Introduction
Renal tuberculosis (TB) is a rare, yet critical
extrapulmonary manifestation of Mycobacterium tuberculosis
infection, often resulting from hematogenous dissemination from a
primary, sometimes subclinical, pulmonary focus (1). Due to its progression through
granulomatous inflammation and fibrosis, diagnosis is often delayed
and is often made after the irreversible renal parenchymal
destruction is manifested (2).
A characteristic diagnostic challenge in renal TB is
the presentation of sterile pyuria with a subacute decrease in
kidney function, mostly where invasive tests are not available.
Case report
The present study describes the case of a
72-year-old male patient affected by type II diabetes mellitus,
arterial hypertension and a positive history for NSTEMI in 2006 and
for the aneurysmal dilation of the left anterior descending artery
in 2018. The first indication of CKD was found in 2021, staged
G3bA0. A worsening of CKD was found in May, 2024, when an estimated
glomerular filtration rate (eGFR) of 20 ml/min/1.73 m2
was detected. This impairment of renal function had been related to
new therapy with the SGLT2 inhibitor (dapaglifozin at 10 mg/die),
which commenced in February, 2024. Subsequently, he developed
prostatitis and a further deterioration of renal function with an
eGFR of 16 ml/min/1.73 m2.
He was admitted to the ward of Nephrology and
Dialysis of the Hospital Umberto I (Enna, Itlay) in December, 2024
following 7 days of diarrhea and vomiting, followed by hypovolemia
and oliguria. Laboratory tests revealed non-anion gap metabolic
acidosis with a Delta/Delta ratio <1, a creatinine level of 9.4
mg/dl and a blood urea nitrogen level of 254 mg/dl. Urine analysis
and a chest X-ray yielded negative results. Volume status was
corrected, and high-dose sodium bicarbonate (1.4% 1,000 cc) and
furosemide (from 100 mg to 250 mg/die) were administered, without
an improvement in renal function until renal replacement therapy
was required.
An ultrasound evaluation suggested chronic
pyelonephritis, which added to the history of prostatitis and a
decrease in eGFR advised for bacterial uropathies. The
QuantiFERON-TB Gold test was performed when repeated negative
microbiological urine tests were negative, which revealed a
positive values >64 IU/ml. Moreover, an extensive diagnostic
workup, including serological tests for autoimmune diseases,
granulomatosis diseases, vasculitis, amyloidosis and sarcoidosis,
was performed, and all were negative, further limiting the
differential diagnosis to renal TB. A confirmatory QuantiFERON test
was performed 1 month thereafter, which was also positive, but
revealed lower values compared to the initial result. With the
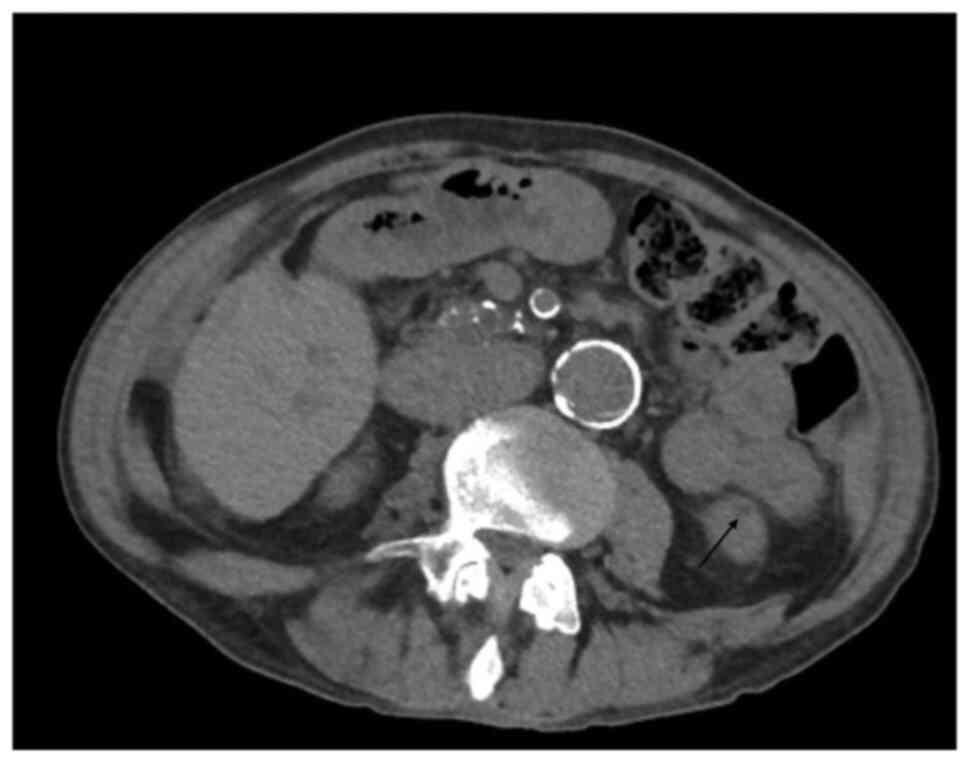
suspicion of renal TB, a computed tomography scan of the abdomen
with contrast was performed, documenting the presence of a ‘small
hypodense area plausibly related to an initial stage of
granulomatous inflammation’ (Fig.
1). The presence of granulomas may explain the negative
Ziehl-Neelsen staining, which is often negative in the chronic
phase of the disease (3-5).
Ziehl-Neelsen microscopic Urine Bk Detection was performed as
follows: Centrifugation of 10-15 ml of fresh urine for 15 min at
3,000 x g/min at room temperature. The supernatant was discarded
and the sediment was used for the smear (3 micron) and placed in a
uniform layer onto a slide. This was then air-dried and heat-fixed
(2-3 quick passes over a flame), The slide was coated with 0.4%
fuchsin (Liofilchem) and left for ~10 min, followed by rinsing with
water, This was followed by destaining with acid-alcohol for 2-3
min, and rinsing with water. The slide was then coated with blue
0.3% methylene (Liofilchem) for 1 min and rinsed with water and
air-dried. The section was observed under a microscope with a 100X
objective and oil immersion. All staining was performed at room
temperature. Note that as no bacteria were detected, no images were
saved.
The patient is currently receiving anti-TB therapy
and received mono-weekly hemodialysis treatment for 9 months.
Treatment commenced without a histopathological confirmation due to
the refusal of the patient to undergo an invasive procedure, but
with the necessity to admission to the transplantation program. The
progression of renal failure was definitively halted, with a mild
improvement of the eGFR from 5 ml/min/1.73 mq to 9 ml/min/1.73
m2, terminating dialysis and starting with a
conservative and low-protein-added to ketoanalogues management of
the disease for 3 weeks, with a weekly monitor of the laboratory
findings.
Discussion
The present study describes a rare case of renal TB,
where the renal localization of the micobacterium was the only site
of disease, without pulmonary or other organ diseases. Renal TB is
notorious for presenting with sterile pyuria, a hallmark that, if
overlooked, invariably leads to delays in diagnosis (2). This delay is particularly detrimental
in renal TB, which progresses insidiously through granulomatous
inflammation and fibrosis, often culminating in irreversible renal
parenchymal destruction requiring dialysis treatment.
The QuantiFERON test detects T-cell-mediated immune
response to Mycobacterium tuberculosis-specific antigens,
providing a substantial advantage over the traditional tuberculin
skin test (TST) in immunocompromised hosts, which includes patients
with advanced CKD and those undergoing hemodialysis (6). Indeed, research consistently
indicates that the uremic environment impairs cellular immunity,
leading to a higher proportion of false negatives at the TST in a
significant proportion of recipients of renal replacement therapy
(7). Conversely, the QuantiFERON
test generally maintains a higher specificity and sensitivity even
in patients with severe immunosuppression.
In keeping with this characteristic, it can be
pivotal and is accessible in any center for the diagnosis of
tuberculosis pathologies. Herein, the serial monitoring aspect,
with a positive, but lower confirmatory value 1 month later, is a
noteworthy feature. While some studies have investigated the
utility of serial QuantiFERON to monitor the treatment response in
active TB, the fluctuation observed herein may reflect the dynamic
interplay of the active disease burden (8-10).
Limiting the diagnostic workup to the more
conventional microbiological and imaging investigations, typically
used to investigate the presence of infectious diseases, while
precluding the use of the QuantiFERON test and the serial
monitoring of this value (9), can
lead to an incomplete clinical image, consequently delaying the
diagnosis and worsening the outcome of the patient. Radiological
findings range from early non-specific changes to classical
manifestations, such as papillary necrosis and calyceal
destruction, ureteral strictures, or a non-functioning, calcified
‘autonephrectomized’ kidney. The absence of cavitary lesions
suggests that the renal involvement, while causing severe
functional decline, was relatively at an early chronic phase, where
the granulomatous interstitial nephritis can precede the visible,
destructive radiological sequelae (11).
In the present case report, even though the
initiation of appropriate anti-TB therapy, as granulomas represent
chronic disease, was not sufficient to markedly improve the
residual kidney function, it is necessary for a prospective
transplantation hypothesis (12).
Although the lack of the gold standard
histopathological or microbiological confirmation represents an
important limit, the slight response after 3 months of therapy can
present a compelling ex juvantibus argument in support of
the diagnosis.
In conclusion, the critical take-home message
supported by the present case report is a reminder that renal RB
needs to be a prioritized differential diagnosis in any patient
with subacute kidney function decline, particularly when
conventional infectious workups, including urine cultures, are
negative. Although rare, TB presenting solely with renal
manifestation must be considered in patients with a subacute
decline in renal function when a comprehensive differential
diagnosis has been ruled out and the gold standard confirmation is
unfeasible. The limitations of relying solely on conventional
diagnostic tools in immunocompromised cohorts can lead to a
critical diagnostic inertia. The successful diagnosis hinged upon
the decisive utilization of the QuantiFERON test, demonstrating its
robust capability to detect active TB observed in the setting of
advanced renal failure. This suggests a clinical update to advocate
for the earlier and routine deployment of QuantiFERON in high-risk
patients with CKD presenting with unexplained renal function
deterioration. Future research is required to focus on refining the
interpretation of QuantiFERON values as a potentially prognostic
tool in patients and establishing clinical protocols for managing
highly probable renal TB in the absence of a confirmatory
biopsy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MRS and VC Conceptualized the study. VC and GR were
involved in reporting the methodology described in the text. VC,
MRS and SG were involved in the writing and preparation of the
original draft of the manuscript. AR, VF and EB were involved in
obtaining the medical history and laboratory data of the patient,
including the results of the Ziehl-Neelsen test. SG and EB were
involved in the writing, reviewing and editing of the manuscript.
All authors have read and agreed to the published version of the
manuscript. MRS and VC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient described in the present study. All procedures performed in
studies involving human participants were in accordance with the
ethical standards of the institutional and/or national research
committee and with the Declaration of Helsinki 1964 and its later
amendments or comparable ethical standards.
Patient consent for publication
Written informed consent was obtained from the
patient described in the present study for the publication of the
present case report and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nunes M, Mourão N, Marques BR, Certal M
and Pinto C: Beyond the lungs: An atypical presentation of renal
tuberculosis. Cureus. 17(e77272)2025.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Figueiredo AA and Lucon AM: Urogenital
tuberculosis: Update and review of 8961 cases from the world
literature. Rev Urol. 10:207–217. 2008.PubMed/NCBI
|
|
3
|
Latus J, Amann K, Braun N, Alscher MD and
Kimmel M: Tubulointerstitial nephritis in active tuberculosis-a
single center experience. Clin Nephrol. 78:297–302. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Goel MM and Budhwar P: Immunohistochemical
localization of Mycobacterium tuberculosis complex antigen
with antibody to 38 kDa antigen versus Ziehl Neelsen staining in
tissue granulomas of extrapulmonary tuberculosis. Indian J Tuberc.
54:24–29. 2007.PubMed/NCBI
|
|
5
|
Chapagain A, Dobbie H, Sheaff M and Yaqoob
MM: Presentation, diagnosis, and treatment outcome of
tuberculous-mediated tubulointerstitial nephritis. Kidney Int.
79:671–677. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grant J, Jastrzebski J, Johnston J,
Stefanovic A, Jastrabesky J, Elwood K, Roscoe D, Balshaw R and
Bryce E: Interferon-gamma release assays are a better tuberculosis
screening test for hemodialysis patients: A study and review of the
literature. Can J Infect Dis Med Microbiol. 23:114–116.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Winthrop KL, Nyendak M, Calvet H, Oh P, Lo
M, Swarbrick G, Johnson C, Lewinsohn DA, Lewinsohn DM and Mazurek
GH: Interferon-gamma release assays for diagnosing Mycobacterium
tuberculosis infection in renal dialysis patients. Clin J Am
Soc Nephrol. 3:1357–1363. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Petruccioli E, Chiacchio T, Vanini V,
Cuzzi G, Codecasa LR, Ferrarese M, Schininà V, Palmieri F, Ippolito
G and Goletti D: Effect of therapy on Quantiferon-Plus response in
patients with active and latent tuberculosis infection. Sci Rep.
8(15626)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee SW, Lee CT and Yim JJ: Serial
interferon-gamma release assays during treatment of active
tuberculosis in young adults. BMC Infect Dis.
10(300)2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ledesma JR, Ma J, Zheng P, Ross JM, Vos T
and Kyu HH: Interferon-gamma release assay levels and risk of
progression to active tuberculosis: A systematic review and
dose-response meta-regression analysis. BMC Infect Dis.
21(467)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Merchant S, Bharati A and Merchant N:
Tuberculosis of the genitourinary system-Urinary tract
tuberculosis: Renal tuberculosis-Part I. Indian J Radiol Imaging.
23:46–63. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zeng J, Zhu D, Zhang H, Lin T and Song T:
IGRA-based INH regimen for prevention of active tuberculosis after
kidney transplantation: A single-centre retrospective study. Int J
Antimicrob Agents. 63(107093)2024.PubMed/NCBI View Article : Google Scholar
|