Introduction
Epithelioid hemangioendothelioma is a rare vascular
tumor arising from endothelial cells, which can occur in the liver,
lungs, bones and soft tissues. When it involves the lungs, it is
known as pulmonary epithelioid hemangioendothelioma (PEH), which is
characterized by a low to moderate malignant potential (1,2).
Historically, PEH was initially described in the medical literature
as an ‘intravascular bronchoalveolar tumor’. Over time, its
nomenclature and understanding have greatly evolved; however, its
rarity has limited comprehensive research and the development of
standardized treatment protocols (3). The etiology of PEH remains largely
unclear, although it is deemed to be associated with a combination
of genetic predispositions and environmental exposures. The
condition predominantly affects young women and is often identified
incidentally upon imaging analyses, typically presenting as
multiple bilateral pulmonary nodules (1). The clinical behavior of PEH exhibits
considerable variability among patients, ranging from indolent
disease progression in some cases, to rapid advancement and
metastasis in others (4,5).
The clinical presentation of PEH is non-specific,
with some patients remaining asymptomatic until the disease reaches
the advanced stages. By contrast, others may develop symptoms, such
as dyspnea or chest pain as a result of tumor growth or associated
complications (4). Given its
rarity, PEH is often misdiagnosed as other pulmonary conditions.
Its incidence is markedly low, estimated at <1 case per million
individuals annually, with ~250 cases reported in the medical
literature (1). The present case
report describes a case of symptomatic PEH in a young male worker
found upon computed tomography (CT) angiography. The case was
written in accordance with the CaReL guidelines (6). In addition, all references were
checked to avoid citing non-peer-reviewed data (7).
Case report
Patient information
On August, 2024, a 31-year-old male worker presented
to Smart Health Tower (Sulaymaniyah, Iraq) with a 1-month history
of left-sided chest pain without any associated symptoms. He had a
significant smoking history of 10 years, although he denied any
past medical or surgical history. An analysis of family history did
not reveal any notable findings. The patient initially sought
evaluation by a cardiologist for his chest pain. A cardiac workup,
including echocardiography and a CT coronary angiogram, revealed
normal coronary vessels. However, the CT coronary angiogram
revealed pulmonary nodules, warranting further investigation. He
was subsequently referred to the Thoracic and Vascular Department
at Smart Health Tower (Sulaymaniyah, Iraq).
Clinical findings
The vital signs (body temperature, pulse rate,
respiratory rate, and blood pressure) of the patient were within
the normal range. Auscultation revealed good bilateral air entry
without added sounds.
Diagnostic assessment
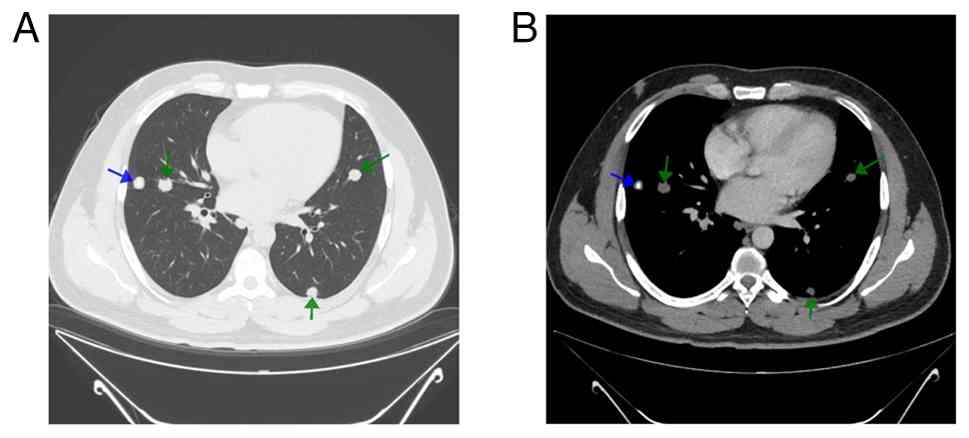
A contrast-enhanced chest CT scan demonstrated
multiple well-defined pulmonary nodules scattered throughout both
lungs (Fig. 1). The largest
nodule, measuring 15 mm, was located in the right upper lobe. Some
nodules exhibited central calcifications and variable post-contrast
enhancement, with no cavitation observed. The patient underwent a
CT-guided core needle biopsy. A histopathological examination was
performed by the hospital laboratory as follows: The analysis was
performed on 5-µm-thick, paraffin-embedded sections. The sections
were stained with 10% neutral buffered formalin at room temperature
for 24 h. They were then stained with hematoxylin and eosin
(H&E; Bio Optica Co, Italy for 1-2 min at room temperature and
examined under a light microscope (Leica Microsystems GmbH). The
histopathological examination revealed benign alveolar tissue,
skeletal muscle tissue and hyalinized material (data not shown).
Although a few atypical cells were noted, the tissue sample was
insufficient for a definitive diagnosis or proper immune staining
assessment. The case was reviewed by a multidisciplinary team,
which recommended a video-assisted thoracoscopic surgery (VATS) to
obtain a biopsy for a more comprehensive evaluation.
Intervention
Under general anesthesia, the patient underwent VATS
for a biopsy of bilateral lung nodules, pleura and mediastinal
lymph nodes. A thoracoscope was inserted through double ports to
access the pulmonary parenchyma and pleural surfaces.
Representative nodules from the left lung and pleura were excised
in addition to a mediastinal lymph node biopsy. The procedure and
postoperative period were uneventful. The chest tube was removed 48
h following the procedure, and the patient was discharged in a
stable condition. A histopathological examination of the lung
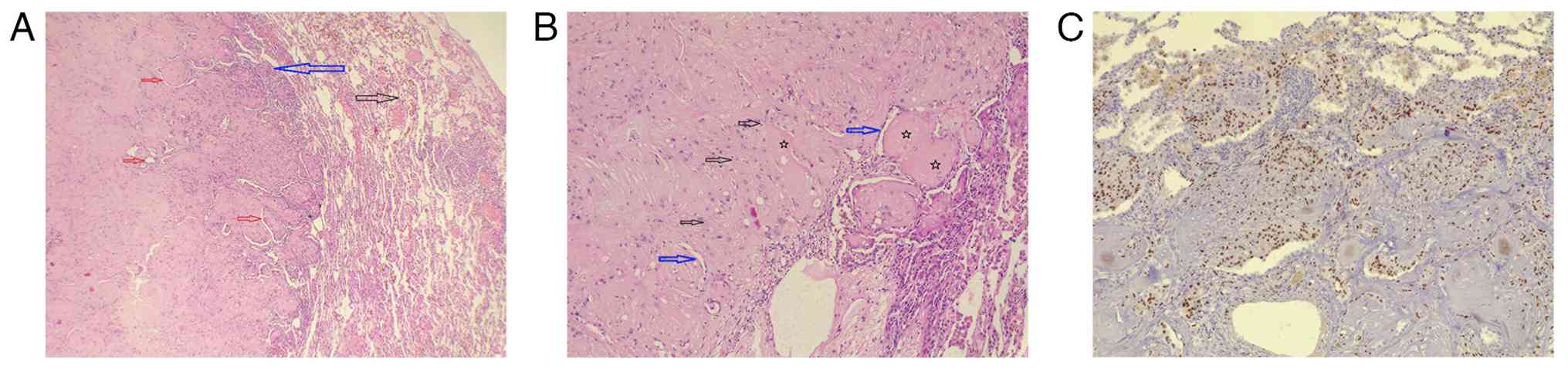
nodules (performed as described above) revealed two well-defined
masses with centrally hyalinized stroma, amorphous eosinophilic
deposits and focal coagulative necrosis (Fig. 2). Slit-like spaces within the
masses were lined by plump, eosinophilic, epithelioid cells with
bland, round-to-oval nuclei, some of which contained inclusions.
There was no marked atypia or significant mitotic activity. The
surrounding lung parenchyma exhibited only mild lymphocytic
infiltration. Immunohistochemistry was performed on 4-µm-thick
paraffin-embedded sections and heat-induced epitope retrieval
(HIER) was performed using citrate buffer (pH 6.0) for AE1/AE3 and
TTF and EDTA buffer (pH 9.0) for ERG. Retrieval was carried out at
95-98˚C for 20-30 min, followed by cooling to room temperature.
Endogenous peroxidase activity was blocked using 3% hydrogen
peroxide for 10 min at room temperature, followed by blocking of
nonspecific binding with 5% normal serum for 20 min at room
temperature. The sections were incubated with primary antibodies
against ERG (1:100; clone EP111, cat. no. GA65961-2), TTF-1 (1:100;
clone 8G7G3/1, cat. no. M3575) and AE1/AE3 (1:100; cat. no.
GA05361-2) (all from Dako; Agilent Technologies, Inc.) for 30-60
min at room temperature, followed by a polymer-based HRP-conjugated
secondary detection system (EnVision™, cat. no. K4003; Dako;
Agilent Technologies, Inc.) for 30 min at room temperature and
visualization with 3,3'-diaminobenzidine. The slides were
counterstained with hematoxylin (Thermo Fisher Scientific, Inc.)
for 30 sec at room temperature, dehydrated and coverslipped and
then examined under a light microscope (Leica Microsystems GmbH) at
magnifications ranging from x4 to x10. Immunohistochemistry with
Congo red and periodic acid-Schiff staining (MilliporeSigma; with
and without diastase digestion; used for 20 min at room
temperature) yielded negative results in the amorphous deposits.
The histological picture, along with epithelioid cell positivity
for ERG and negativity for TTF1 and AE1/AE3, supported a diagnosis
of PEH (Fig. 2). The pleural
biopsy did not reveal any notable findings, and the mediastinal
lymph nodes were benign.
Follow-up
The patient was referred to an oncologist for
further evaluation and follow-up. During routine follow-up, a right
axillary lymph node appeared suspicious on ultrasound. Fine-needle
aspiration was performed, which was negative for malignancy. The
patient was prescribed a pazopanib tablet (400 mg/day). At the
3-month follow-up, the CT scan demonstrated stable disease with no
evidence of progression.
Discussion
Pulmonary epithelioid hemangioendothelioma is a
rare, low-grade vascular tumor characterized by a low to
intermediate potential for malignancy. It typically presents as
multiple small nodules in both lungs (8). Due to its rarity, data on this
disease remain limited. A study reported a male-to-female ratio of
1:1 among 16 patients with PEH (9). Conversely, another study indicated
that the disease predominantly affects women, with an incidence
rate of 2- to 4-fold higher in females compared to males (2). The age range for the diagnosis of PEH
exhibits vast variability. A previous study reported an age range
of 25 to 54 years (4), while
another study identified an average onset age of ~47.75 years
(9). Similarly, Xuan et al
(2) reported an average age of
onset of ~40 years. Herein, in reviewing 12 cases of PEH (2,5,10-16),
the age of patients was found to range from 35 to 70 years, with a
mean age of 53.9 years, including 7 males and 5 females. The
disease was equally distributed, with unilateral and bilateral
involvement observed in 6 cases each (50%) (Table I). The case presented herein was
that of a male smoker in his thirties who presented with bilateral
PEH.
 | Table ISummary of 12 cases of pulmonary
epithelioid hemangioendothelioma identified in the literature. |
Table I
Summary of 12 cases of pulmonary
epithelioid hemangioendothelioma identified in the literature.
| First author, year of
publication | Age, years | Sex | Presentation | Site of disease | Radiological
findings | Tumor size (cm) | Metastasis |
Immunohistochemistry | Treatment | Adjuvant therapy | Follow-up and
outcome | (Refs.) |
|---|
| Xuan, 2024 | 50 | F | Recurrent coughing,
excess phlegm production | LULL | CT: A mass with lymph
node enlargement; PET: Increased SUV | 5.2 | Bones, soft tissues,
and multiple lymph nodes | Negative: Napsin A,
TTF-1, CK (pan), Vimentin, CgA, CD56, Syn, Calretinin, CK7, EMA;
ositive: CD34, INI-1, BRG1, Fli-1, CD31, ERG | Thoracoscopic surgery
(wedge resection) | Chemotherapy
(ifosfamide, epirubicin, paclitaxel), targeted therapy
(bevacizumab, anlotinib), immunotherapy (cardonilizumab) | Succumbed to the
disease | (2) |
| Chen, 2023 | 55 | M | Incidental
finding | LULL | CT: Partial
enhancement in soft tissue | 1.3 | Not reported | Negative: S-100, PCK,
CK8, CK7, TTF-1, P63, HMB45, Syn, CgA, CD56, SMA, PAX8, WT-1,
TFE-3; Positive: CAMTA-1, CD34, CD31, EMA | Lobectomy by VATS,
lymph node dissection | N/A | Alive (no recurrence
within 5 months post-surgery) | (10) |
| Aung, 2020 | 58 | F | Chest tightness,
shortness of breath | LLLL | CT: Bilateral ground
glass opacities; PET: No increased SUV | 0.8 | Not reported | Negative:
Pancytokeratin, TTF-1, Pax-8, CAM 5.2, EMA, CDX2, CK20, CK7;
Positive: CD31, ERG | Conservative
(N/A) | N/A | Alive (no progression
after one year of follow-up) | (11) |
| Da Silva, 2020 | 45 | M | Incidental
finding | Bilateral | X-ray and CT: Solid
lung nodules | >1 | Not reported | N/A | Only follow up | N/A | Alive (stable after 9
years of follow-up) | (12) |
| | 70 | F | Cough, dyspnea,
weight loss | Bilateral | CT: Bilateral
nodules, gross calcifications, bronchial stenosis | N/A | Not reported | N/A | Endobronchial
prosthesis, high-dose external radiotherapy | Targeted therapy with
pazopanib | Died due to massive
hemoptysis, cardio-pulmonary arrest | (12) |
| Xiong, 2020 | 54 | F | Incidental
finding | Bilateral | CT: Multiple small
nodules with no calcification | N/A | Not reported | N/A | Wedge resection by
VATS | Watchful
waiting | Alive (no
progression at 3-month follow-up) | (13) |
| Mao, 2017 | 43 | M | Chest pain | Bilateral | CT: Multiple small
nodules | 2.2 | Not reported | Negative:
Cytokeratin-7, CD56, thyroid transcription factor 1, and Syn;
Positive: CD31, CD34, Vimentin, Bcl-2, and partially for CD99 | Palliative
thoracotomy: wedge resection of the right lower lobe | N/A | N/A | (14) |
| Mesquita, 2017 | 35 | M | Incidental
finding | Bilateral | X-ray and CT:
Multiple small bilateral nodules | <10 | Not reported | Positive: CD34 | Only follow up | N/A | Alive (after 48
months of follow-up) | (5) |
| | 67 | F | Cough, hematic
sputum, back pain | Bilateral | X-ray: Left lung
opacification; CT: Multiple nodules with necrosis and
atelectasis | N/A | Left hilar
lymphadenopathy, splenic nodules | Positive: CD31,
CD34, Factor VIII | Thoracic surgery
(wedge resection) | Chemotherapy
(carboplatin/paclitaxel, doxorubicin/cyclophos-phamide) | Succumbed due to
respiratory failure | (5) |
| Zheng, 2017 | 44 | M | Recurrent
hemoptysis | RMLL | CT: Multiple small
nodules, pleural effusion with calcification, and metastases | 5 | Chest wall, pleura,
liver, and mediastinum | Negative: CD34;
Positive: CD31, CK, Vimentin | Lobectomy, pleural
decortication | Apatinib
monotherapy | Succumbed due to
respiratory failure after six months | (15) |
| Soo, 2016 | 59 | M | Progressive
breath-lessness, productive cough | RULL | Heterogeneous
enhancing mass | N/A | Not reported | Positive: CD31,
CD34, and factor VIII | N/A | N/A | Succumbed due to
respiratory failure | (16) |
| | 67 | M | Hemiplegia | RULL | CT: Mass with
spiculated margins | N/A | Brain | Positive: CD31,
CD34, and factor VIII | Traditional
treatment (N/A) | N/A | Succumbed within 1
month | (16) |
The etiology of PEH remains uncertain. Although an
association with estrogen receptors has been observed in some
cases, its occurrence in both sexes challenges the hormonal
hypothesis (4). No definitive risk
factors or causes have been identified for this condition, and it
often presents with nonspecific symptoms (10,12,13).
Clinical manifestations vary significantly, with common symptoms
including cough, shortness of breath and chest pain. In a previous
study, 17 of 18 patients exhibited respiratory symptoms, such as
cough and shortness of breath (17). Similarly, another study reported
that 7 of 16 patients experienced symptoms such as cough, sputum,
shortness of breath, hemoptysis and chest pain (9). Some cases may remain asymptomatic and
are detected incidentally during imaging for unrelated indications
(10,12,13).
Pleural thickening and pleural effusion have been observed,
particularly in cases involving pleural metastases (9). A previous case report described a
patient presenting with a loculated pleural effusion, which was
initially misdiagnosed as necrotizing pneumonia (18). Less commonly, hemoptysis and
abdominal pain have been reported (5,15,17).
In a previous study, abdominal pain was noted in a single case
where respiratory symptoms were absent (17). In the present study, among the 12
reviewed cases, the most common presenting symptoms were coughing
(33.3%), dyspnea (25%), chest pain or tightness (16.7%) and
hemoptysis (16.7%). However, 33.3% of cases were incidentally
diagnosed. The only symptom in the current case was chest pain, and
he had a smoking history for 10 years.
Pulmonary epithelioid hemangioendothelioma generally
has a favorable prognosis, with a median survival of 4.6 years and
a 5-year survival rate of ~60%. However, PEH tends to have a worse
prognosis compared to soft tissue or bone primaries, particularly
when bilateral pulmonary involvement, pleural invasion, or
concomitant liver disease is present. This contributes to a
relatively higher mortality risk in PEH cases. By contrast,
epithelioid hemangioendothelioma originating in soft tissue or skin
often behaves more indolently and is associated with better
outcomes after surgical resection (19).
Key factors influencing outcomes include anemia,
pleural invasion, and lymph node and distant metastases (2). A multinodular pattern on CT scans is
linked to improved survival, while pleural involvement with
malignant effusion is associated with poorer outcomes (11). The literature review by Amin et
al (20), reviewing >90
cases, identified the male sex, symptomatic presentation, and
pleural effusion as independent predictors of reduced survival. The
tumor can metastasize, involving pleural and mediastinal lymph
nodes, as well as distant organs such as the liver, skin, bones,
spleen, and central nervous system (2). In a study of 18 patients with PEH,
extrapulmonary involvement was identified in 7 cases, notably
affecting the liver and bones (21). A total of 4 cases (33.3%) reviewed
in the present study were metastatic (2,5,15,16).
The metastatic regions included bones, soft tissues, lymph nodes,
spleen, chest wall, liver, pleura, mediastinum and brain. In the
present case report, despite the positron emission tomography (PET)
scan not yet being performed, an initial follow-up ultrasound
revealed a suspicious right axillary lymph node. However,
fine-needle aspiration was negative for malignancy.
On CT imaging, PEH typically manifests as numerous
small nodules distributed across both lungs, generally measuring
<2 cm in diameter. These nodules are frequently located near
blood vessels, reflecting their vascular endothelial origin
(21). In some cases, punctate
calcifications may be present within the nodules, and associated
pleural thickening can also be observed (8). PET/CT scans often reveal increased
fluorodeoxyglucose uptake in PEH lesions, signifying heightened
metabolic activity and potential metastasis. A positive association
exists between lesion size and maximum standardized uptake values
(21). Isolated lesions in PEH are
uncommon; when present, they may exceed 5 cm in their largest
dimension (2). The diagnosis of
PEH depends on a histopathological examination supported by
immunohistochemistry, as the disease lacks specific clinical or
imaging features. Microscopically, tumor cells form irregular
nests, strips, or sheets with eosinophilic, polygonal cytoplasm
that may contain vacuoles. Primitive vascular lumens with
erythrocytes are occasionally seen. Tumor cells often extend along
alveolar septal blood vessels, with reactive hyperplasia in
adjacent alveolar epithelium. Rarely, they invade alveolar or
bronchial walls in an infiltrative pattern. Immunohistochemistry
reveals the positivity of the tumor cells for vascular endothelial
markers, such as CD31, CD34, Fli-1 and ERG, with CD31 being highly
specific (2). Histological atypia
(high nuclear grade, necrosis, high mitoses), larger tumor size and
multi-organ involvement are pathological features associated with
higher metastasis risk and poorer prognosis in epithelioid
hemangioendothelioma, whereas routine immunostaining profiles and
fusion status primarily aid diagnosis rather than prognosis
(22).
Among the cases reviewed herein,
immunohistochemistry data were available for 9 cases, all of which
were positive for CD31. In the present case, immunohistochemical
staining supported the PEH diagnosis with ERG positivity and TTF1
and AE1/AE3 negativity.
Due to its rarity, PEH lacks a standard treatment
approach (2). In patients with
localized PEH or disease confined to one lung or discrete lesions,
complete surgical resection remains the mainstay of potentially
curative therapy. It is associated with favorable local control and
improved long-term outcomes when feasible, whereas the benefit of
surgery in multifocal or systemic disease is less clear (2,23).
For patients with multifocal pulmonary nodules, bilateral disease,
pleural involvement, or distant metastases not amenable to surgery,
the role of standard cytotoxic chemotherapy is limited, and no
consensus regimen exists. Combination chemotherapy has yielded
mixed results and generally limited efficacy in PEH (3). Given its vascular origin, therapies
targeting angiogenesis are rational options in advanced disease.
Agents that inhibit the vascular endothelial growth factor (VEGF)
signaling pathway, such as tyrosine kinase inhibitors, like
pazopanib, have demonstrated prolonged disease stabilization and
symptomatic improvement, while other VEGF/VEGFR pathway inhibitors,
including sorafenib, sunitinib, and bevacizumab, have shown
variable responses (24,25). In selected patients with indolent
or asymptomatic disease, a watchful waiting/active surveillance
approach may also be considered, as some PEH cases exhibit slow
progression (23).
Among the 12 cases reviewed herein, 6 patients
succumbed to disease progression and related complications.
Following 3 months of follow-up of the present case, the CT scan
demonstrated stable disease with no evidence of progression. A
notable limitation of the present report is the lack of long-term
follow-up data to assess outcomes and consequences. Other
limitations include unretrievable histopathology figure of initial
CT-guided core needle biopsy and the lack of molecular testing for
WWTR1-CAMTA1 fusion or immunohistochemistry for CAMTA1, CD31 and
CD34 to support the diagnosis further.
In conclusion, PEH may present with non-specific
symptoms and may often be diagnosed incidentally during
checkups.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FHK and AMA were major contributors to the
conception of the study and to the literature search for related
studies. FHK, RHA, HOA and MNH contributed to the clinical
management of the patient, assisted with data acquisition and
interpretation, and participated in the literature review and
manuscript preparation. HKA, BAA and SSA contributed to the
conception and design of the study, the literature review, the
critical revision of the manuscript and the processing of the
table. SHT was the radiologist who performed the assessment of the
case. AMA, RMA and HAY were the pathologists who conducted the
histopathological diagnosis of the case. FHK and AMA confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient for participation in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di̇rol H, Özbudak Ö and Özbudak İ: A rare
primary lung tumor: Pulmonary epithelioid hemangioendothelioma and
a literature review. Turk J Oncol. 35:340–344. 2020.
|
|
2
|
Xuan R, Cui Z and Zhao L: Isolated
pulmonary epithelioid hemangioendothelioma: A case report. Medicine
(Batimore). 103(e40959)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ye B, Li W, Feng JI, Shi JX, Chen Y and
Han BH: Treatment of pulmonary epithelioid hemangioendothelioma
with combination chemotherapy: Report of three cases and review of
the literature. Oncol Lett. 5:1491–1496. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shao J and Zhang J: Clinicopathological
characteristics of pulmonary epithelioid hemangioendothelioma: A
report of four cases and review of the literature. Oncol Lett.
8:2517–2522. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mesquita RD, Sousa M, Trinidad C, Pinto E
and Badiola IA: New insights about pulmonary epithelioid
hemangioendothelioma: Review of the literature and two case
reports. Case Rep Radiol. 2017(5972940)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Prasad S, Nassar M, Azzam AY, José FGMS,
Jamee M, Sliman RK, Evola G, Mustafa AM, Abdullah HO, Abdalla BA,
et al: CaReL guidelines: A consensus-based guideline on case
reports and literature review (CaReL). Barw Med J. 2:13–19.
2024.
|
|
7
|
Abdullah HO, Abdalla BA, Kakamad FH, Ahmed
JO, Baba HO, Hassan MN, Bapir R, Rahim HM, Omar DA, Kakamad SH, et
al: Predatory publishing lists: A review on the ongoing battle
against fraudulent actions. Barw Med J. 2:26–30. 2024.
|
|
8
|
Jang YC, Hung WC, Su TC and Wu WP: Primary
pulmonary epithelioid hemangioendothelioma. BMJ Case Rep.
16(e254915)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu JX, Xie Q, Zhong AH, Lin QH and Lan
CQ: Clinical analysis of 16 cases of pulmonary epithelioid
hemangioendothelioma. Zhonghua Jie He He Hu Xi Za Zhi. 44:966–971.
2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
10
|
Chen X, Wang Y, Che G and Shen C: An
extremely rare case of pulmonary epithelioid hemangioendothelioma.
Thorac Cancer. 14:2519–2522. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aung TT, Chu A, Kondapi D, Markabawi D,
Mirchia K and Kaul P: A case of pulmonary epithelioid
hemangioendothelioma with literature review. Case Rep Oncol Med.
2020(8048056)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Da Silva TP, Taveira MD, Guzman HO and
Guimaraes MD: Pulmonary epithelioid hemangioendothelioma: Two case
reports of a rare neoplasm with an unpredictable prognosis. SN
Comprehensive Clinical Medicine. 2:2427–2432. 2020.
|
|
13
|
Xiong W, Wang Y, Ma X and Ding X: Multiple
bilateral pulmonary epithelioid hemangioendothelioma mimicking
metastatic lung cancer: Case report and literature review. J Int
Med Res. 48(0300060520913148)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mao X, Liang Z, Chibhabha F, Ou W, Li N,
Xu P and Wang S: Clinico-radiological features and next generation
sequencing of pulmonary epithelioid hemangioendothelioma: A case
report and review of literature. Thorac Cancer. 8:687–692.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zheng Z, Wang H, Jiang H, Chen E, Zhang J
and Xie X: Apatinib for the treatment of pulmonary epithelioid
hemangioendothelioma: A case report and literature review. Medicine
(Batimore). 96(e8507)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Soo CI, Ng BH, Tan EL and Hamid FA:
Ambiguous presentations of pulmonary epithelioid
hemangioendothelioma: Two case reports of a rare pulmonary
malignancy. SAGE Open Med Case Rep.
4(2050313X16650323)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Han J, Wei JG, Gao XZ, Xu Y, Zhang L, Xie
YL, Liu YQ, Fan XY, Li WC and Li SL: Clinicopathological features
of pulmonary epithelioid hemangioendothelioma: A study of 18 cases.
Zhonghua Bing Li Xue Za Zhi. 49:550–555. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nguyen T, Chagani F, Khasawneh M,
Khasawneh T and Jamalifard F: Epithelioid hemangioendothelioma
presenting as necrotizing pneumonia. Cureus.
15(e39328)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Z and He S: Epithelioid
hemangioendothelioma: Incidence, mortality, prognostic factors, and
survival analysis using the surveillance, epidemiology, and end
results database. J Oncol. 2022(2349991)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Amin RM, Hiroshima K, Kokubo T, Nishikawa
M, Narita M, Kuroki M and Nakatani Y: Risk factors and independent
predictors of survival in patients with pulmonary epithelioid
haemangioendothelioma. Review of the literature and a case report.
Respirology. 11:818–825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu H, Wang J, Lang J and Zhang X:
Pulmonary epithelioid hemangioendothelioma: Imaging and clinical
features. J Comput Assist Tomogr. 45:788–794. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang S, Liang Y, Yang Y, Guo L, Li W and
Shi S: Clinicopathological features, risk model and prognosis of
115 cases of epithelioid hemangioendothelioma: A single-center
study. Front Oncol. 15(1577968)2025.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsuchihashi K and Baba E: Epithelioid
hemangioendothelioma-its history, clinical features, molecular
biology and current therapy. Jpn J Clin Oncol. 54:739–747.
2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Semenisty V, Naroditsky I, Keidar Z and
Bar-Sela G: Pazopanib for metastatic pulmonary epithelioid
hemangioendothelioma-a suitable treatment option: Case report and
review of anti-angiogenic treatment options. BMC Cancer.
15(402)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Park MS, Ravi V and Araujo DM: Inhibiting
the VEGF-VEGFR pathway in angiosarcoma, epithelioid
hemangioendothelioma, and hemangiopericytoma/solitary fibrous
tumor. Curr Opin Oncol. 22:351–355. 2010.PubMed/NCBI View Article : Google Scholar
|