1. Introduction
Viruses, as obligate intracellular parasites,
represent a significant yet often overlooked component of the oral
microbiome, particularly within the context of periodontal health
and disease. Viruses are submicroscopic infectious agents composed
of genetic material enclosed within a protein shell, often
enveloped by a lipid membrane. These entities are incapable of
self-replication and rely entirely on the host cellular machinery
for propagation (1). Despite their
minimalistic structure, viruses are greatly efficient in hijacking
host mechanisms, often resulting in chronic, latent, or even
oncogenic infections. Within the oral cavity, viruses have been
identified in gingival crevicular fluid (GCF), periodontal pockets
and oral mucosal tissues, indicating their active involvement in
the periodontal microenvironment (2).
Traditionally, periodontal diseases have been viewed
as bacteria-driven inflammatory conditions. However, the emerging
understanding of the oral microbiome has revealed a complex
interplay between host immunity and polymicrobial communities,
including viruses. Unlike bacteria, viruses exert their influence
primarily by modulating immune responses and enhancing the
pathogenicity of resident bacteria (3). Persistent infections, immune
suppression and viral reactivation are all mechanisms through which
viruses can exacerbate periodontal inflammation and accelerate the
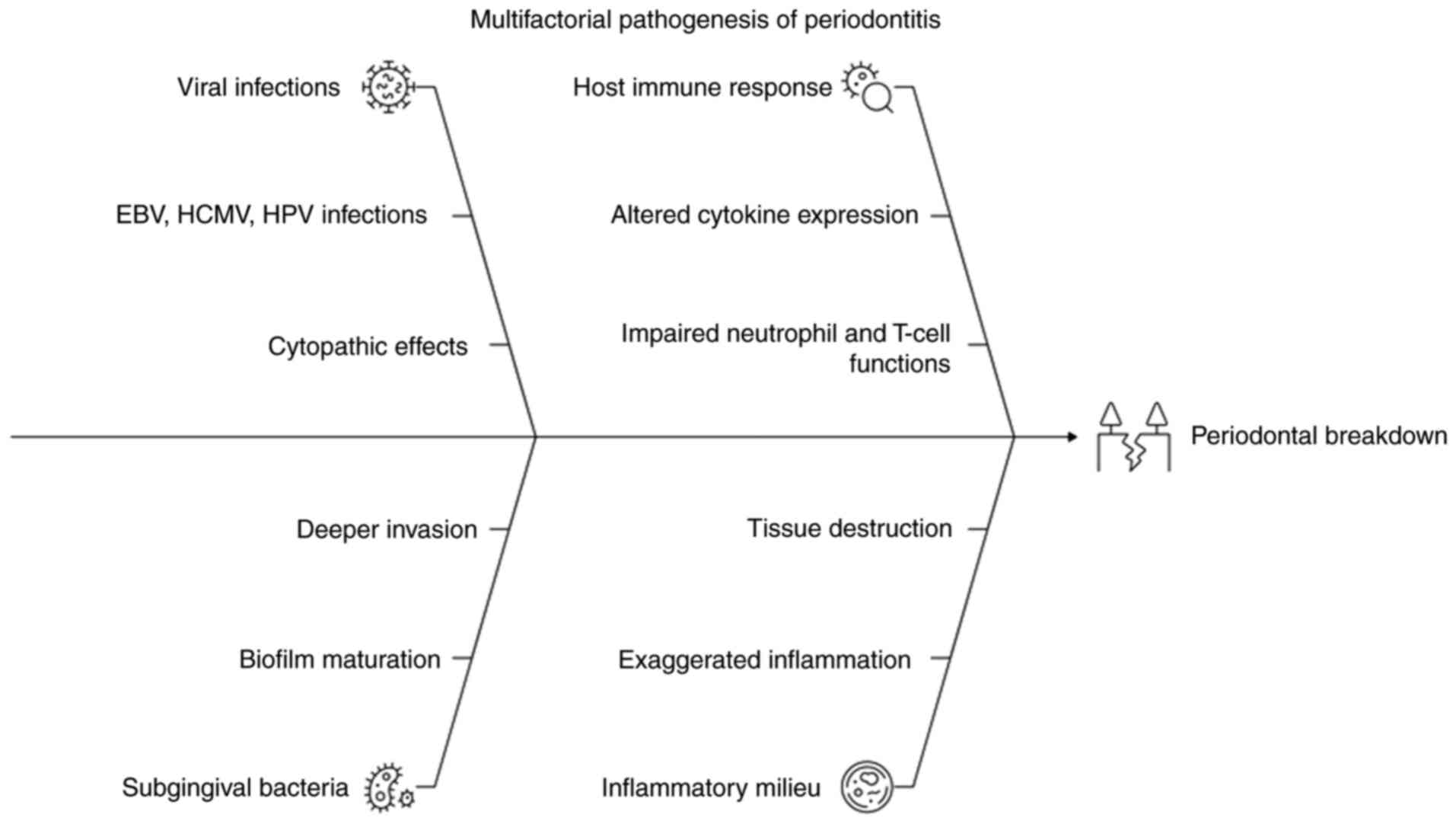
breakdown of periodontal tissues (4). A schematic illustration summarizing
viral-host-bacterial interactions in periodontal pathogenesis is
provided in Fig. 1.
Epidemiological studies have (5-7)
reported herpesvirus detection in 30-60% of chronic periodontitis
sites, with Epstein-Barr virus (EBV) and human cytomegalovirus
(HCMV) frequently isolated from deep pockets (8,9).
Previous meta-analyses have suggested a several-fold higher
prevalence of these viruses in diseased sites compared to healthy
controls (10,11).
2. Literature search methods
A structured literature search was conducted across
the PubMed, Scopus, Web of Science and Google Scholar databases.
The search strategy used combinations of the following key words
and Boolean operators: ‘viruses AND periodontitis’, ‘herpesviruses
AND periodontal disease’, ‘HIV oral manifestations’,
‘viral-bacterial synergism’, ‘periodontal virome’, ‘SARS-CoV-2 and
oral health’ and ‘salivary viral biomarkers’. The search included
articles published between 1990 and 2025, and was restricted to
English-language publications. Both clinical trials, observational
studies, molecular studies and review articles were included,
whereas editorials, non-peer reviewed sources, and articles lacking
relevance to viral periodontal involvement were excluded. Of note,
two authors (AS and VRA) independently screened titles and
abstracts, followed by full-text assessment. Data extraction
focused on viral mechanisms, immune modulation, diagnostic
approaches, clinical manifestations and therapeutic strategies. Any
disagreement was resolved by consensus.
3. Evolution and classification of
viruses
The evolutionary origin of viruses is a subject of
ongoing debate. Three prominent theories provide potential
explanations. The virus-early hypothesis suggests that viruses
predate cellular life, having evolved from primordial
self-replicating molecules. This theory implies that viruses may
represent remnants of precellular life, providing insight into
early molecular evolution (12).
The regression hypothesis posits that viruses are degenerate forms
of parasitic cells that gradually lose essential genes over time,
reducing themselves to genetic elements capable of hijacking only
host cells. Finally, the escaped gene hypothesis theorizes that
viruses originate from fragments of host genetic material, such as
plasmids or transposons, that gain the ability to exit and enter
other cells, becoming infectious in the process (1).
Despite their origin, viruses have co-evolved with
host species for millions of years, developing sophisticated
mechanisms to persist, evade immune detection and manipulate
cellular functions. These evolutionary traits are particularly
relevant in chronic infections such as periodontitis, where viruses
can remain undetected, yet actively modulate host-pathogen
interactions and inflammation (13).
As regards the classification of viruses, according
to the Baltimore Classification of Viruses, introduced by Nobel
Laureate David Baltimore in 1971, categorizes viruses into seven
classes on the basis of their genome type and replication strategy
(Table I). This system is
particularly relevant in periodontology, where viral infections can
modulate host immunity and contribute to the pathogenesis and
progression of periodontal diseases (14).
 | Table IBaltimore classification of
viruses. |
Table I
Baltimore classification of
viruses.
| Class | Genome type and
replication strategy | Examples | Relevance to
periodontology |
|---|
| Class I | Double-stranded DNA
(dsDNA) | Herpesviridae
(HSV), Varicella-zoster virus (VZV), Papillomaviridae
(HPV) | • Latent infections
in the periodontium • Can reactivate under stress or
immunosuppression • HPV associated with oral cancers affecting
periodontal tissues |
| Class II | Single-stranded DNA
(ssDNA) |
Parvoviridae | • Less studied in
periodontology • May integrate into host genome • Potential role in
chronic oral lesions and gingivitis |
| Class III | Double-stranded RNA
(dsRNA) | Reoviridae (e.g.,
Rotavirus) | • Rarely cause
chronic oral infections • Systemic infections may impair immune
responses, indirectly impacting periodontal health |
| Class IV | Positive-sense
single-stranded RNA (+ssRNA) | Picornaviruses
(Enteroviruses, Rhinoviruses) | • Can alter immune
response • May exacerbate existing gingivitis or oral infections
indirectly |
| Class V | Negative-sense
single-stranded RNA (-ssRNA) | Paramyxoviruses
(measles, mumps), rhabdoviruses (rabies) | • Not directly
involved in periodontal disease • May lead to immunosuppression,
increasing susceptibility to periodontal pathogens |
| Class VI | Single-stranded RNA
with reverse transcription | Retroviruses (e.g.,
HIV) | • Profound effect
on immune system (CD4+ T-cell depletion) • Strongly
associated with severe periodontal diseases (e.g., necrotizing
periodontitis) • Disrupts microbial-immune balance in the
periodontium |
| Class VII | Double-stranded DNA
with reverse transcription | Hepadnaviruses
(e.g., hepatitis B virus) | • Not directly
pathogenic to periodontal tissues • Can cause immune dysfunction •
Liver disease affects host response to periodontal pathogens |
4. Viral components and structure, and
replication and spread in the body
Components and structure of a
virus
A virus particle, or virion, consists of several key
components: The genome (either DNA or RNA) that encodes viral
proteins, a protective protein shell called the capsid, and, in
enveloped viruses, an outer lipid bilayer derived from the host
cell membrane (6,15). This envelope typically adorns
glycoproteins or peplomers that facilitate viral attachment to
specific host cell receptors, allowing the virus to enter the host
cell (16).
Some viruses, such as retroviruses, also carry
enzymes, such as reverse transcriptase or integrase, which are
essential for replication within host cells. The capsid structure
can range from simple icosahedral or helical forms to more complex
arrangements, which influence the ability of a virus to interact
with host cells, evade immune responses and withstand environmental
challenges. In the context of periodontal health, these viral
components play crucial roles in the pathogenesis of periodontal
diseases (17).
For example, viruses, such as herpesviruses can
persist in periodontal tissues by evading immune detection through
latency, contributing to chronic inflammation. The presence of
viral particles in periodontal pockets may not only increase the
severity of gingival inflammation, but can also alter the balance
between host immunity and pathogenic bacteria, promoting the
progression of conditions, such as periodontitis, particularly in
immunocompromised individuals (18).
Viral replication and spread
The Modes of viral replication and spread in the
human body are summarized in Table
II. There are various steps of viral replication and systemic
dissemination with relevance to periodontal tissues (12). In systemic infections, the virus
can spread through the bloodstream, nerves, or lymphatic system,
where it can reach distant sites in the body, including the oral
cavity. Oral tissues, such as the gingival mucosa and periodontal
tissues, can function as reservoirs for certain viruses. For
example, herpesviruses can remain latent in host cells, such as the
sensory ganglia, and re-emerge under certain conditions, such as
immunosuppression or stress. This periodic reactivation results in
the development of recurrent oral lesions, such as cold sores or
ulcers (19). In the context of
periodontal health, viral replication and spread can exacerbate
existing periodontal conditions by altering the immune response and
interacting with periodontal bacteria. Viral infections can lead to
persistent inflammation in the periodontium, contributing to tissue
breakdown, the destruction of periodontal ligaments and even
alveolar bone loss. Over time, viral reactivation and chronic
inflammation can aggravate periodontal disease, particularly in
immunocompromised individuals (12).
 | Table IIMode of viral spread and
replication. |
Table II
Mode of viral spread and
replication.
| Step | Process | Description |
|---|
| 1 | Attachment | Virus binds to
specific receptors on the host cell surface, ensuring target
specificity. |
| 2 | Entry | Virus enters the
host cell via endocytosis or direct fusion with the cell
membrane. |
| 3 | Uncoating | Viral genetic
material (DNA or RNA) is released into the cytoplasm or nucleus of
the host. |
| 4 | Transcription and
translation | Virus hijacks the
host machinery to transcribe its genome and translate viral
proteins using host ribosomes and enzymes. |
| 5 | Assembly | Newly synthesized
viral genomes and proteins are assembled into new virions. |
| 6 | Release | New virions exit
the host cell by budding (for enveloped viruses) or by cell lysis
(for non-enveloped viruses), often leading to host cell
destruction. |
5. Host immune response to viruses
The immune system responds to viral infections
through a coordinated network of innate and adaptive mechanisms.
Innate responses include those involving interferons, natural
killer cells and macrophages, which serve as the first line of
defense. Interferons function as antiviral cytokines, promoting an
antiviral state in neighboring cells and enhancing antigen
presentation (20).
Adaptive immunity involves both humoral and
cell-mediated responses (21).
B-lymphocytes produce neutralizing antibodies (IgG and IgA) that
bind viral particles, preventing entry into host cells. In the oral
cavity, secretory IgA plays a critical role in mucosal defense.
Cytotoxic t-lymphocytes (CD8+) recognize and destroy
virus-infected cells by detecting viral peptides presented on MHC
class I molecules (22).
Viruses counteract host defenses by downregulating
MHC molecules, secreting immunomodulatory proteins, inhibiting
apoptosis, and mutating antigenic epitopes to escape recognition.
These immune evasion strategies allow chronic persistence and
immune dysregulation, a hallmark of virus-related periodontal
pathogenesis (23).
6. Diagnostic methods in virology
The accurate identification of viral infections in
periodontal tissues requires both direct and indirect diagnostic
techniques. Direct methods include the detection of viral antigens
via enzyme-linked immunosorbent assays or immunofluorescence,
visualization via electron microscopy and polymerase chain reaction
(PCR) for viral nucleic acid amplification. PCR has become the gold
standard for detecting herpesviruses and human immunodeficiency
virus (HIV) in oral tissues due to its high sensitivity and
specificity (17).
Indirect methods involve serological tests that
detect host antibodies against viral proteins and virus isolation
in tissue cultures to observe cytopathic effects (24). Light microscopy can also identify
viral inclusion bodies within infected tissues. These methods,
particularly when used in combination, allow clinicians to detect
latent or active viral infections in periodontal lesions,
contributing to targeted therapy and prognosis assessment (25).
7. Different viruses and their periodontal
implications
A summary of viral pathogenesis in periodontitis
based on the mechanisms of action of viruses is presented in
Table III. There are various
viruses involved in periodontal diseases:
 | Table IIIClassification of viral pathogenesis
in periodontitis based on the mechanisms of action. |
Table III
Classification of viral pathogenesis
in periodontitis based on the mechanisms of action.
| Category of
pathogenesis |
Definition/focus | Key viruses | Mechanism in
periodontitis |
|---|
| 1. Direct viral
cytopathic effects | Viral destruction
of host cells, leading to localized lesions or tissue
necrosis. | Herpes simplex
virus (HSV)-1/2, human papillomavirus (HPV), picornaviruses (e.g.,
coxsackievirus) | The virus directly
infects and destroys epithelial and fibroblast cells, causing
ulceration, vesiculation and tissue breakdown, particularly in
primary or acute infections. HPV infection can lead to epithelial
dysplasia in the sulcus. |
| 2. Indirect
immune-mediated effects | Viruses manipulate
and dysregulate the host immune response, creating chronic
inflammation and systemic immune deficiency. | Human
immunodeficiency virus (HIV), Epstein-Barr virus (EBV), human
cytomegalovirus (HCMV) | Viruses establish
latency and periodic reactivation, actively suppressing local
immune surveillance (e.g., inhibiting MHC molecules or reducing
neutrophil function) and driving chronic, non-resolving
inflammation via the induction of pro-inflammatory cytokines (e.g.,
IL-6, TNF-α). |
| 3. Viral-bacterial
synergism in periodontal pockets | Viruses create a
pathological microenvironment that enhances the survival and
virulence of periodontopathic bacteria. | EBV and HCMV
(co-infection), viruses that cause immunosuppression (e.g.,
HIV) | Viral immune
dysregulation and inflammation favor dysbiosis by impairing the
host's ability to clear bacteria. This allows the proliferation and
increased virulence of key periodontopathogens (e.g., P.
gingivalis), leading to accelerated and more severe tissue
destruction than either pathogen could achieve alone. |
The Herpesviridae family
Herpesviridae family includes several viruses
implicated in periodontal diseases: Herpes simplex virus (HSV-1 and
HSV-2), EBV and HCMV. These are double-stranded DNA viruses capable
of establishing lifelong latency with periodic reactivation.
EBV infects B lymphocytes and epithelial cells and
is frequently detected in patients with aggressive periodontitis.
It can promote the production of pro-inflammatory cytokines, reduce
neutrophil function and synergize with periodontopathogenic
bacteria. EBV has also been shown to be associated with oral hairy
leukoplakia (OHL), particularly in HIV-positive individuals
(26). In EBV, latent membrane
protein-1 activates the NF-κB and JNK pathways, promoting the
release of pro-inflammatory cytokines and impairing neutrophil
oxidative burst (27).
HCMV exhibits broader cell tropism, infecting
macrophages, endothelial cells and fibroblasts. It is commonly
found in deep periodontal pockets and is associated with severe
inflammation and immune suppression. Co-infection with EBV and HCMV
in lesions in periodontitis is associated with greater tissue
destruction and greater probing depths (23). HCMV expresses immunomodulatory
proteins, such as US28, a viral chemokine receptor that
dysregulates leukocyte trafficking and enhances inflammatory
mediator production. These molecular strategies collectively weaken
host defense and facilitate bacterial overgrowth, amplifying
periodontal tissue destruction (28).
HSV-1 and HSV-2 are known for their ulcerative
manifestations in the oral cavity. HSV-1 primarily causes
oral-facial infections and has been linked to necrotizing
periodontal diseases, particularly in immunocompromised hosts.
These viruses possess the ability to modulate host immunity and
trigger apoptosis, contributing to rapid tissue breakdown (29).
Human papillomavirus (HPV)
HPV is a non-enveloped DNA virus with a predilection
for epithelial tissues. Of note, >200 types have been
identified, with HPV-16 and HPV-18 classified as high-risk due to
their association with oral and oropharyngeal squamous cell
carcinoma. In periodontal tissues, HPV DNA has been isolated from
gingival sulci, periodontal pockets and inflamed gingiva,
suggesting a possible etiological or contributory role (30).
The E6 and E7 proteins of HPV interfere with tumor
suppressor proteins, such as p53 and Rb, promoting cell cycle
dysregulation and potential epithelial dysplasia. Although the
direct link between HPV and periodontitis remains under
investigation, its detection in diseased periodontal tissues,
particularly in HIV-infected patients, highlights its potential
role in modulating epithelial barrier integrity and immune
surveillance (31). Recent
research has shown that HPV-associated epithelial dysregulation may
enhance microbial adhesion and alter gingival epithelial immune
signaling (32).
Picornaviruses and
paramyxoviruses
Although less frequently implicated in periodontal
diseases, picornaviruses and paramyxoviruses may affect the oral
cavity. Picornaviruses, such as coxsackievirus A, are
single-stranded RNA viruses responsible for hand-foot-and-mouth
disease and herpangina, both of which feature vesicular and
ulcerative oral lesions. These typically affect children and may
cause discomfort, poor oral hygiene and transient gingival
inflammation (33).
Paramyxoviruses, such as the measles virus and mumps
virus can also be present in the oral cavity. Measles present with
Koplik's spots, whereas mumps cause parotitis. Although these
viruses are not directly involved in periodontal tissue
destruction, they can alter host immunity and mucosal barriers,
indirectly influencing periodontal health, particularly in
susceptible populations (33). RNA
viruses may modify interferon pathways and innate antiviral
signaling, indirectly heightening periodontal tissue
vulnerability.
HIV
The most clinically significant retrovirus in
periodontology is HIV. HIV is a single-stranded RNA virus that
integrates into host DNA via reverse transcriptase and targets
CD4+ T-lymphocytes, leading to progressive
immunosuppression. The oral cavity is a sentinel site for
HIV-related manifestations and often reveals early signs of
systemic disease (20).
In the context of periodontal disease, HIV is
associated with linear gingival erythema (LGE), necrotizing
ulcerative gingivitis (NUG), necrotizing ulcerative periodontitis
(NUP), and necrotizing ulcerative stomatitis (NUS). These
conditions are characterized by the rapid and severe destruction of
soft and hard tissues, spontaneous bleeding and halitosis. They are
frequently accompanied by systemic symptoms and often occur at low
CD4+ counts (<200 cells/µl) (34).
HIV also facilitates co-infections with
herpesviruses, HPV and fungal pathogens, such as Candida
albicans. Saliva and gingival crevicular fluid in HIV-positive
individuals often harbor detectable levels of HIV RNA, indicating
the role of the oral cavity as a potential reservoir and site of
viral transmission (35).
While previous studies have established associations
between herpesviruses, HIV, HPV and periodontal destruction, the
strength of evidence varies considerably (5,34).
Mechanistic insights are strongest for EBV and HCMV, whereas HPV
and RNA viruses rely largely on correlative or preliminary reports.
Conflicting findings, particularly regarding virus detection across
populations and methodologies, suggest the need for standardized
sampling and molecular protocols. Future studies are warranted to
integrate longitudinal designs and metagenomic approaches to
clarify whether viruses initiate, accelerate, or merely amplify
periodontal breakdown.
8. Clinical oral manifestations of viral
infections
The oral manifestations of viral infections
represent a critical aspect of oral diagnostics (20), particularly in immunocompromised
individuals, such as those with HIV/AIDS. These manifestations can
range from lesions and gingival inflammation to severe forms of
periodontal disease, underscoring the need for a careful assessment
of the viral etiology of oral pathologies (25).
OHL
OHL is a distinctive condition caused by EBV. It
typically presents as white, non-removable plaques on the lateral
borders of the tongue and is often associated with HIV-positive
patients, particularly those with advanced immunosuppression. These
plaques are asymptomatic, but can be diagnostic markers for HIV
progression, indicating a decline in immune function. Lesions are
considered a marker of immunocompromised status rather than a
direct indicator of malignancy, although their presence may
increase the risk of further oral lesions and opportunistic
infections (19).
Candidiasis
Although candidiasis is a fungal infection, it often
co-exists with viral infections, particularly in individuals with
HIV or other forms of immunosuppression. It is considered a
diagnostic marker of immune decline, particularly when it presents
as oropharyngeal candidiasis (thrush). Fungal infection caused by
Candida albicans can be exacerbated by viral infections,
particularly HIV, as the capacity of the immune system to fight
opportunistic infections is significantly diminished. Candidiasis
manifests as white patches on mucosal surfaces and is often
accompanied by redness or ulceration in severe cases. Its recurrent
presence in patients with HIV is often associated with advanced
immunosuppression and serves as an indicator for assessing immune
system function and the progression of the underlying viral
infection (20).
LGE
LGE is closely associated with HIV and presents as a
distinct red band along the gingival margin, characterized by
minimal plaque accumulation and a poor response to conventional
treatment. It is a manifestation of immune dysregulation and the
inflammatory response within the gingiva and is often observed in
individuals with HIV/AIDS. Gingival inflammation is not typically
caused by bacterial plaque accumulation, but rather by the immune
response of the host to the presence of oral pathogens, including
viral and fungal elements. LGE may be one of the first clinical
signs of immune decline in patients with HIV, and is a key oral
manifestation requiring careful management alongside antiviral
therapy and periodontal care (36).
Necrotizing gingival and periodontal
diseases (NUG/NUP/NUS)
NUG, NUP and NUS are severe oral conditions often
observed in individuals with HIV/AIDS, and they are frequently
complicated by viral co-infections [e.g., HSV and cytomegalovirus
(CMV)]. These conditions are characterized by rapid and severe
tissue necrosis in the gingiva and periodontium, leading to painful
ulcers, bleeding and a foul odor (37). Viral co-infections aggravate the
clinical presentation, as they suppress the immune response and
hinder the ability of the body to effectively manage both viral and
bacterial pathogens. The rapid progression of these diseases can
result in significant tooth loss and oral disfigurement if not
promptly treated with a combination of antiviral and antibacterial
therapies (34).
Ulcers and erosions
Oral ulcers and erosions are common manifestations
of viral infections triggered by viruses, such as HSV,
Coxsackievirus and paramyxoviruses. These painful, shallow ulcers
typically appear on non-keratinized mucosal surfaces, such as the
buccal mucosa, tongue and soft palate. Characteristic painful
lesions are often recurrent, particularly in individuals with HSV
infections, which can be reactivated by stress, immune suppression,
or other triggers. Viral ulcers can greatly impair oral function,
causing difficulty in eating and speaking, and are often
accompanied by swelling and redness. The presence of herpetic
ulcers in the oral cavity is often an indication of active viral
replication, requiring appropriate antiviral treatment to control
symptoms and reduce the risk of transmission (38).
9. Clinical implications and management
The recognition of viral involvement in periodontal
disease requires a multifaceted management approach that not only
targets the viral pathogen, but also addresses the immune status of
the patient and the complexity of coinfections. Given the
increasing interplay between viral infections and periodontal
diseases, it is essential for clinicians to implement comprehensive
strategies to control viral replication, maintain oral health and
reduce the risk of disease progression, particularly in
immunocompromised patients, such as those living with HIV/AIDS
(34).
Antiviral therapy
In the management of viral periodontal diseases,
antiviral medications play a critical role in reducing the viral
load and preventing the progression of lesions:
i) Acyclovir, ganciclovir and valganciclovir are
commonly used in the treatment of HSV and CMV infections. These
drugs inhibit viral replication by targeting viral DNA synthesis,
and they are particularly effective in preventing recurrent
outbreaks of HSV and controlling CMV-related oral manifestations,
including oral ulcers and gingivitis (39).
ii) Highly active antiretroviral therapy (HAART) is
the cornerstone for the management of HIV and preventing the
immunosuppressive effects that exacerbate periodontal disease.
HAART functions by inhibiting various stages of the HIV replication
cycle, thereby controlling the viral load and improving the immune
response, which can help reduce the severity of oral manifestations
such as oral candidiasis, OHL and gingival inflammation associated
with HIV. By restoring immune function, HAART can reduce the
frequency of periodontal infections and complications related to
immunosuppression (40).
Combined antiviral and antibacterial
therapies
In a number of cases, particularly those involving
coinfections (viral and bacterial), a combination of antiviral and
antibacterial therapies may be necessary. This is particularly true
for conditions, such as NUG or NUP, where viral infections such as
HSV or CMV cause bacterial infection (41). The use of antibiotics such as
metronidazole (effective against anaerobic bacteria) and
doxycycline (which also has anti-inflammatory properties) helps
control bacterial overgrowth, preventing further tissue
destruction. In such cases, the dual approach ensures that both the
viral and bacterial components are managed effectively, reducing
the risk of systemic spread and improving oral outcomes (39).
Standard periodontal therapy
Standard periodontal therapy remains an essential
part of managing viral periodontal diseases, particularly when
combined with antiviral medications, such as the following: i)
Scaling and root planing helps to remove bacterial biofilms and
calculus deposits, reducing inflammation and increasing the
effectiveness of antiviral treatments (42). ii) Chlorhexidine irrigation can be
used to irrigate periodontal pockets, reducing plaque accumulation
and controlling bacterial growth, while allowing antiviral
treatments to target the viral load more effectively. iii)
Antibiotic coverage (e.g., metronidazole or doxycycline) is often
administered to manage the bacterial component of coinfections,
which can exacerbate the clinical presentation of viral periodontal
diseases.
However, beyond mechanical and antimicrobial
management, it is crucial to consider the systemic health and
immune status of the patient. In patients with HIV, the regular
monitoring of CD4+ T-cell counts and the viral load is
necessary. These markers help assess the degree of immune
suppression, predict periodontal risk and guide treatment
modifications. Lower CD4+ counts are associated with
more severe periodontal disease progression, and higher viral loads
suggest poorer immune control, necessitating more aggressive
periodontal and antiviral management (43).
Recent periodontal consensus reports emphasize
adjunctive host-modulation therapies, such as sub-antimicrobial
doxycycline, NSAID-modulating regimens, and complement-targeting
agents, which may be particularly valuable when viral-driven immune
dysregulation underlies periodontal destruction (44,45).
Preventive strategies
Prevention plays a critical role in reducing viral
transmission and disease recurrence in individuals who are at a
high risk of developing viral infections:
i) Vaccination against human papillomavirus (HPV)
and varicella (chickenpox) can significantly reduce the risk of
developing virus-related oral diseases, such as oral cancers or
varicella-zoster-related oral lesions. The HPV vaccine, in
particular, has been shown to reduce the incidence of oral cancers,
whereas varicella vaccination helps prevent reactivation of the
virus, which can result in shingles with oral involvement (39).
ii) Immune support through the use of nutritional
supplements, such as vitamins D and C, zinc and
prebiotics/probiotics (46),
appropriate antiretroviral therapy (ART), typically a combination
of drugs, such as non-nucleoside reverse transcriptase inhibitors,
nucleoside reverse transcriptase inhibitors and protease inhibitors
(47), and other immune-boosting
treatments, including certain immunomodulatory cytokines (e.g.,
IL-2 or IFN-γ in select contexts) or therapeutic vaccines (48), can help strengthen the defence
system of the body against opportunistic viral infections.
iii) Strict oral hygiene practices are essential for
reducing the risk of developing oral viral infections and
preventing the recurrence of candidiasis and other opportunistic
infections. Brushing with fluoride toothpaste, regular flossing and
antiseptic mouthwashes (such as those containing chlorhexidine) can
help reduce the bacterial and viral loads in the mouth (43).
10. Emerging viral pathogens and periodontal
health
Coronavirus disease 2019 (COVID-19), caused by
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has
been implicated in various systemic effects that in turn affect
periodontal health. SARS-CoV-2, an RNA virus, is capable of
inducing widespread immune system dysregulation and inflammatory
responses, both of which can exacerbate pre-existing periodontal
diseases. Patients with COVID-19 have been reported to experience
oral manifestations, such as dry mouth, oral lesions and an altered
taste, which contribute to periodontal inflammation and tissue
breakdown (49,50).
COVID-19 is also associated with increased levels of
cytokines and pro-inflammatory mediators, which may exacerbate
gingival inflammation and accelerate the progression of
periodontitis. Furthermore, the ability of the virus to suppress
immune function and cause immunosuppression in severe cases
enhances susceptibility to secondary infections, including
bacterial periodontal pathogens. This immunosuppression, along with
increased viral load, can lead to worsening periodontal conditions,
particularly in patients with underlying chronic diseases such as
diabetes or HIV (51).
Additionally, patients with COVID-19 may have a
greater incidence of co-infection with periodontal pathogens, which
can increase the severity of periodontal destruction. The stress
associated with the pandemic and reduced access to dental care may
also contribute to the neglect of oral hygiene, further
exacerbating the progression of periodontal disease. These factors
collectively underscore the need for comprehensive oral health care
and preventive measures during and after COVID-19 infection
(52).
Recent studies have demonstrated that
SARS-CoV-2-induced lymphopenia, elevated levels of IL-6, and ACE2
overexpression in the gingival epithelium may heighten periodontal
inflammatory responses (49,50).
11. Future directions: Unlocking new avenues
in viral periodontology
The recognition that viruses are not bystanders, but
silent saboteurs in periodontal disease necessitates a paradigm
shift in both research and clinical management. To translate this
knowledge into tangible clinical benefits and to strengthen the
multifactorial etiology model of periodontitis, future
investigations must prioritize the following innovative areas:
i) Non-invasive viral biomarkers (salivary
diagnostics): Future research is required to focus on developing
highly sensitive, rapid and chair-side assays to detect and
quantify the viral load (e.g., EBV, HCMV and HIV) directly in
non-invasive samples, such as salivary fluid and GCF. Salivary
diagnostics provide a potential tool for early risk assessment,
monitoring disease activity and predicting the need for aggressive
antiviral/antibacterial therapies, particularly in
immunocompromised individuals (34).
ii) Omics technologies for the periodontal
microbiome: Employing advanced technologies, such as shotgun
metagenomics and RNA sequencing will allow for the simultaneous
analysis of the complete viral, bacterial, and fungal communities
(the ‘virome’, ‘bacteriome’ and ‘mycobiome’) within periodontal
lesions. This approach will move beyond simply detecting presence
to understanding the precise transcriptomic and proteomic
interactions that facilitate tissue destruction (6), providing a high-resolution map of the
viral-host-bacterial synergy described in the present review.
iii) Novel antivirals and targeted therapies:
Research is required to investigate next-generation therapeutic
agents beyond conventional antivirals (e.g., acyclovir and
ganciclovir) and HAART. This includes exploring the potential of
host-modulation therapies and advanced gene-editing techniques such
as CRISPR-based technologies to target and inactivate the specific
latent viral DNA of persistent viruses like herpesviruses (EBV and
HCMV) within periodontal host cells. Such targeted approaches may
provide a cure for virus-driven chronic inflammation (39).
iv) Vaccine development for viral periodontal risk:
Given the strong association between viruses, such as EBV, HCMV and
HPV with severe and aggressive forms of periodontitis, public
health strategies should consider the impact of viral vaccination.
Widespread vaccination against these specific
periodontitis-associated viruses, similar to existing HPV and
varicella vaccines, may offer a potent long-term preventive measure
against disease initiation and recurrence.
Emerging approaches, such as salivary virome assays,
metagenomic sequencing and CRISPR-based antiviral platforms may
enable personalized risk prediction and targeted suppression of
latent viral reservoirs in periodontal tissues. Integrating these
tools into diagnostic workflows could transform early detection and
individualized periodontal care.
12. Conclusion
Viruses are no longer considered passive bystanders
in periodontal diseases. The ability of viruses to establish
latency, evade host immunity, modulate inflammatory pathways and
synergize with bacterial pathogens positions them as significant
contributors to periodontal pathogenesis. Their presence in
periodontal pockets and gingival tissues underscores the need to
expand current diagnostic frameworks beyond bacteria-focused
models. Integrating viral detection, through molecular diagnostics,
salivary biomarkers and chairside assays into routine periodontal
evaluation represents a critical future direction that may
transform risk assessment and early diagnosis.
Furthermore, the complex interplay among viruses,
host immunity and the oral microbiome highlights the necessity for
interdisciplinary collaboration among periodontists, virologists,
immunologists and infectious disease specialists. Such
collaborations are essential for developing targeted antivirals,
vaccines, host-modulation strategies and personalized treatment
protocols.
As the understanding of viral contributions deepens,
periodontology should embrace a more holistic, multi-pathogen
approach to prevention, diagnosis and management, paving the way
for more accurate prognostication and improved clinical
outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AS and VRA were involved in the conceptualization of
the present review, as well as in data curation, in the literature
search, the evaluation of studies form the literature for inclusion
in the review, project administration, validation, visualization
and in the writing of the original draft, and in the writing,
reviewing and editing of the manuscript. SUN and DGK were involved
in the conceptualization of the present review, in data curation,
investigation, in the literature search, in project administration,
validation and study supervision. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Wankhede A, Wankhede S and Wasu S: Role of
genetic in periodontal disease. J ICDRO. 9(53)2017.
|
|
2
|
Popova C, Dosseva-Panova V and Panov V:
Microbiology of periodontal diseases. A review. Biotechnol
Biotechnol Eq. 27:3754–3759. 2013.
|
|
3
|
Abdulkareem AA, Al-Taweel FB, Al-Sharqi
AJB, Gul SS, Sha A and Chapple ILC: Current concepts in the
pathogenesis of periodontitis: From symbiosis to dysbiosis. J Oral
Microbiol. 15(2180743)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cekici A, Kantarci A, Hasturk H and Van
Dyke TE: Inflammatory and immune pathways in the pathogenesis of
periodontal disease. Periodontol 2000. 64:57–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Contreras A and Slots J: Herpesviruses in
human periodontal disease. J Periodontal Res. 35:3–16.
2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Parra B and Slots J: Detection of human
viruses in periodontal pockets using polymerase chain reaction.
Oral Microbiol Immunol. 11:289–293. 1996.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Botero A, Betancourth M, Jaramillo A,
Montoya Y and Hincapié O: Cytomegalovirus and Epstein-Barr virus in
chronic periodontitis and aggressive periodontitis. Rev Clín
Periodoncia Implantol Rehabil Oral. 3:65–71. 2010.
|
|
8
|
Slots J: Herpesviruses in periodontal
disease-an update. J Oral Microbiol. 2004;3(1).
|
|
9
|
Kamma JJ, Nakou M and Baehni PC:
Association of herpesviruses with the progression of periodontal
lesions. Oral Microbiol Immunol. 16:282–288. 2001.
|
|
10
|
Zhu C, Li F, Ce Y, Zhang S, Li Z and Zhou
X: Association between herpesviruses and chronic periodontitis: A
meta-analysis based on case-control studies. PLoS One.
10(e0144319)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li F, Zhu C, Deng FY, Wong MCM, Lu HX and
Feng XP: Herpesviruses in etiopathogenesis of aggressive
periodontitis: A meta-analysis based on case-control studies. PLoS
One. 12(e0186373)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Slots J: Human viruses in periodontitis.
Periodontol 2000. 53:89–110. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Belibasakis GN, Belstrøm D, Eick S, Gursoy
UK, Johansson A and Könönen E: Periodontal microbiology and
microbial etiology of periodontal diseases: Historical concepts and
contemporary perspectives. Periodontol 2000. 91:12–32.
2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Koonin E V..Krupovic M and Agol VI: The
Baltimore Classification of Viruses 50 Years Later: How Does It
Stand in the Light of Virus Evolution? Microbiol Mol Biol Rev.
85(e0005321)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Satija A and Rai JJ: Viruses: A Paradox in
Etiopathogenesis of Periodontal Diseases. IOSR Journal of Dental
and Medical Sciences (IOSR-JDMS) e-ISSN [Internet]. 2015;14:58-64.
Available from: www.iosrjournals.org.
|
|
16
|
Teles F, Collman RG, Mominkhan D and Wang
Y: Viruses, periodontitis, and comorbidities. Periodontology.
89:190–206. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gupta C and Dhruvakumar D: Role of
periodontopathogenic virus in periodontal disease: a review. Tanta
Dental J. 14:51–55. 2017.
|
|
18
|
Bhagat M, Tapashetti R, Fatima G and
Bhutani N: Role of Viruses in Periodontal Diseases. Galore Int J
Health Sci Res. 5:89–97. 2020.
|
|
19
|
Slots J: Oral viral infections of adults.
Periodontol 2000. 49:60–86. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Belardelli F: Role of interferons and
other cytokines in the regulation of the immune response. APMIS.
103:161–179. 1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Murphy K and Weaver C: Janeway's
Immunobiology. 9th edition. Garland Science, New York, NY,
2017.
|
|
22
|
Tizard I: Veterinary Immunology: An
Introduction. 10th edition. Elsevier, St. Louis, MO, 2018.
|
|
23
|
Contreras A, Zadeh HH, Nowzari H and Slots
J: Herpesvirus infection of inflammatory cells in human
periodontitis. Oral Microbiol Immunol. 14:206–212. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Taneja N, Kudva P, Goswamy M, Bhat G and
Kudva HP: Role of viruses in periodontal diseases: A review. Int J
Curr Microbiol Appl Sci. 6:1481–1488. 2017.
|
|
25
|
Chandran W, Dharmadhikari S and Shetty D:
Viruses in periodontal disease: A literature review. Int J Applied
Dental Sci. 8:242–245. 2022.
|
|
26
|
Slots J: Herpesviral-bacterial
interactions in periodontal diseases. Periodontol 2000. 52:117–140.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao Z, Liu W and Luo B: Role of Oxidative
Stress in the Epstein–Barr Virus Lifecycle and Tumorigenicity.
Future Virology. 18:465–477. 2023.
|
|
28
|
Botero JE, Rodríguez-Medina C,
Jaramillo-Echeverry A and Contreras A: Association between human
cytomegalovirus and periodontitis: A systematic review and
meta-analysis. J Periodontal Res. 55:551–558. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Izzat AW, Al-Ghurabi H, Adham LS and
Shaker ZF: Emerging Role of Viral Infection in Periodontal Disease.
Dentistry 3000: 13. https://doi.org/10.5195/d3000.2025.938.
|
|
30
|
Tejnani A, Mani A, Marawar P and Pawar B:
Herpes virus: A key missing piece of the periodontopathogenic
jigsaw puzzle. Chronicles of Young Scientists. 3(245)2012.
|
|
31
|
Kazi MMAG and Bharadwaj R: Role of
herpesviruses in chronic periodontitis and their association with
clinical parameters and in increasing severity of the disease. Eur
J Dent. 11:299–304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Feng X, Patel EU, White JL, Li S, Zhu X,
Zhao N, Shi J, Park DE, Liu CM, Kaul R, et al: Association of Oral
Microbiome With Oral Human Papillomavirus Infection: A Population
Study of the National Health and Nutrition Examination Survey,
2009-2012. J Infect Dis. 230:726–735. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rajapandi S, Sukumaran ES, Prasad KRN,
Venkatesan AV, Ravichandran TAS and Sabarathinam S: Viral Invasion
of the Oral Cavity: A Review of Viral Impact on Oral Health and the
Potential Use of Saliva as a Diagnostic Tool. Front Biosci (Elite
Ed). 17(33494)2025.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Patton LL, Phelan JA, Ramos-Gomez FJ,
Nittayananta W, Shiboski CH and Mbuguye TL: Prevalence and
classification of HIV-associated oral lesions. Oral Dis. 20 (Suppl
1):S5–S12. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cappuyns I, Gugerli P and Mombelli A:
Viruses in periodontal disease-A review. Oral Dis. 11:219–229.
2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lomelí-Martínez SM, González-Hernández LA,
Ruiz-Anaya A, Lomelí-Martínez MA, Martínez-Salazar SY, Mercado
González AE, et al: Oral Manifestations Associated with HIV/AIDS
Patients. Medicina (Kaunas). 58(1214)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Malek R, Gharibi A, Khlil N and Kissa J:
Necrotizing ulcerative gingivitis. Contemp Clin Dent. 8:496–500.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xu X, Zhang Y and Li Q: Characteristics of
herpes simplex virus infection and pathogenesis suggest a strategy
for vaccine development. Rev Med Virol. 29(e2052)2019.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Forghieri F, Cordella S, Marchesi F, Itri
F, Del Principe MI, Cavalieri E, Pasciolla C, Bonanni M, Criscuolo
M, Fiorentini A, et al: Antiviral prophylaxis to prevent herpes
simplex virus (HSV) and varicella zoster virus (VZV) reactivation
in adult patients with newly diagnosed acute leukemia: results of a
survey submitted to Italian centers belonging to SEIFEM
(Sorveglianza Epidemiologica Infezioni nelle Emopatie) group. Ann
Hematol. 103:4329–4332. 2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nuwagaba J, Li JA, Ngo B and Sutton RE: 30
years of HIV therapy: Current and future antiviral drug targets.
Virology. 603(110362)2025.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sadowski LA, Upadhyay R, Greeley ZW and
Margulies BJ: Current drugs to treat infections with herpes simplex
viruses-1 and -2. Viruses. 13(1228)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Parashar A, Sanikop S, Zingade A, Parashar
S and Gupta S: Virus-associated periodontal diseases. Futuristic
implications. J Dent Oral Disord Ther. 3:1–5. 2015.
|
|
43
|
Kim YJ, Song YW, Park SY, Cha JK, Lee HJ,
Yang SM, Park JB and Koo KT: Current understanding of the etiology,
diagnosis, treatment, and management of peri-implant diseases: a
narrative review for the consensus report of the Korean Academy of
Periodontology. J Periodontal Implant Sci. 54:377–392.
2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang CW: Emerging opportunity to implement
host modulation therapy in non-surgical periodontal therapy-The
role of probiotics and future perspectives. J Dent Sci.
19:1305–1306. 2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Herrera D, Tonetti MS, Chapple I,
Kebschull M, Papapanou PN, Sculean A, Abusleme L, Aimetti M,
Belibasakis G, Blanco J, et al: Consensus Report of the 20th
European Workshop on Periodontology: Contemporary and Emerging
Technologies in Periodontal Diagnosis. J Clin Periodontol. 52
(Suppl 29):4–33. 2025.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sinopoli A, Sciurti A, Isonne C, Santoro
MM and Baccolini V: The Efficacy of Multivitamin, Vitamin A,
Vitamin B, Vitamin C, and Vitamin D Supplements in the Prevention
and Management of COVID-19 and Long-COVID: An Updated Systematic
Review and Meta-Analysis of Randomized Clinical Trials. Nutrients.
16(1345)2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sax PE: Treatment of HIV infection. N Engl
J Med. 377:1590–1591. 2017.
|
|
48
|
Peluso MJ, Sandel DA, Deitchman AN, Kim
SJ, Dalhuisen T, Tummala HP, Tibúrcio R, Zemelko L, Borgo GM and
Singh SS: Correlates of HIV-1 control after combination
immunotherapy. Nature: Dec 1, 2025 (Epub ahead of print).
|
|
49
|
Schwartz J, Capistrano KJ, Gluck J,
Hezarkhani A and Naqvi AR: SARS-CoV-2, periodontal pathogens, and
host factors: The trinity of oral post-acute sequelae of COVID-19.
Rev Med Virol. 34(e2543)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Riad A, et al: Oral manifestations of
COdVID-19 infection: A systematic review. J Clin Med.
10(3063)2021.
|
|
51
|
Basso L, Chacun D, Sy K, Grosgogeat B and
Gritsch K: Periodontal Diseases and COVID-19: A Scoping Review. Eur
J Dent. 15:768–775. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Al-Hadeethi T, Charde P, Sunil S, Marouf N
and Tamimi F: Association Between Periodontitis and COVID-19. Curr
Oral Health Rep. 11:1–7. 2024.
|