Introduction
Overactive bladder (OAB) syndrome is defined by the
International Continence Society (ICS) as a symptom of urinary
urgency, with or without incontinence. It is usually associated
with nocturia and urinary frequency (1). OAB is divided into two types: i) Dry,
when urinary incontinence is not present; and ii) wet, when it is
present (2). Epidemiological
studies have consistently demonstrated that OAB is common among
both men and women, with prevalence increasing with age and symptom
profiles differing by sex (3,4).
However, a study conducted in Indonesia revealed no difference in
prevalence between the two sexes, suggesting the need for a
different approach to OAB in the Indonesian population (4).
In both men and women, lower urinary tract symptoms
(LUTS) associated with OAB can substantially impair daily
functioning and overall quality of life. In men, OAB has been
linked to a higher prevalence of sexual dysfunction, particularly
reductions in erectile function, sexual activity and sexual
satisfaction (5). Similar effects
have been observed in women: The cross-sectional study by Lin et
al (6) demonstrated that women
with OAB had significantly lower scores on several domains of the
female sexual function index (FSFI), including desire, arousal,
lubrication, orgasm and sexual satisfaction, with these reductions
strongly associated with the severity of OAB. Despite its
non-life-threatening nature, OAB is frequently under-recognised and
undertreated by both patients and clinicians, even though it can
lead to considerable deterioration in quality of life (7).
OAB can be managed using pharmacological therapy,
typically antimuscarinic agents or β3-adrenoceptor
agonists, or through conservative approaches such as lifestyle
modification and behavioural interventions, including pelvic floor
muscle training and bladder training. The most commonly used
first-line pharmacological agents are antimuscarinics, which reduce
detrusor smooth muscle activity and decrease bladder outlet
resistance (8,9). Current guidelines from the American
Urological Association and the European Association of Urology
recommend behavioural therapy as the initial treatment option for
OAB, with pharmacotherapy introduced as the following step when
needed (10). Both pharmacological
and behavioural approaches have demonstrated efficacy in reducing
OAB symptoms across multiple clinical parameters and validated
scoring systems (11).
The previous systematic review and meta-analysis by
La Rosa et al (12) also
found that both therapies have a similar efficacy for the treatment
of OAB. However, this statement should be interpreted with caution
due to the high risk of bias (12). The combination of both drugs and
behavioural therapy could enhance the each other's efficacy or may
be more effective than single therapy. However, to date, only a
limited number of studies have reported differences between
combination therapy and single therapy in patients with OAB
(11,12). Shapiro and Brucker (13) found that the combination of
behavioural therapy and pharmacological intervention was effective
for OAB syndrome. Therefore, the present systematic review and
meta-analysis aimed to compile and quantitatively measure the
difference between monotherapy and combination therapy in treating
the symptoms of OAB.
Data and methods
The present study was a systematic review and
meta-analysis conducted based on the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement
(14).
Study eligibility criteria
All studies on the effect of combination therapy in
both male and female patients with OAB syndrome were included. The
articles identified during the literature search were first
filtered based on their title and abstract to exclude irrelevant
studies. The full texts of the selected studies were then
thoroughly assessed. The inclusion criteria were as follows: i)
Studies reporting the effect of combination therapy on patients
with OAB; ii) studies with a follow-up period with ≥8 weeks; iii)
studies that contained a detailed explanation of the study
treatment, particularly the behavioural therapy conducted; iv)
studies that reported the outcome using measurable metrics, such as
bladder diary parameters or scoring systems, such as the Overactive
Bladder Symptom Score (OABSS). The exclusion criteria were the
following: i) Case series studies and case reports; ii) literature
reviews; iii) studies without full text available; iv) studies not
reported in English or Indonesian.
Literature search strategy
A systematic literature search was conducted on the
PubMed, Cochrane Library and ScienceDirect databases to identify
studies on the effect of combination therapy using both drugs and
behavioural therapy on reducing symptoms of OAB in male and female
patients. The search was conducted in August, 2024 using the key
words presented in Table I.
 | Table IKey words used during systematic
searching. |
Table I
Key words used during systematic
searching.
| Database | Key words | Hits |
|---|
| PubMed |
(pharmacological[All Fields] OR
pharmacological therapy[All Fields] OR pharmacological
treatment[All Fields] OR pharmacotherapy[All Fields] OR
antimuscarinic[All Fields] OR mirabegron[All Fields]) AND
(behavioural[All Fields] OR behavioural therapy[All Fields] OR
bladder training[All Fields] OR pelvic floor muscle training[All
Fields] OR pelvic floor training[All Fields] OR cognitive
behavioural therapy[All Fields]) AND (combination[All Fields] OR
multimodal[All Fields] OR integrated[All Fields] OR combined
modality therapy[All Fields]) AND (overactive bladder[All Fields]
OR oab[All Fields]) | 48 |
| Cochrane | #1
(pharmacotherapy):ti,ab,kw OR (pharmacological):ti,ab,kw OR
(antimuscarinic):ti,ab,kw OR (mirabegron):ti,ab,kw OR
(‘pharmacological treatment’):ti,ab,kw #2 (behavioural):ti,ab,kw OR
(‘behavioural therapy’):ti,ab,kw OR (‘bladder training’):ti,ab,kw
OR (‘pelvic floor muscle training’):ti,ab,kw OR (‘cognitive
behavioural therapy’):ti,ab,kw #3 (combination):ti,ab,kw OR
(‘multimodalities’):ti,ab,kw OR (‘combined modality
therapy’):ti,ab,kw OR (integrated):ti,ab,kw #4 (‘overactive bladder
syndrome’):ti,ab,kw OR (‘overactive bladder’):ti,ab,kw OR (‘OAB
syndrome’):ti,ab,kw OR (OAB):ti,ab,kw #5 #1 AND #2 AND #3 AND
#4 | 42 |
| ScienceDirect | ((‘pharmacological
therapy’ OR ‘antimuscarinic’ OR Mirabegron’) AND (‘behavioural
therapy’ OR ‘pelvic floor muscle training’) AND (Combination) AND
(‘overactive bladder’ OR OAB) | 326 |
Risk of bias assessment
The selected studies were tested for their risk of
bias based on their study designs. Randomised controlled trial
(RCT) studies were assessed using the Cochrane's Risk of Bias tool,
integrated into the Review Manager (RevMan) software version
5.4(15). Cohort studies were
assessed using the Newcastle-Ottawa Scale (NOS) that reviews three
risk of bias domains: Selection, comparability and outcome
(16). Cross-sectional studies
were assessed using the analytical cross-sectional risk of bias
checklist from the Joanna-Briggs Institute (JBI) (16).
Data extraction
The data extracted from the selected studies were
the following: i) Authors and year of publication; ii) study
design; iii) sex prevalence; iv) pharmacological and behavioural
therapy regimen; v) sample size in each subgroup; vi) follow-up
period; and vii) outcome parameters reported. The data of the
general characteristics were then compiled into a table. The
outcomes were analysed qualitatively in a systematic review and,
when possible, quantitatively in a meta-analysis.
Statistical analysis
The outcomes were analysed qualitatively in a
systematic review and, where appropriate, quantitatively in a
meta-analysis. For the quantitative synthesis, continuous outcomes
were analysed using mean differences (MDs), while categorical
outcomes were analysed using odds ratios (ORs), each with
corresponding 95% confidence intervals (CIs). Meta-analyses were
performed using the inverse-variance method under a random-effects
model to account for potential clinical and methodological
heterogeneity across studies, irrespective of the observed
I2 values, in accordance with the recommendations of the
Cochrane Handbook for Systematic Reviews of Interventions. Forest
plots were generated to visually present individual study estimates
and pooled effect sizes. Pooled estimates and 95% CIs were
calculated using study-specific effect measures, and P-values for
overall effects were derived from Z-tests.
Statistical heterogeneity was assessed using
Cochran's Q (χ2) test and quantified using the
I2 statistic, with I2 values >50%
indicating substantial heterogeneity. Publication bias was assessed
qualitatively by visual inspection of funnel plot symmetry, where
applicable. A two-sided P-value <0.05 was considered to indicate
a statistically significant difference. All quantitative analyses
were conducted using Review Manager (RevMan) version 5.4 (Cochrane
Collaboration).
Results
Literature search
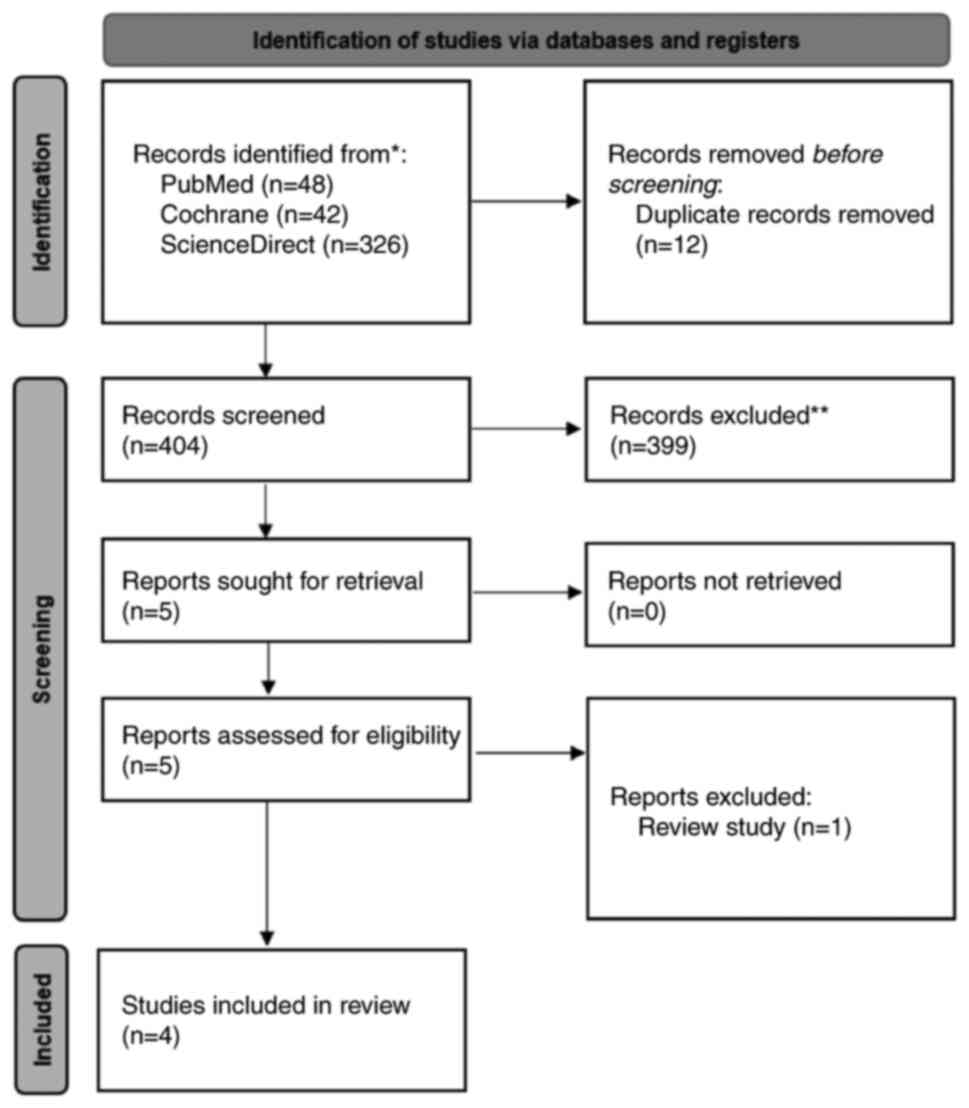
A total of 416 records were identified (Fig. 1). After removing 12 duplicates in
Zotero, 404 titles and abstracts were screened, of which 399 were
excluded based on the pre-defined criteria: Studies not involving
OAB populations, not evaluating combined
behavioural-pharmacological therapy, using ineligible designs (case
reports, case series, reviews and editorials), involving non-adult
populations, or lacking measurable clinical outcomes. In total,
five full-text articles were assessed, and one article was excluded
as it was a narrative review. Thus, four studies met all the
eligibility criteria and were included in the present systematic
review.
Characteristics of the included
studies
The characteristics of the studies included in the
present systematic review and meta-analysis are presented in
Table II. The table summarises
four studies evaluating the effectiveness of monotherapy and
combination therapy for overactive bladder syndrome. Burgio et
al (17) conducted an RCT in
an all-female group, assessed the impact of oxybutynin chloride
combined with pelvic floor exercises and urgency control strategies
over 8 weeks, focusing on urinary frequency. The second study was
by Klutke et al (18), who
conducted a cohort study with 86% female participants and examined
the effect of daily tolterodine extended-release (4 mg), alongside
behavioural interventions such as pelvic floor training, over a
period of 12 weeks. Their study measured the total micturition,
urgency, and nocturia of the patients'. Subsequently, Burgio et
al (11), conducted another
RCT, but in an all-male population. Their study explored the
combination of tolterodine and tamsulosin with behavioural therapy
and compared it with patients receiving drug therapy alone and
behavioural therapy alone over a period of 12 weeks. The
researchers measured the frequency, urgency and nocturia of the
patients. The final analysed study was by Lin et al
(19), who also conducted an RCT
in an all-male group and investigated the effects of 50 mg of
mirabegron daily combined with urge control strategies over a
period of 12 weeks. Their study measured the OABSS of the patients,
the International Index of Erectile Function-5 (IIEF-5), and
International Prostate Symptom Score (IPSS). These studies
collectively demonstrate the varied approaches to managing urinary
symptoms, with a focus on specific outcomes depending on the
demographic and intervention used, providing valuable insight into
the effectiveness of these therapies across different patient
groups.
 | Table IICharacteristics of the included
studies. |
Table II
Characteristics of the included
studies.
| Authors, year of
publication | Study design | Sex prevalence | Pharmacological
therapy | Combination
therapy | No. of samples | Follow-up
duration | Outcome
measured | (Refs.) |
|---|
| Burgio, et
al 2000 | RCT | All female | Oxybutynin
chloride, titrated from 2.5 to 15 mg daily | Oxybutynin
chloride, titrated from 2.5 to 15 mg daily + PFMT exercises,
urgency control strategies, and a home exercise program | BT group, 55; DT
group, 19; CT group, 35 | 8 weeks | Frequency | (17) |
| Klutke, et
al 2009 | Cohort | 86% female | Tolterodine
Extended Release (ER) 4 mg once daily | Tolterodine
extended release at 4 mg once daily + pelvic floor muscle training,
urgency control strategies, and lifestyle modifications | Total, 416 | 12 weeks | Frequency, urgency,
nocturia | (18) |
| Burgio, et
al 2020 | RCT | All male | Tolterodine
(sustained-release, 4 mg once daily) and tamsulosin (0.4 mg once
daily) | Tolterodine
(sustained-release, 4 mg and Tamsulosin 0,4 mg + pelvic floor
muscle training, urge suppression strategies, delayed voiding, and
lifestyle modifications | BT group, 71; DT
group, 68; CT group, 65 | 12 weeks | Frequency, urgency,
nocturia | (11) |
| Lin, et al
2023 | RCT | All male | Mirabegron at 50 mg
once daily | Mirabegron 50 mg +
urge control strategy | BT group, 36; CT
group, 65 | 12 weeks | Voiding diary OABSS
IIEF-5 IPSS | (19) |
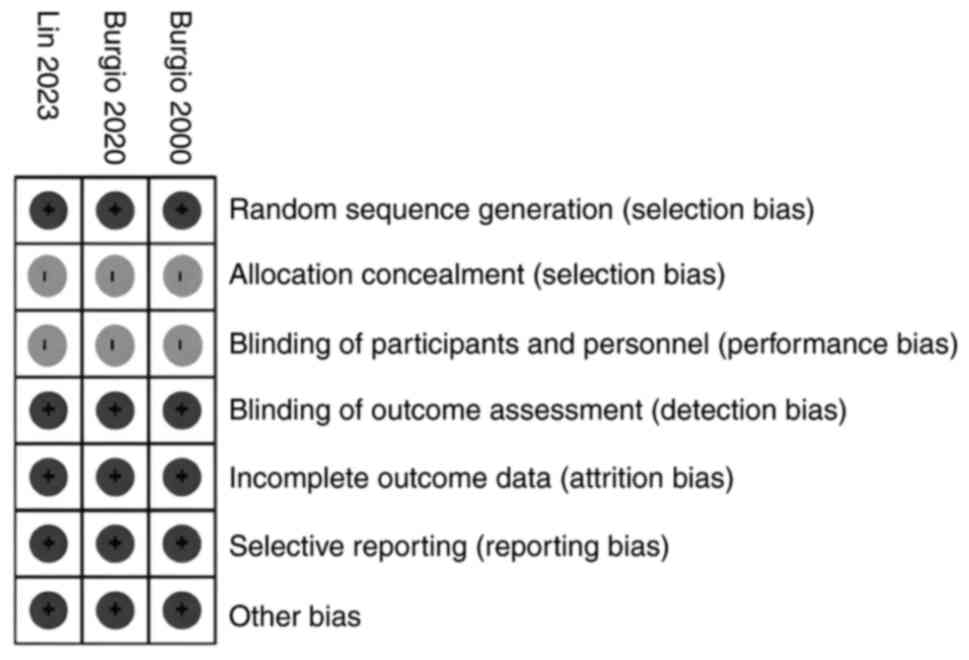
Risk of bias analysis
Risk of bias analysis was conducted based on the
designs of the selected studies. For RCT studies, the Cochrane
checklist was used, and the results revealed that these studies had
a low risk of bias (Fig. 2).
However, there was an unclear risk of bias due to the lack of
description on how the study concealed the allocation for each
patient. The unclear risk of bias was also due to the nature of the
study treatment, making it impossible to apply blinding.
For the cohort study by Klutke et al, the
risk of bias was assessed using the Newcastle-Ottawa scale
(Table III). The results
revealed that the study had a low risk of bias with a total score
of 9 (Table III). Publication
bias was not formally assessed using funnel plots or statistical
tests due to the limited number of included studies, as such
methods are not recommended when <10 studies are available.
 | Table IIIRisk of bias analysis for the study
by Klutke et al (18). |
Table III
Risk of bias analysis for the study
by Klutke et al (18).
| Risk of bias
parameters | Klutke et al
(18) |
|---|
| Representativeness
of the exposed cohort (1) | 1 |
| Selection of the
non-exposed cohort (1) | 1 |
| Ascertainment of
exposure (1) | 1 |
| Demonstration that
outcome of interest was not present at start of study (1) | 1 |
| Comparability of
cohorts on the basis of the design or analysis (2) | 2 |
| Assessment of
outcome (1) | 1 |
| Was follow-up long
enough for outcomes to occur? (1) | 1 |
| Adequacy of follow
up of cohorts (1) | 1 |
| Total | 9 |
Bladder diary variable
A total of two studies compared changes in bladder
diary data between combination and single therapy regimens. Burgio
et al (11) reported
baseline, post-treatment and the change in the number of voiding
frequencies, mean nocturia, mean urgency score and maximum urgency
score. Their study found that compared to behavioural therapy or
drug therapy, combination therapy was superior in reducing daytime
frequency (reduction of 3.8±2.1 vs. 3.7±2.3 vs. 3.2±2.5 episodes;
P=0.33), mean nocturia (reduction of 1.0±1.0 vs. 0.9±1.0 vs.
0.8±1.0 episodes; P=0.34), mean urgency (reduction of 0.3±0.6 vs.
0.1±0.6 vs. 0.2±0.6 episodes; P=0.06) and maximum urgency score
(reduction of 0.6±0.8 vs. 0.3±0.7 vs. 0.4±0.7 episodes; P=0.07).
However, after 12 weeks, there was no statistically significant
difference between the three groups (11).
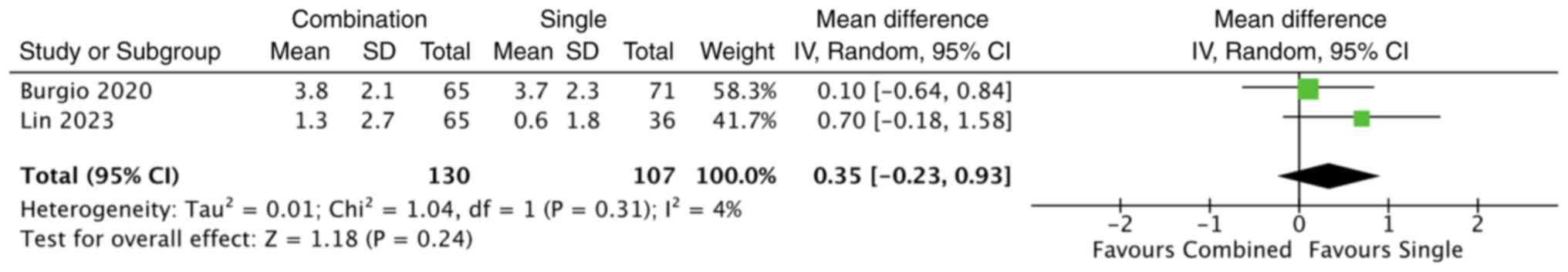
Lin et al (19) compared urge control behavioural
therapy alone with a combination of behavioural therapy and
mirabegron 50 mg. At 12 weeks, combination therapy resulted in a
greater reduction in daytime voiding frequency compared with
behavioural therapy alone (mean reduction, 1.3±2.7 vs. 0.6±1.8
episodes), although this difference did not reach statistical
significance (P=0.198) (19). A
quantitative synthesis of the two included studies using a
random-effects model demonstrated no statistically significant
difference between combination therapy and behavioural therapy
alone in reducing daytime frequency (MD, 0.35; 95% CI, -0.23 to
0.93; P=0.24) (Fig. 3).
Heterogeneity across studies was low (I2=4%).
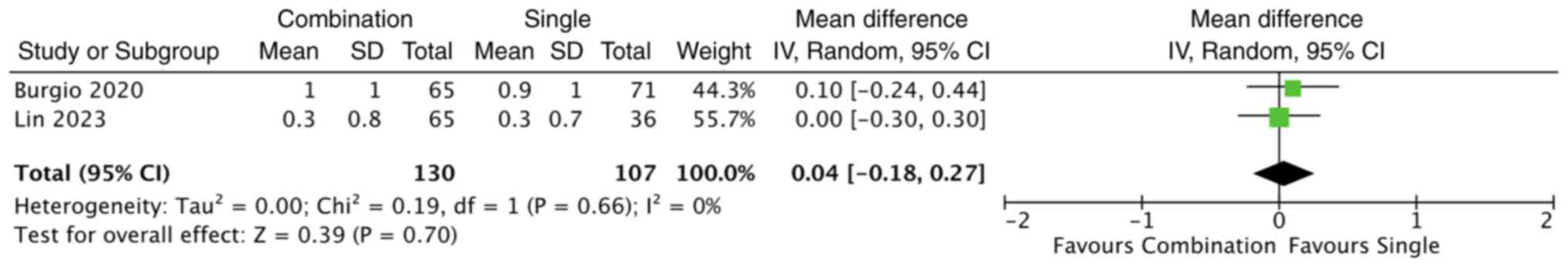
The studies by both Burgio et al (11) and Lin et al (19) evaluated changes in nocturia
episodes; however, their findings were inconsistent. Burgio et
al (11) reported a greater
reduction in nocturia episodes with combination therapy, whereas
Lin et al (19) observed no
significant differences between the treatment groups. Consequently,
a random-effects meta-analysis was conducted to estimate the
overall effect of combination therapy compared with behavioural
therapy alone. The pooled analysis demonstrated no statistically
significant difference in nocturia reduction between the two groups
(MD, 0.04; 95% CI, -0.18 to 0.27; P=0.70) (Fig. 4). No significant heterogeneity was
observed among the studies (I2=0%).
Klutke et al (18) conducted a one-group cohort study to
assess the combined effects of tolterodine extended release and
behavioural intervention in patients with OAB syndrome who were
dissatisfied with their prior antimuscarinic treatments. Their
study was conducted over a period of 16 weeks and included 416
participants who received 4 mg tolterodine extended release along
with a self-administered behavioural intervention for the first 8
weeks. In their study, significant improvements were observed in
the bladder diary variable, including up to -3.0 episodes reduced
in total micturition per 24 h, -5.0 episodes reduced in
urgency-related micturition, -1.0 episodes reduced in urgency
urinary incontinence and -1.0 episode reduced in nocturnal
micturitions (18).
Burgio et al (17) examined the effects of combination
therapy and monotherapy on reducing incontinence episodes,
specifically among patients converting from monotherapy to
combination therapy. Their study initially assessed the
effectiveness of monotherapies, either using oxybutynin therapy or
behavioural therapy through pelvic floor muscle training and urge
control strategy. The group that received behavioural therapy alone
demonstrated a mean reduction in incontinence of 84.1%. By
contrast, the group treated with oxybutynin alone exhibited a mean
reduction of 71.8%. The placebo group, which received no active
treatment, exhibited a mean reduction in incontinence of 60.5%
(17).
Upon completion of the initial monotherapy for at
least 8 weeks, some participants opted to switch to a combination
therapy, adding either behavioural therapy to their drug regimen or
vice versa. The transition to combined therapy yielded significant
improvements in incontinence reduction for these participants. The
participants who initially received behavioural therapy and
subsequently added oxybutynin experienced an increase in
incontinence reduction from 57.5% (±29.4%) after only receiving
behavioural therapy to 88.5% (P=0.034) following the combination
therapy (17). Similarly,
participants who began with oxybutynin and then incorporated
behavioural therapy into their treatment plan saw their
incontinence reduction rise from 72.7% (±24.9%) after only drug
therapy to 84.3% (P<0.001) following the combination therapy
(17).
OABSS
Lin et al (19) reported a detailed assessment of
OABSS changes between combination therapy and monotherapy. Both
groups began with similar baseline scores. After treatment, both
showed improvements across all subgroups, including daytime
frequency, nocturia, urgency, urinary incontinence, and total OABSS
score. The changes were slightly more pronounced in the combination
group, particularly in urgency and total OABSS score. However, the
differences between the groups were not statistically significant
(P>0.05). Overall, both therapies were effective, with no clear
superiority of one over the other (19). The study by Burgio et al
(11) demonstrated that combining
behavioural therapy with drug therapy achieved superior outcomes
compared to either treatment alone. This was evident in the
Overactive Bladder Questionnaire (OAB-q) scores, where the
combination therapy group had a much lower mean score [mean (SD):
23.8 (22.1)] than the behavioural therapy group [43.0 (28.2)] and
the drug therapy group [39.5 (30.0)]. The difference was
statistically significant (P<0.001), indicating better symptom
improvement with the combination approach (11).
IPSS score
Lin et al (19) evaluated the effects of combination
therapy on the IPSS of patients with OAB syndrome. Their results
revealed that both the behavioural and combination therapy groups
experienced improvements over period of 12 weeks. However, the
changes in the combined therapy group did not differ significantly
from those of the monotherapy group, indicating the comparable
efficacy of both therapies (19).
However, the study by Burgio et al (11) on the effects of combination therapy
on the IPSS of patients demonstrated that combination therapy was
more favourable, with a lower mean score [mean (SD): 9.2 (4.8)]
compared to behavioural therapy alone [11.4 (5.3)] and drug therapy
alone [11.5 (5.8)]. This finding was also statistically significant
(P<0.001), further supporting the greater efficacy of the
combination treatment in managing symptoms (11).
Sexual function
The study by Lin et al (19) also used the IIEF-5 to assess the
effects of combination therapy on sexual function. Their data
demonstrated that both behavioural and combination therapies led to
slight improvements in sexual function over a period of 12 weeks.
However, the changes in the IIEF-5 scores were not statistically
significant (19).
Moreover, the same study used the Male Sexual Health
Questionnaire-Ejaculatory Dysfunction Short Form (MESH EjD SF) to
investigate the effects of combination therapy on sexual function
(19). The MESH EjD SF scores in
that study assessed various aspects of ejaculatory function,
including frequency, strength, semen volume and overall
satisfaction over a period of 12 weeks. At baseline, both groups
had similar scores for frequency, strength and semen volume. Over
the period of 12 weeks, there were slight improvements in each
aspect, although there were no significant differences. The total
scores for Q1-Q3 (frequency, strength and volume) remained
relatively stable, with only minimal differences from the baseline
(19).
Adverse events
Klutke et al (18) reported that the adverse events
observed during their study period were associated with treatment
with tolterodine. Their study demonstrated that the the majority of
common adverse events associated with standard treatments were dry
mouth in 25 patients (6%) and constipation in 20 patients (5%)
(18). The majority of the adverse
events in the study by Burgio et al (11) were observed in patients who
underwent the drug therapy (79% of patients, or 44 of 65), and 2%
(1 of 65) were classified as extreme adverse events (11).
Discussion
The present systematic review and meta-analysis
evaluated the efficacy of a combination of behavioural and
pharmacological therapy compared with monotherapy in patients with
OAB syndrome. Across the four included studies, the pooled analyses
revealed no significant differences between combination therapy and
monotherapy in reducing voiding frequency (MD, 0.35; 95% CI,
-0.23-0.93; P=0.24) or nocturia episodes (MD, 0.04; 95% CI,
-0.18-0.27; P=0.70) over the short-term follow-up. However,
individual studies reported trends favouring combination therapy
for specific symptoms, particularly urgency scores and quality of
life measures, suggesting that the combination approach may offer
modest additional benefits in selected patients.
The absence of statistically significant differences
in the meta-analysis may be partially explained by variability in
the behavioural interventions used across studies. For instance,
pelvic floor muscle training (PFMT) as implemented in the study by
Burgio et al (11)
demonstrated substantial improvements in the bladder diary
parameter even when delivered without pharmacotherapy, indicating
that well-structured behavioural therapy can independently produce
strong clinical responses. By contrast, the urge-control strategy
used in the study by Lin et al (19) appears to yield more modest effects,
which may reduce the incremental value observed when
pharmacotherapy is added. These differences highlight the
importance of the type, intensity and quality of behavioural
interventions when evaluating the effectiveness of combined therapy
for OAB.
Furthermore, PFMT has been proven effective in
controlling OAB symptoms and is recommended by the ICS (1). Its benefits are attributed to three
key mechanisms: i) Enhancing pelvic floor muscle strength through
repetitive contractions that support the urethra; ii) improving
timing and coordination, enabling anticipatory contractions during
urgency or leakage triggers; and iii) engaging core musculature,
which promotes reflexive co-activation of the pelvic floor
(20-22).
Evidence across diverse populations supports these mechanisms: The
study by Bo et al (20)
found that five of 11 RCTs demonstrated a significant improvement
in OAB symptoms among pregnant women. In another study, Tibaek
et al (21) demonstrated
reductions in urinary frequency in patients post-stroke; and the
study by Hagovska et al (23) reported improved LUTS in men with
benign prostatic hyperplasia when PFMT was combined with Silodosin.
Together, these findings highlight PFMT as a versatile and
effective behavioural intervention for the management of OAB.
The minor differences between combination therapy
and monotherapy observed in the studies included in the present
systematic review and meta-analysis may be due to the short
follow-up durations, which were limited to a maximum of 12 weeks.
Early improvements were consistently noted, such as the symptom
reductions at 4 weeks in the study by Lin et al (19) and at 6 weeks in the study Burgio
et al (11). However, these
advantages did not persist by week 12, where the outcomes of
combination therapy and monotherapy converged (11,19)
Similar early responses were reported in the studies by Burgio
et al (17) and Klutke
et al (18), both of which
demonstrated symptom improvement by 8 weeks. This pattern suggests
that OAB treatments often produce an initial response followed by a
therapeutic plateau, reducing the observable differences between
treatment strategies over time (24).
Another contributing factor is poor long-term
treatment adherence, which is a well-recognised challenge in the
management of OAB. Enemchukwu et al (25) reported that treatment adherence
within 1 year decreased to 32% for behavioural therapy and 15-40%
for pharmacotherapy due to side-effects, limited perceived benefit,
treatment-related fatigue and communication barriers. Even for
mirabegron, which is known to have better adherence than
solifenacin or oxybutynin, only 25% of women continued therapy
after 1 year. These adherence limitations likely blunt the
sustained effects of prolonged treatment, limiting the ability of
short-term studies to capture meaningful differences between
combination therapy and monotherapy (24,25).
Moreover, Lin et al (19) did not directly compare the
behavioural urge control strategy alone with pharmacological
mirabegron alone. Thus, it is unclear which type of therapy has a
greater impact on clinical improvement in patients with OAB
(19,24). However, the finding that
combination therapy is not significantly different from behavioural
therapy alone suggests that the addition of mirabegron therapy to
patients with OAB already undergoing urge control therapy does not
provide significant clinical benefits (25). Similar findings were also reported
in male sexual function, measured by IIEF-5 and IPSS. Mirabegron is
a selective β3-adrenoceptor agonist that inhibits
β-adrenergic receptors in detrusor muscles, causing relaxation of
those smooth muscles (26).
Studies have demonstrated that, compared to the placebo, mirabegron
is as effective as peripheral tibial nerve stimulation in reducing
LUTS symptoms in patients with OAB (24,27).
Studies comparing combination therapy with
monotherapy for OAB have yielded mixed results. Lin et al
(19) reported improvements in
OABSS and IPSS scores in both groups, with slightly greater,
although not statistically significant, reductions in urgency and
total scores in the combination group (P>0.05). By contrast,
Burgio et al (11) observed
significantly improved outcomes with combined behavioural and
pharmacological therapy, including lower OAB-q and IPSS scores
(P<0.001), suggesting a potential advantage for combination
approaches. However, all the studies included in the present
systematic review had relatively short follow-up durations, with
none extending beyond 12 weeks. Earlier studies, such as those of
Burgio et al (17) and
Klutke et al (18)
demonstrated symptom improvement by 8 weeks, while the studies by
Burgio et al (11) and Lin
et al (19) reported early
benefits at 6 and 4 weeks, respectively. Notably, although both
studies observed better early improvements with combination
therapy, these differences were no longer significant by week 12
(11,19). This pattern suggests that the
long-term efficacy of combination therapy may converge with that of
monotherapy, although longer follow-up studies are needed to
confirm this trend (24,25).
The findings of the present systematic review and
meta-analysis indicate that both behavioural and pharmacological
therapies remain effective options for the management of OAB, and
combination therapy may provide modest short-term advantages in
certain domains, such as urgency and quality of life outcomes.
However, the lack of sustained superiority at 12 weeks underscores
the need for clinicians to set realistic expectations with patients
regarding the expected trajectory of symptom improvement and the
potential for therapeutic plateau (24,25).
As urge-control strategies and PFMT can independently produce
significant improvements, sometimes comparable to those achieved
with medication, behavioural therapy should remain a cornerstone of
first-line management (20,21).
Pharmacotherapy, including mirabegron, may still be valuable as an
adjunct, particularly for patients who do not achieve adequate
relief with behavioural therapy alone. However, clinicians should
emphasise the importance of treatment adherence to achieve durable
symptom control (26,27). Overall, individualised treatment
selection that considers patient preference, symptom burden, and
motivation is essential to optimise real-world outcomes in the
management of OAB.
The present systematic review has several
limitations. First, all the included studies had short follow-up
durations of a maximum of 12 weeks, which is a key limitation given
that OAB is a chronic condition requiring long-term management.
Second, treatment adherence remains a major challenge in the
treatment of OAB, particularly for pharmacotherapy, and poor
compliance may influence the observed outcomes across studies.
Another limitation concerns the variability in treatment protocols;
both behavioural interventions and physical therapies, such as
PFMT, differed across studies, making direct comparisons difficult.
Additionally, heterogeneity in outcome reporting meant that many
parameters were derived from a single study, and only two studies
contributed data to the meta-analysis of voiding frequency,
nocturia and OABSS. The generally small sample sizes further
limited the robustness and generalisability of the findings.
Despite these limitations, however, the present
systematic review has several strengths. It included three
randomised controlled trials with low risk of bias, providing a
relatively strong evidence base. The diversity of reported outcomes
allowed the review to explore the impact of combination therapy
across multiple clinical domains, including LUTS symptoms, sexual
function and adverse effects. Collectively, these strengths
contribute valuable insight into the comparative effectiveness of
combination therapy vs. monotherapy in the management of OAB.
In conclusion, the present systematic review and
meta-analysis found no significant difference between combined
behavioural-pharmacological therapy and monotherapy in reducing
voiding frequency or nocturia in patients with OAB over a
short-term follow-up period. Although some studies reported modest
early advantages with combination therapy, these benefits were not
sustained by 12 weeks. Behavioural interventions, particularly PFMT
and urge-control strategies, demonstrated potent independent
effects, while pharmacotherapy remains a useful adjunct for
selected patients. Overall, both treatment approaches are
effective, and clinical decisions should be individualised based on
symptom burden, patient preference, and likelihood of adherence.
Further high-quality trials with longer follow-up periods are
required to clarify the long-term comparative effectiveness of
combination therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AMMA and FW conceived and designed the study. AMMA
conducted the literature search and data extraction. FW and HER
analysed and interpreted the data. AMMA drafted the manuscript. FW
and HER critically revised the manuscript. FW and HER confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Bo K, Frawley HC, Haylen BT, Abramov Y,
Almeida FG, Berghmans B, Bortolini M, Dumoulin C, Gomes M, McClurg
D, et al: An International Urogynecological Association
(IUGA)/International Continence Society (ICS) joint report on the
terminology for the conservative and nonpharmacological management
of female pelvic floor dysfunction. Neurourol Urodyn. 36:221–244.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Scarneciu I, Lupu S, Bratu OG, Teodorescu
A, Maxim LS, Brinza A, Laculiceanu AG, Rotaru RM, Lupu AM and
Scarneciu CC: Overactive bladder: A review and update. Exp Ther
Med. 22(1444)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eapen RS and Radomski SB: Review of the
epidemiology of overactive bladder. Res Rep Urol. 8:71–76.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sumardi R, Mochtar CA, Junizaf Santoso BI,
Setiati S, Nuhonni SA, Trihono PP, Rahardjo HE and Syahputra FA:
Prevalence of urinary incontinence, risk factors and its impact:
Multivariate analysis from Indonesian nationwide survey. Acta Med
Indones. 46:175–182. 2014.PubMed/NCBI
|
|
5
|
Millman AL, Cheung DC, Hackett C and
Elterman D: Overactive bladder in men: A practical approach. Br J
Gen Pract. 68:298–299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin XD, Lin N, Ke ZB, Xu N, Jiang P and Li
H: Effects of overactive bladder syndrome on female sexual
function. Medicine (Baltimore). 100(e25761)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Edmonds VS and Khan AA: Overactive
Bladder: The Patient Perspective. Curr Bladder Dysfunct Rep.
19:89–94. 2024.
|
|
8
|
Jiang YH and Kuo HC: Current optimal
pharmacologic therapies for overactive bladder. Expert Opin
Pharmacother. 24:2005–2019. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kreydin EI, Gomes CM and Cruz F: Current
pharmacotherapy of overactive bladder. Int Braz J Urol.
47:1091–1107. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cameron AP, Chung DE, Dielubanza EJ,
Enemchukwu E, Ginsberg DA, Helfand BT, Linder BJ, Reynolds WS,
Rovner ES, Souter L, et al: The AUA/SUFU guideline on the diagnosis
and treatment of idiopathic overactive bladder. J Urol. 212:11–20.
2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Burgio KL, Kraus SR, Johnson TM II,
Markland AD, Vaughan CP, Li P, Redden DT and Goode PS:
Effectiveness of combined behavioural and drug therapy for
overactive bladder symptoms in men: A Randomized clinical trial.
JAMA Intern Med. 180:411–419. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
La Rosa VL, Duarte de Campos da Silva T,
Rosa de Oliveira A, Marques Cerentini T, Viana da Rosa P and Telles
da Rosa LH: Behavioural therapy versus drug therapy in individuals
with idiopathic overactive bladder: A systematic review and
meta-analysis. J Health Psychol. 25:573–585. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shapiro KK and Brucker BM: Treatment of
overactive bladder in men: Is it really different? Neurourol
Urodyn. 41:1975–1982. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: a revised tool for assessing risk of bias in
randomised trials. BMJ. 366(l4898)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wells G, Shea B, O'Connel D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. The Ottawa Hospital Research Institute. https://ohri.ca/en/who-we-are/core-facilities-and-platforms/ottawa-methods-centre/newcastle-ottawa-scale.
Accessed April 13, 2025.
|
|
17
|
Burgio KL, Locher JL and Goode PS:
Combined behavioural and drug therapy for urge incontinence in
older women. J Am Geriatr Soc. 48:370–374. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Klutke CG, Burgio KL, Wyman JF, Guan Z,
Sun F, Berriman S and Bavendam T: Combined effects of behavioural
intervention and tolterodine in patients dissatisfied with
overactive bladder medication. J Urol. 181:2599–2607.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin CC, Kuo HC, Li JR and Chuang YC:
Comparative study between behavior therapy and behavior therapy
plus mirabegron 50 mg in sexually active men with bothersome
overactive bladder symptoms-a multicenter, randomized study. Int
Neurourol J. 27:182–191. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bo K, Fernandes ACNL, Duarte TB, Brito LGO
and Ferreira CHJ: Is pelvic floor muscle training effective for
symptoms of overactive bladder in women? A systematic review.
Physiotherapy. 106:65–76. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tibaek S, Gard G, Dehlendorff C, Iversen
HK, Biering-Soerensen F and Jensen R: Is pelvic floor muscle
training effective for men with poststroke lower urinary tract
symptoms? A single-blinded randomized, controlled trial. Am J Mens
Health. 11:1460–1471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sheng Y, Carpenter JS, Ashton-Miller JA
and Miller JM: Mechanisms of pelvic floor muscle training for
managing urinary incontinence in women: A scoping review. BMC
Womens Health. 22(161)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hagovska M, Svihra J Sr, Macko L, Breza J
Jr, Svihra J Jr, Luptak J and Lachvac L: The effect of pelvic floor
muscle training in men with benign prostatic hyperplasia and
overactive bladder. World J Urol. 42(287)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu P, Li Y, Shi B, Zhang Q and Guo H:
Comparison of different types of therapy for overactive bladder: A
systematic review and network meta-analysis. Front Med (Lausanne).
9(1014291)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Enemchukwu EA, Subak LL and Markland A:
Barriers and facilitators to overactive bladder therapy adherence.
Neurourol Urodyn. 41:1983–1992. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Carlson KV, Rovner ES, Nair KV, Deal AS,
Kristy RM and Hairston JC: Persistence with mirabegron or
antimuscarinic treatment for overactive bladder syndrome: Findings
from the PERSPECTIVE registry study. Low Urin Tract Symptoms.
13:425–434. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Duckett J and Balachandran A: Tolerability
and persistence in a large, prospective case series of women
prescribed mirabegron. Int Urogynecol J. 27:1163–1167.
2016.PubMed/NCBI View Article : Google Scholar
|