening, suggestive of a malignancy of the
bladder (Fig. 4). The patient was
then diagnosed with a bladder tumor T2N0M0. Radical cystectomy with
chemoradiation was initially recommended; however, the patient
declined surgery due to concerns over its invasiveness and impact
on quality of life, opting instead for bladder-preserving trimodal
therapy. The patient received four cycles of gemcitabine-cisplatin
and radiation (60-70 Gy) in 33 fractions, which was completed in
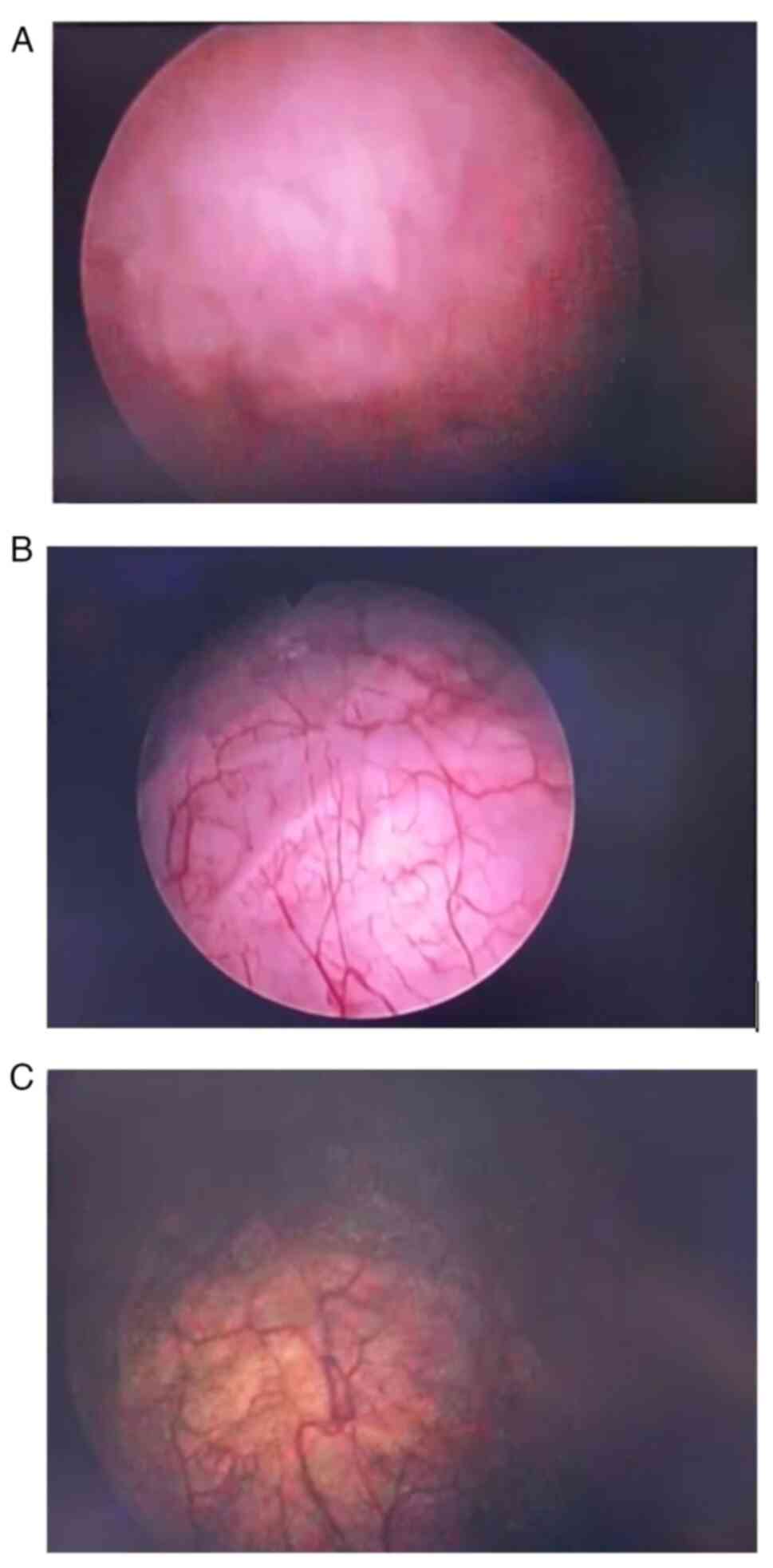
December 2022. The patient underwent routine cystoscopy every 3
months beginning from February 2023 (Fig. 5). The patient does not have any
risk factors for bladder cancer, such as smoking or a family
history of cancer, apart from treatment with cyclophosphamide.
Discussion
Mechanisms of cyclophosphamide-induced
carcinogenesis
Cyclophosphamide is an alkylator of the nitrogen
mustard group, which causes alkylation in DNA, thereby inhibiting
DNA synthesis and function. B- and T-cells are equally inhibited by
cyclophosphamide, although the toxicity is greater for B-cells
(6). Cyclophosphamide is a prodrug
which is converted to both active and inactive metabolites by the
hepatic P450 enzymatic system. Cyclophosphamide is transformed into
4-hydroxy-cyclophosphamide and its tautomer aldophosphamide in the
liver via the cytochrome p-450 monooxygenase system. During β
elimination, aldophosphamide releases acrolein and the alkylating
chemical phosphoramide mustard (Fig.
6) (13,14).
The inactive (non-alkylating) metabolite acrolein is
considered to cause cystitis. Although there is no concrete
evidence, acrolein is deemed to be a likely contributor to bladder
cancer as well. Similar to idiopathic bladder cancers, the vast
majority of bladder cancers that develop following treatment with
cyclophosphamide are transitional cell carcinomas; nevertheless,
there have been a few isolated occurrences of sarcomas and other
forms of bladder carcinoma documented (13). The patient in the present study was
diagnosed with bladder transitional cell carcinoma after 7 years of
terminating the use of cyclophosphamide as a breast cancer therapy.
In a previous study, a review of 54 cases of breast cancer with
bladder metastasis revealed that the median interval from breast
cancer diagnosis to bladder metastasis was 5.6 years. The majority
of these instances pertained to invasive ductal carcinoma, and the
bladder metastases were histologically congruent with the initial
breast cancer (15).
Cyclophosphamide therapy is a known risk factor for
carcinogenesis due to its mutagenic qualities, particularly in the
development of bladder cancer. When cyclophosphamide is
administered, its active metabolite, 4-hydroxy-cyclophosphamide,
diffuses into cancer cells and is responsible for the alkylating
ability of cyclophosphamide. The alkylating effects of
cyclophosphamide, such as mutations in the p53 tumor suppressor
gene, are more likely to be the molecular changes that mediate the
carcinogenic consequences (9,16).
Epidemiology and risk factors
The mode of cyclophosphamide administration that has
generated the most concern with regards to bladder toxicity is
daily oral dosing, as the duration of treatment and the total
cumulative exposure are generally higher compared with intermittent
intravenous dosing (11,13). The main risk factor is a cumulative
dose >20 g, and the median time from treatment to bladder cancer
diagnosis is 7 years (11). The
study by Yilmaz et al (10)
identified 17 cases of hemorrhagic cystitis and 2 cases of bladder
cancer among 1,018 patients treated with cyclophosphamide for
autoimmune diseases. The median time from initial diagnosis to the
onset of hemorrhagic cystitis was 10 months, while the median time
to the development of bladder cancer was 8 years. The median
cumulative dose of cyclophosphamide in their cystitis patients was
10 g. The only significant risk factor for hemorrhagic cystitis
identified in the risk factor analysis was cumulative
cyclophosphamide dosage (10).
In the case in the present study, the bladder cancer
was diagnosed 7 years after receiving cyclophosphamide therapy.
However, cyclophosphamide was not administered orally; it was
instead used as a chemotherapeutic regimen for breast cancer
treatment. Research has also demonstrated the association between
cyclophosphamide therapy and bladder cancer (17). Such cases of bladder cancer
occurring years after cyclophosphamide therapy in breast cancer
patients are rare.
The administration of cyclophosphamide, particularly
via oral dosage, has elicited considerable apprehension regarding
its long-term impact on the bladder; nevertheless, other factors,
including preoperative hydronephrosis and renal insufficiency, may
also significantly influence the advancement of bladder cancer.
Increased blood urea nitrogen and serum creatinine values,
indicative of renal impairment, have been identified as independent
predictors of locally progressed bladder cancer, highlighting the
necessity for thorough renal function monitoring in patients
undergoing cyclophosphamide treatment (18).
In a previous study, it was demonstrated that ~50%
of 145 patients with Wegener's granulomatosis who received
cyclophosphamide experienced hematuria, and 7 of these individuals
went on to develop bladder cancer (19). Among those who subsequently
developed bladder cancer, the median cumulative dose reached ~113 g
over a median treatment duration of 86 months, compared with only
25 g in patients without bladder cancer. Each additional 10 g of
cyclophosphamide was associated with a doubling of the risk of
developing bladder cancer (19).
In another study by Stillwell et al (20), only 5 of the 100 patients in a
trial with cyclophosphamide-induced hemorrhagic cystitis went on to
develop bladder cancer. For these 5 patients, the average dose of
cyclophosphamide was 195 g for 60 months, which was significantly
greater than the dosage for the other patients (20). Hemorrhagic cystitis and bladder
cancer were related in each of these investigations (9).
The risk of cyclophosphamide-induced bladder cancer
is associated with the high dose and long duration of therapy.
Exposure to cyclophosphamide for a period >13 months has been
shown to be associated with an almost 8-fold increased risk of
developing bladder cancer (21).
In the study by Radis et al (22), 9 of the 50 cancers observed in 37
of the 119 individuals with rheumatoid arthritis who received
cyclophosphamide were bladder cancers, and 19 of the cancers were
skin cancers, as opposed to no bladder cancers and six skin cancers
in the control group. Cyclophosphamide had stopped being used for
14, 16, and 17 years when three bladder tumors developed. The
patients who developed cancer received a median cumulative dose of
100 g. However, it is not known whether it was a superficial or a
muscle-invasive bladder tumor (22).
In the patient in the present study, although she
received only six cycles of cyclophosphamide at 600
mg/m2 per cycle, an estimated cumulative dose well below
the established risk threshold, she developed MIBC 7 years later.
This suggests that even lower cumulative doses, in the absence of
acute urinary toxicity, may contribute to carcinogenesis in
susceptible individuals.
Latency to diagnosis has been found to range from 0
to 14 years, with a median of 2.7 years. Saoji (9) reported a latency of 5 and 9 years in
2 cases, while Volm et al (5) documented bladder cancer 18 years
following the termination of therapy. The case in the present study
falls within this window. These findings underscore the need for
long-term urologic surveillance in cancer survivors treated with
cyclophosphamide, as bladder cancer may emerge many years after
therapy ends (5,9).Surveillance recommendations and
clinical implications. The incidence of bladder carcinoma is
considered to be lower in patients receiving intravenous
cyclophosphamide, likely due to the lower total cumulative dose
used with this form of therapy (10,11).
In the study conducted by Saoji (9), 3 patients all had advanced disease
when they were first seen, and they all received more than 50 g of
oral cyclophosphamide over a 3-year period. In fact, 2 of the
patients needed a higher dose of cyclophosphamide (100 g) to
control their disease. None of these 3 patients ever expressed any
complaints about urinary symptoms while receiving treatment. While
undergoing treatment and throughout the follow-up period, they
underwent routine examinations, including urine analyses. In
addition to the risk of bladder cancer, cyclophosphamide is
associated with the risk of developing other malignancies, such as
lymphoma, leukemia and squamous cell carcinoma (9).
The risk factors for bladder cancer are demonstrated
in Table I (4). In the case in the present study,
cyclophosphamide therapy may be the cause of bladder cancer when
considering the risk factors for bladder cancer development.
 | Table IRisk factors of urothelial cancer. |
Table I
Risk factors of urothelial cancer.
| Risk factors for
urothelial cancer |
|---|
| Cigarette
smoking |
| Exposure to aryl
amines (workers in organic chemical, dye, rubber, paint
industries) |
| Phenacetin abuse |
| Familial history |
| Cyclophosphamide
therapy |
| Nonglomerular
hematuria |
It is recommended that all patients treated with
cyclophosphamide undergo routine urinalysis every 3 to 6 months,
even after completing treatment, as hematuria is often the first
sign of cyclophosphamide-induced cystitis or bladder cancer.
Although outcome data to support this level of surveillance are
currently lacking, early detection may be critical. In the event
that a patient presents with microscopic or gross hematuria, a
cystoscopy should be performed (5). In cases where no visible lesion is
detected but hematuria persists, repeat cystoscopy every 1 to 2
years is advisable. Stillwell et al specifically recommended
that all patients exposed to cyclophosphamide and with microscopic
hematuria undergo a cystoscopic evaluation. Persistent microscopic
hematuria has been associated with progressive thickening of the
bladder wall (22,23).
In the case in the present study, the patient did
not report hematuria during cyclophosphamide therapy, although mild
dysuria was noted. At 7 years after completing chemotherapy,
including cyclophosphamide treatment, the patient developed
hematuria and dysuria. Cystoscopy revealed a bladder tumor, which
was completely resected via transurethral resection. As the patient
declined radical cystectomy and chemoradiation, bladder-preserving
trimodal therapy was administered, followed by surveillance
cystoscopy every 3 months.
In conclusion, due to its mutagenic characteristics,
notably in the development of bladder cancer, cyclophosphamide
therapy is a known risk factor for carcinogenesis. The high dose
and prolonged length of therapy are linked to an increased risk of
bladder cancer caused by cyclophosphamide. Several years following
the start of treatment, cyclophosphamide-induced bladder cancer may
develop. Consequently, ongoing monitoring is necessary.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Data and materials availability
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SMW, MHW, RR and DH conceived and designed the
study. The methodology was initiated by SMW, GPS, DDK and ZZT. The
software-based analysis was performed by MHW, RR, MIS and DISS.
Data validation was performed by SMW, DH and GPS and FFP. Formal
analysis was performed by RR, DH, CTM, GPS and FFP. Investigation
of the patient's data was performed by SMW, MHW, RR, MIS and DISS.
Resources were provided by SMW, MHW and ZZT (providing
institutional access to databases, computing or mathematical
analysis software, and also sharing reference management tools).
Data curation was performed by SMW, CTM, MHW and RR. SMW, CTM and
RR analyzed the data. The manuscript was drafted by SMW, MHW, DH
and RR. SMW, MHW, DH, GPS, DDK, FFP and CTM. Visualization was
initiated by CTM, MIS and DISS. The study was supervised by SMW,
GPS and FFP. Lastly, project administration was performed by MHW,
RR and ZZT. All authors have read and approved the final
manuscript. RR, CTM, MIS and DISS confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the Health Research Ethics Committee of Universitas Sumatera
Utara under the issued ID with no. 330/KEPK/USU/2025 (approving
institution komiteetik@usu.ac.id) and the ethics committee agreed
with consent for this investigation being obtained verbally. Verbal
informed consent was obtained from the patient for their
participation in the present study.
Patient consent for publication
Verbal informed consent was obtained from the
patient for their anonymized information, and any related images to
be published in the present study (‘I understand that my data will
be published in this article. I consent to this publication,
provided that my identity remains confidential and no identifying
information is disclosed’).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GLOBOCAN: All Cancer. The Global Cancer
Observatory, 2020.
|
|
2
|
Pedersen RN, Esen BÖ, Mellemkjær L,
Christiansen P, Ejlertsen B, Lash TL, Nørgaard M and Cronin-Fenton
D: The incidence of breast cancer recurrence 10-32 years after
primary diagnosis. J Natl Cancer Inst. 114:391–399. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sinanoglu O, Yener AN, Ekici S, Midi A and
Aksungar FB: The protective effects of spirulina in
cyclophosphamide induced nephrotoxicity and urotoxicity in rats.
Urology. 80:1392.e1–e6. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abraham P, Kanakasabapathy I and Sugumar
E: Decrease in the activities of lysosomal enzymes may contribute
to the urotoxicity of cyclophosphamide in the rat. Biomed Res.
18:131–136. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Volm T, Pfaff P, Gnann R and Kreienberg R:
Bladder carcinoma associated with cyclophosphamide therapy for
ovarian cancer occurring with a latency of 20 years. Gynecol Oncol.
82:197–199. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Veitch Z, Khan OF, Tilley D, Tang PA,
Ribnikar D, Stewart DA, Kostaras X, King K and Lupichuk S: Impact
of cumulative chemotherapy dose on survival with adjuvant FEC-D
chemotherapy for breast cancer. J Natl Compr Canc Netw. 8:957–967.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liga EGK, Abdullah N, Tiro E, Maisuri S
and Chalid T: The effect of cyclophosphamide chemotherapy on
ovarian AntiMüllerian hormone levels in breast cancer patients.
Indonesian J Obstetr Gynecol. 6:64–67. 2018.
|
|
8
|
Kang YK, Si YR, An GY and Yuan P: Efficacy
and safety of cyclophosphamide in anthracycline- and Taxane-based
neoadjuvant chemotherapy in breast cancer: A meta-analysis. Gland
Surg. 10:252–261. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saoji VA: Cyclophosphamide-induced cancers
in pemphigus patients-A report of three cases. Indian J Drugs
Dermatol. 6:32–34. 2020.
|
|
10
|
Yilmaz N, Emmungil H, Gucenmez S, Ozen G,
Yildiz F, Balkarli A, Kimyon G, Coskun BN, Dogan I, Pamuk ON, et
al: Incidence of cyclophosphamide-induced urotoxicity and
protective effect of mesna in rheumatic diseases. J Rheumatol.
42:1661–1666. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Emadi A, Jones RJ and Brodsky RA:
Cyclophosphamide and cancer: Golden anniversary. Nat Rev Clin
Oncol. 6:638–647. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Worth PH: Cyclophosphamide and the
bladder. Br Med J. 3(182)1971.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Monach PA, Arnold LM and Merkel PA:
Incidence and prevention of bladder toxicity from cyclophosphamide
in the treatment of rheumatic diseases: A data-driven review.
Arthritis Rheum. 62:9–21. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sohn HJ, Kim SH, Lee GW, Kim S, Kang HJ,
Ahn JH, Kim SB, Kim SW, Kim WK and Suh C: High-dose chemotherapy of
cyclophosphamide, thiotepa, and carboplatin (CTCb) followed by
autologous stem-cell transplantation for metastatic breast cancer
patients: A 6-year follow-up result. Cancer Res Treat. 37:24–30.
2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou H, Liu D, Chen L, Zhang Y, Zhao X, Ge
Y, Liu M and Kong T: Metastasis to the bladder from primary breast
cancer: A case report and literature review. Oncol Lett.
27(249)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gor PP, Su HI, Gray RJ, Gimotty PA, Horn
M, Aplenc R, Vaughan WP, Tallman MS, Rebbeck TR and DeMichele A:
Cyclophosphamide-metabolizing enzyme polymorphisms and survival
outcomes after adjuvant chemotherapy for node-positive breast
cancer: A retrospective cohort study. Breast Cancer Res.
12(R26)2010.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Chou WH, McGregor B, Schmidt A, Carvalho
FLF, Hirsch MS, Chang SL, Kibel A and Mossanen M:
Cyclophosphamide-associated bladder cancers and considerations for
survivorship care: A systematic review. Urol Oncol. 39:678–685.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Warli SM, Prapiska FF, Siregar DIS and
Seja IA: Tumor markers as predictors of acute kidney injury
incidence and staging of the Muscle-invasive bladder cancer
receiving chemoradiation therapy. World J Oncol. 14:423–429.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Knight A, Askling J, Granath F, Sparen P
and Ekbom A: Urinary bladder cancer in Wegener's granulomatosis:
Risks and relation to cyclophosphamide. Ann Rheum Dis.
63:1307–1311. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stillwell TJ, Benson RC Jr and Burgent EO
Jr: Cyclophosphamide-induced hemorrhagic cystitis in Ewing's
sarcoma. J Clin Onco. 6:76–82. 1988.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Warli SM, Prapiska FF, Siregar DIS and
Wijaya WS: Prediction of locally advanced bladder tumor using
preoperative clinical parameters. Urol Ann. 15:412–416.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Radis CD, Kahl LE, Baker GL, Wasko MC,
Cash JM, Gallatin A, Stolzer BL, Agarwal AK, Medsger TA Jr and Kwoh
CK: Effects of cyclophosphamide on the development of malignancy
and on long-term survival of patients with rheumatoid arthritis. A
20-year followup study. Arthritis Rheum. 38:1120–1127.
1995.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tanaka T, Nakashima Y, Sasaki H, Masaki M,
Mogi A, Tamura K and Takamatsu Y: Severe hemorrhagic cystitis
caused by cyclophosphamide and capecitabine therapy in breast
cancer patients: Two case reports and literature review. Case Rep
Oncol. 12:69–75. 2019.PubMed/NCBI View Article : Google Scholar
|