Introduction
Symptoms resembling Parkinson's disease (PD) have
been described in ancient texts (1). However, it was first considered as a
standalone disease by James Parkinson in 1817(2). Currently, PD is the second most
common neurodegenerative condition following Alzheimer's disease,
has the most rapidly increasing prevalence among neurological
disorders (3) and is projected to
affect ~13 million individuals worldwide by the year 2040(4). This disease places a substantial
burden on the population of Georgia. In 2016, 5,900 patients were
diagnosed with PD in the country, which represented an 8.6% change
from the 1990 baseline (5).
Although extrapyramidal motor deficit is the
hallmark of PD, pain is also very frequent during this disorder,
with a prevalence of >80% reported by certain studies (6,7).
Despite its ubiquity, the instruments used to assess PD-related
pain have only begun to be developed recently. The first ever scale
used to assess pain in PD was validated by Chaudhuri et al
(8) in 2015 and is currently
referred as the King's Parkinson's disease Pain Scale (KPPS).
The present study aimed to evaluate the presence and
extent of the association between certain demographic (age and sex)
and PD-related (disease duration, cognitive function, anxiety,
depression and disability) characteristics and KPPS scores in the
Georgian population. To the best of our knowledge, this study
represents the first ever scientific application of KPPS in
Georgia.
Materials and methods
Patient data
Data collection was performed between November 1,
2023, and May 29, 2024. A total of 100 adult patients, aged 45-86
years, were assessed in a single, PD-dedicated facility. Patients
outside this age range, patients with Parkinsonism due to drugs or
other conditions and those with severe cognitive impairment [Mini
Mental State Examination (MMSE) score <20] were excluded. The
baseline characteristics of the study sample are presented in
Table I.
 | Table IBaseline characteristics of the study
population. |
Table I
Baseline characteristics of the study
population.
| Characteristic | No. of participants
(n=100) | Percentage |
|---|
| Sex | | |
|
Female | 58 | 58.0 |
|
Male | 42 | 42.0 |
| Age group (years)
(mean age, 65.03 years; SD, 10.98 years) | | |
|
45-49 | 9 | 9.0 |
|
50-59 | 15 | 15.0 |
|
60-69 | 39 | 39.0 |
|
70-79 | 30 | 30.0 |
|
80-86 | 7 | 7.0 |
| Living
arrangements | | |
|
With
family | 87 | 87.0 |
|
Alone | 13 | 13.0 |
| Marital status | | |
|
Married | 89 | 89.0 |
|
Single | 7 | 7.0 |
|
Widowed | 4 | 4.0 |
| Time since disease
onset (mean, 5.9 years, SD, 4.7 years; range, 0.3-20 years) | | |
|
0-4
years | 60 | 60 |
|
5-10
years | 32 | 32 |
|
11-15
years | 7 | 7 |
|
16-20
years | 5 | 5 |
All patients with a diagnosis of PD who visited The
Movement Disorders Referral Centre at the Khechinashvili University
Hospital, Tbilisi, Georgia were examined. The present study aimed
to assess the correlation between PD-related pain and age, time
since disease onset, disability due to impaired mobility,
depression severity, anxiety severity, cognitive function and PD
severity. Pain was assessed using the KPPS, followed by an
objective neurological examination. According to the research
protocol for patient assessment, the following tools were also
used: The Schwab and England Activities of Daily Living (ADL) scale
was used to quantify disability; Beck's Depression Inventory (BDI)
(9) was used to evaluate the
severity of depression; Generalized Anxiety Disorder Test 7 (GAD-7)
(10) was used to measure anxiety
severity; Montreal Cognitive Assessment (MoCA) (11) was used to assess the cognitive
function of patients; and the Movement Disorder Society-Unified PD
Rating Scale (MDS-UPDRS) (12) was
used to determine the severity of PD. Self-reported questionnaires,
BDI and GAD-7, were completed by the patients on paper in a
clinical setting without assistance. MoCA, KPPS, ADL and MDS-UPDRS
were administered and scored by the first author.
Statistical analysis
Following acquisition, the data were analyzed for
associations by biostatisticians (please see the Acknowledgements
section below) using SPSS software, version 23.0 (IBM, USA).
Pearson's correlation analysis was used for normally distributed
data (Kolmogorov-Smirnov normality test, P>0.05), while
Spearman's Rho was used for non-normally distributed data. KPPS
domain 1 data were analyzed using Spearman's Rho due to short
scoring scale, while Pearson's correlation was used for other
domains and the total score. Correlation coefficients of 0.4-0.69
were considered moderate, while coefficients of 0.1-0.39 were
deemed weak. Multiple regression analyses were then performed
separately for individual domain and KPPS total scores. In
addition, relative risk was calculated between male and female
patients for being positive for each item, and individual domain
means and KPPS domain and total score means were compared between
the sexes. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Data analysis revealed moderate, positive
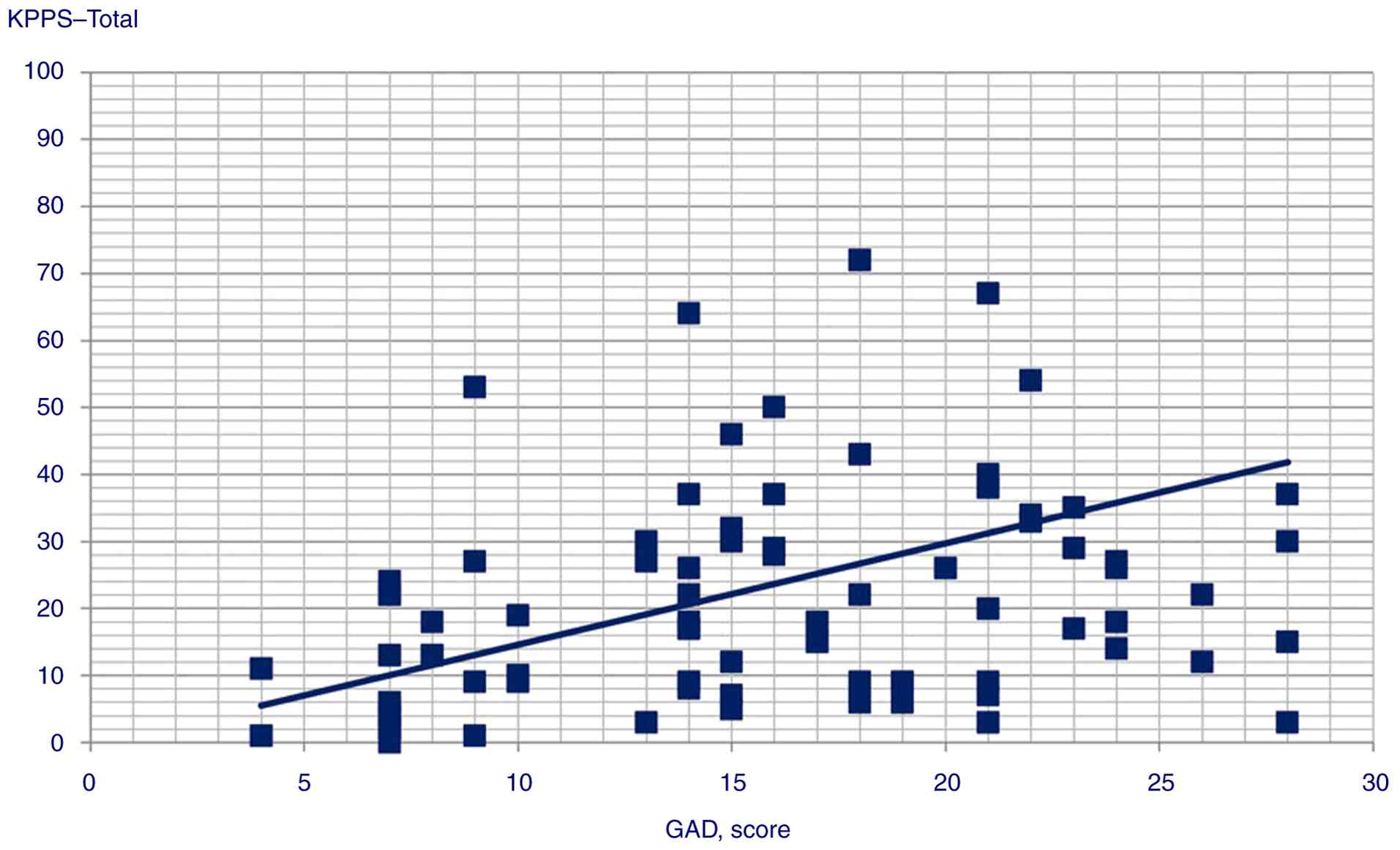
correlations between the following variables: GAD score and KPPS
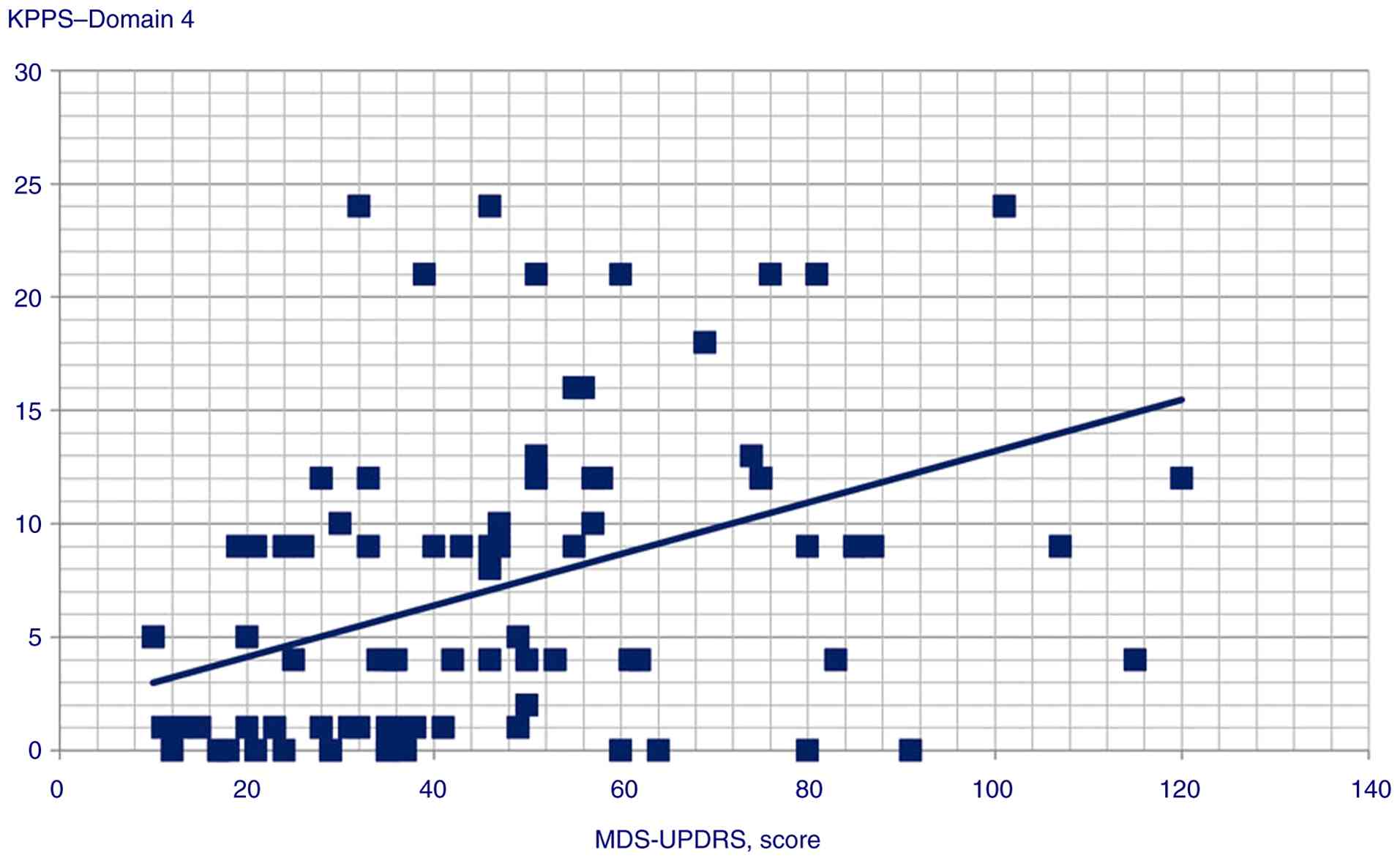
total score (Pearson's r=0.4, P<0.001); MDS-UPDRS and Domain 4
score (Pearson's r=0.414, P<0.001).
In addition, a weak correlation was present between
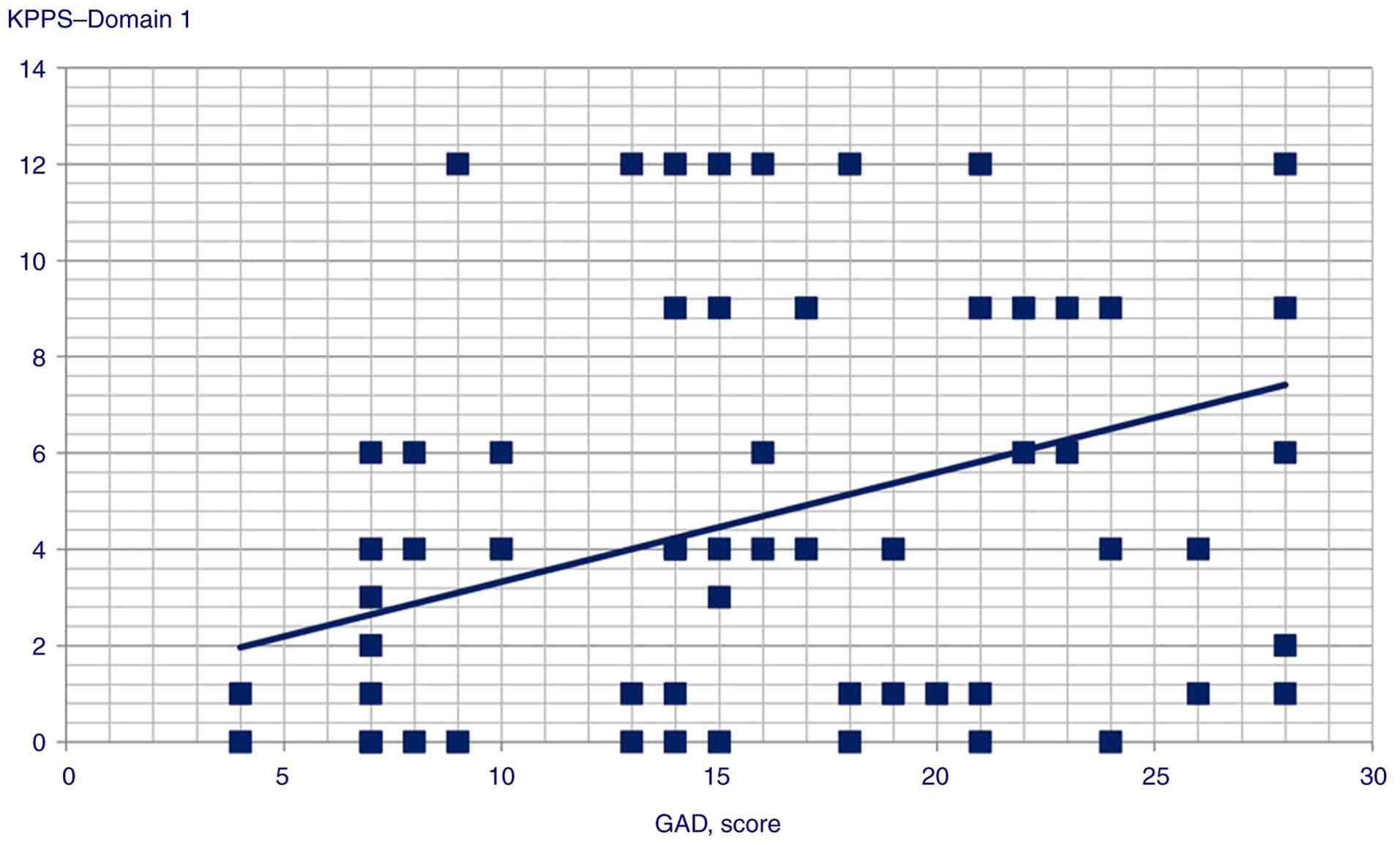
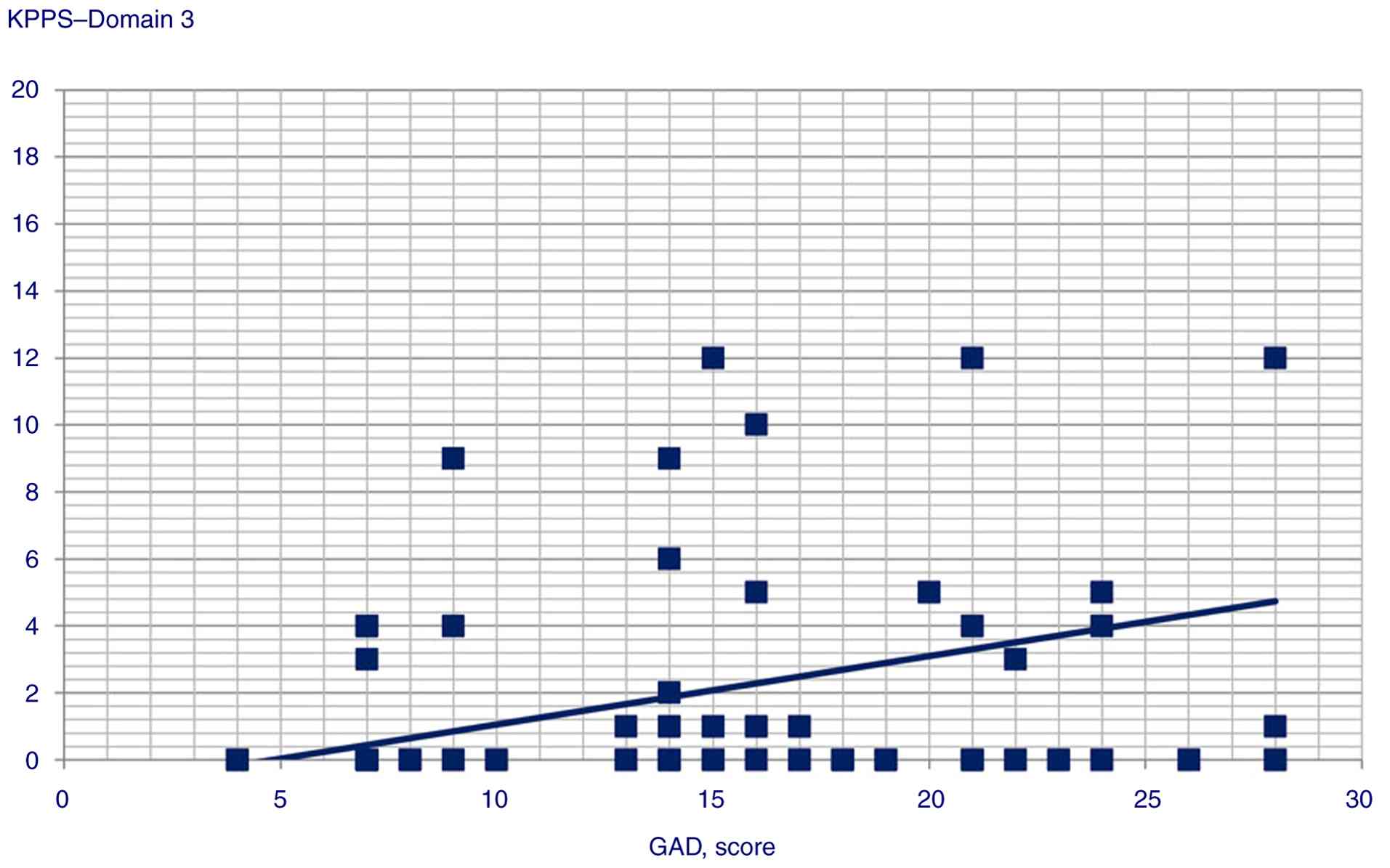
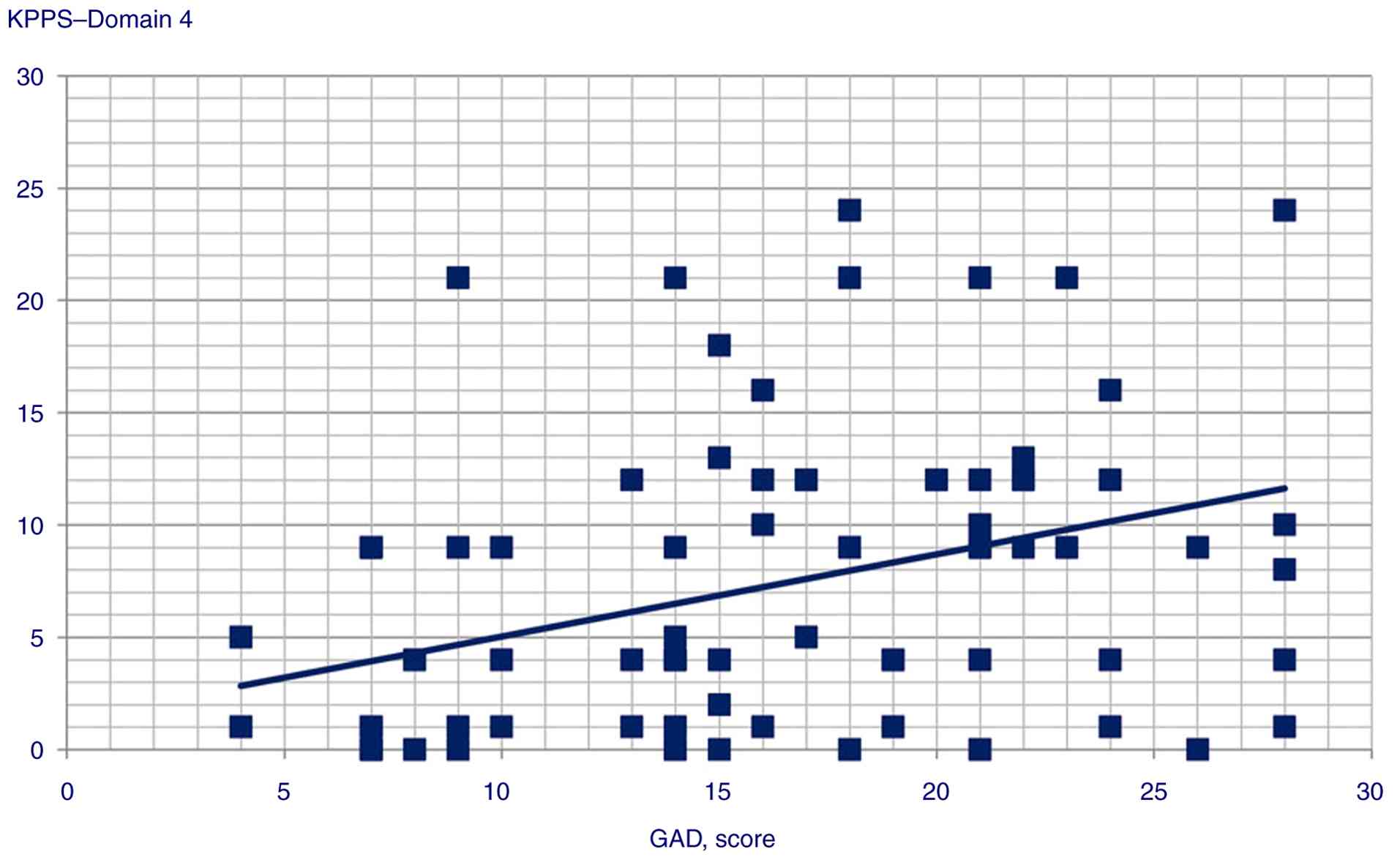
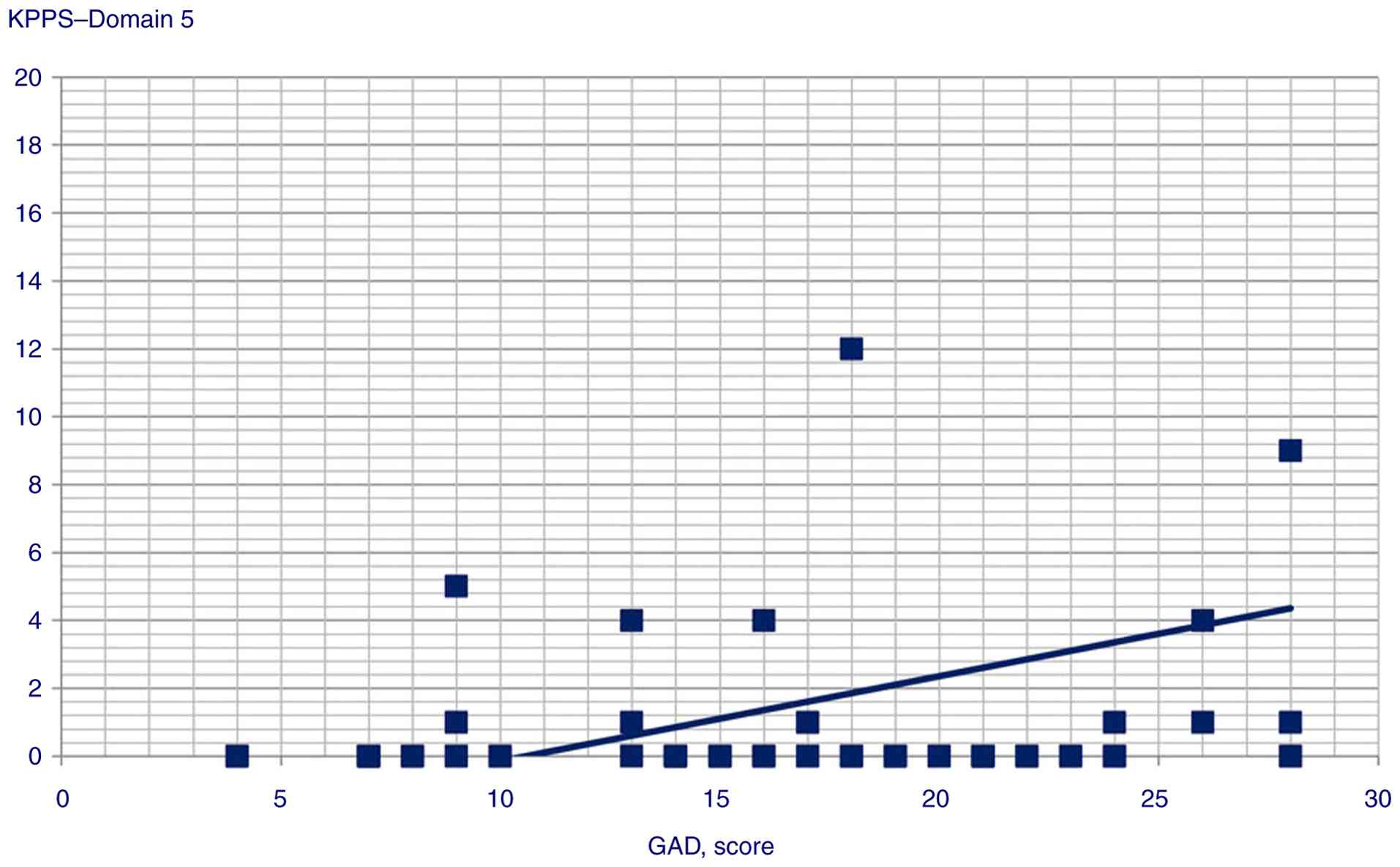
the following variables: i) GAD score and Domain 1 (Spearman's
Rho=0.358, P<0.001), Domain 3 (Pearson's r=0.245, P<0.014),
Domain 4 (Pearson's r=0.359, P<0.001), Domain 5 (Pearson's
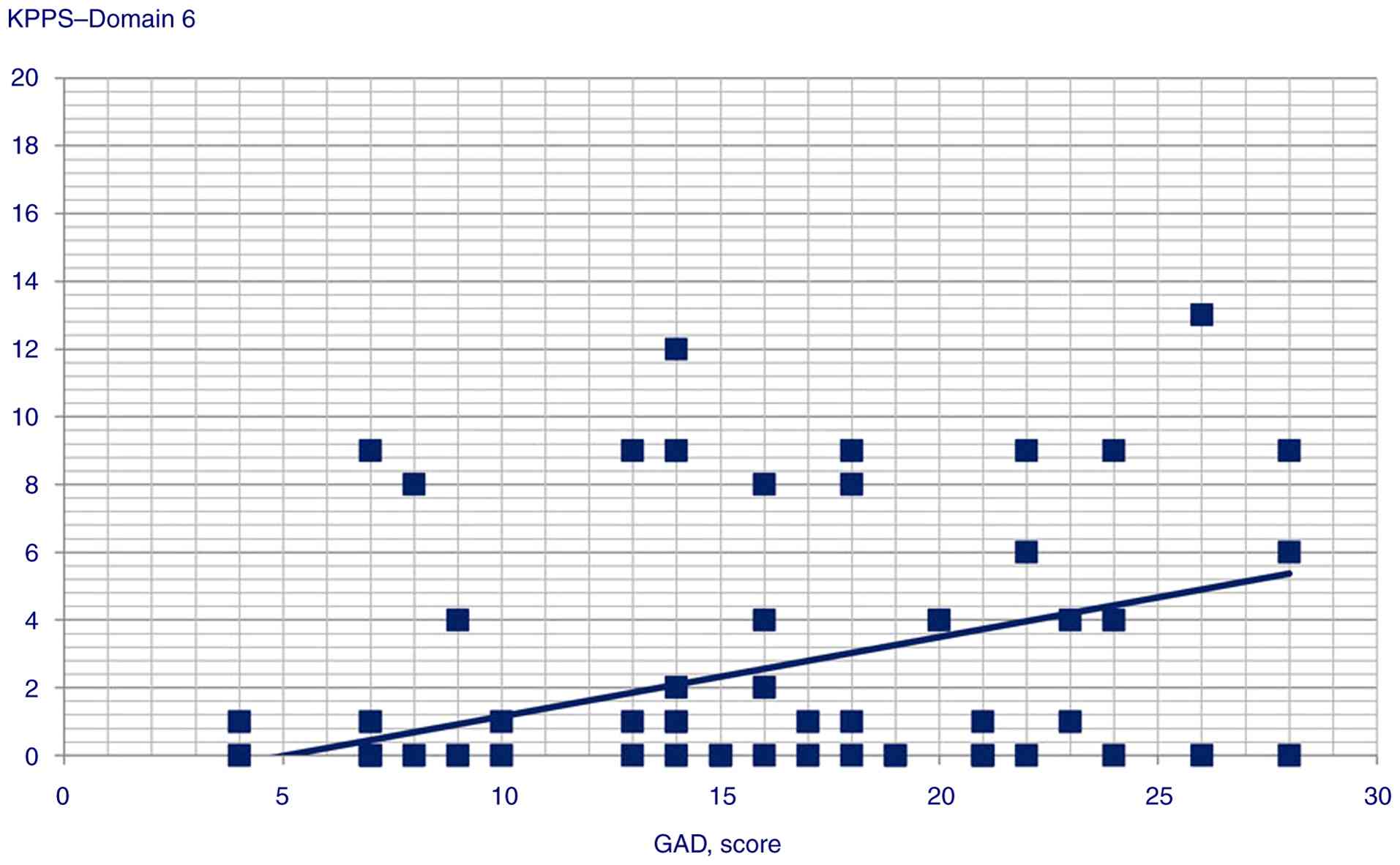
r=0.306, P=0.002) and Domain 6 scores (Pearson's r=0.34,
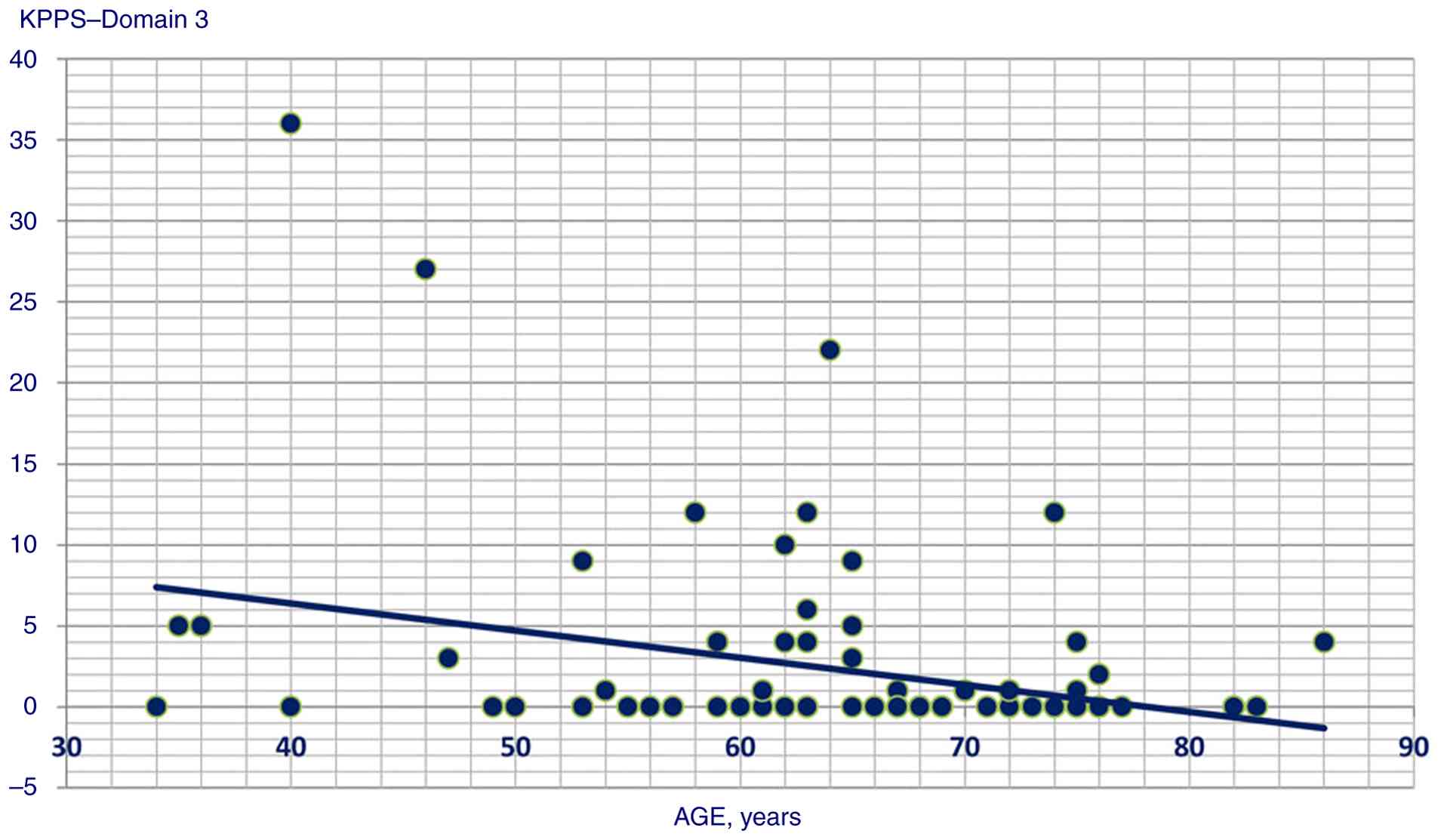
P<0.001); ii) age and KPPS Domain 3 (Spearman's Rho=-0.271,
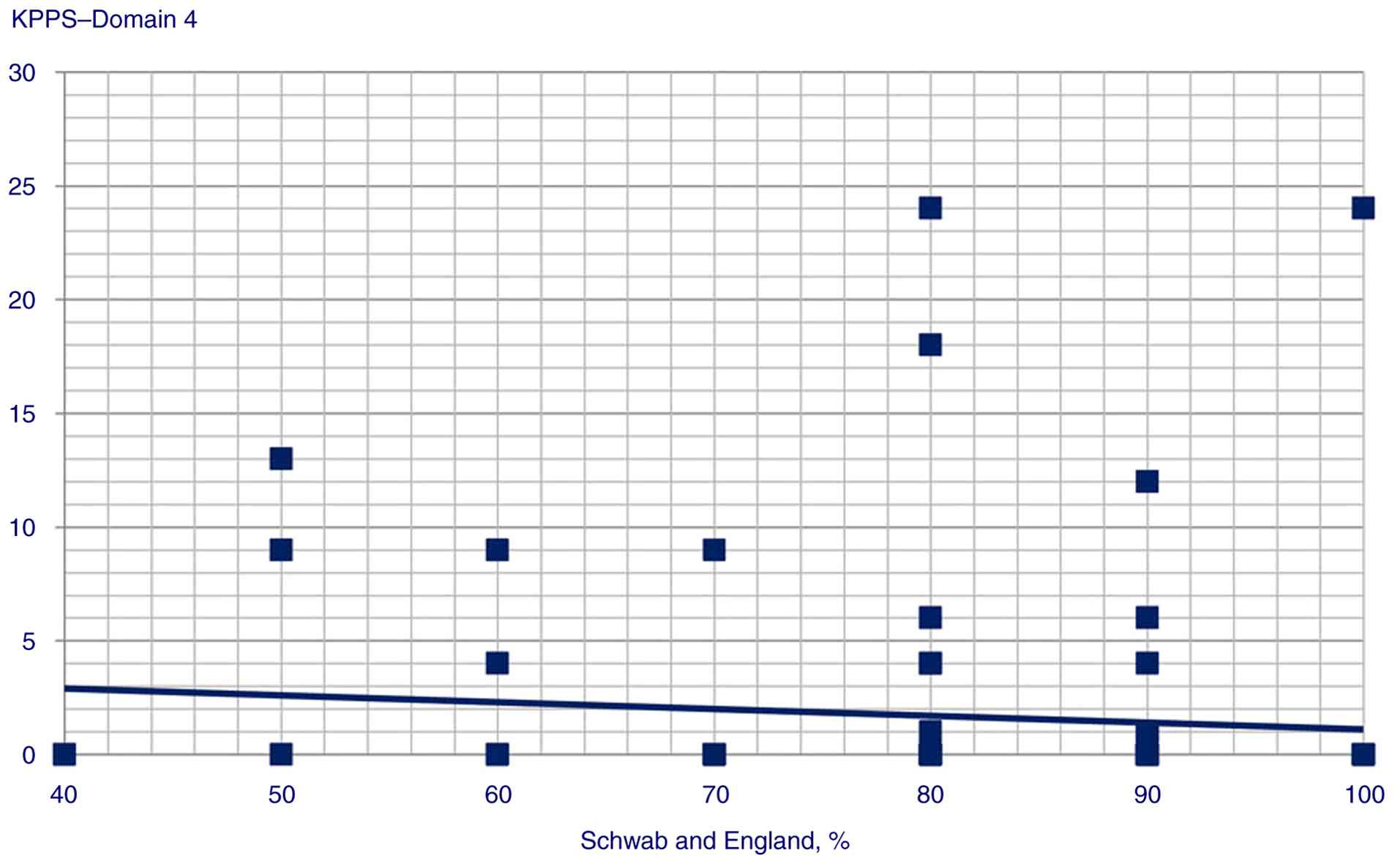
P=0.006); iii) Schwab and England ADL score and KPPS Domain 4
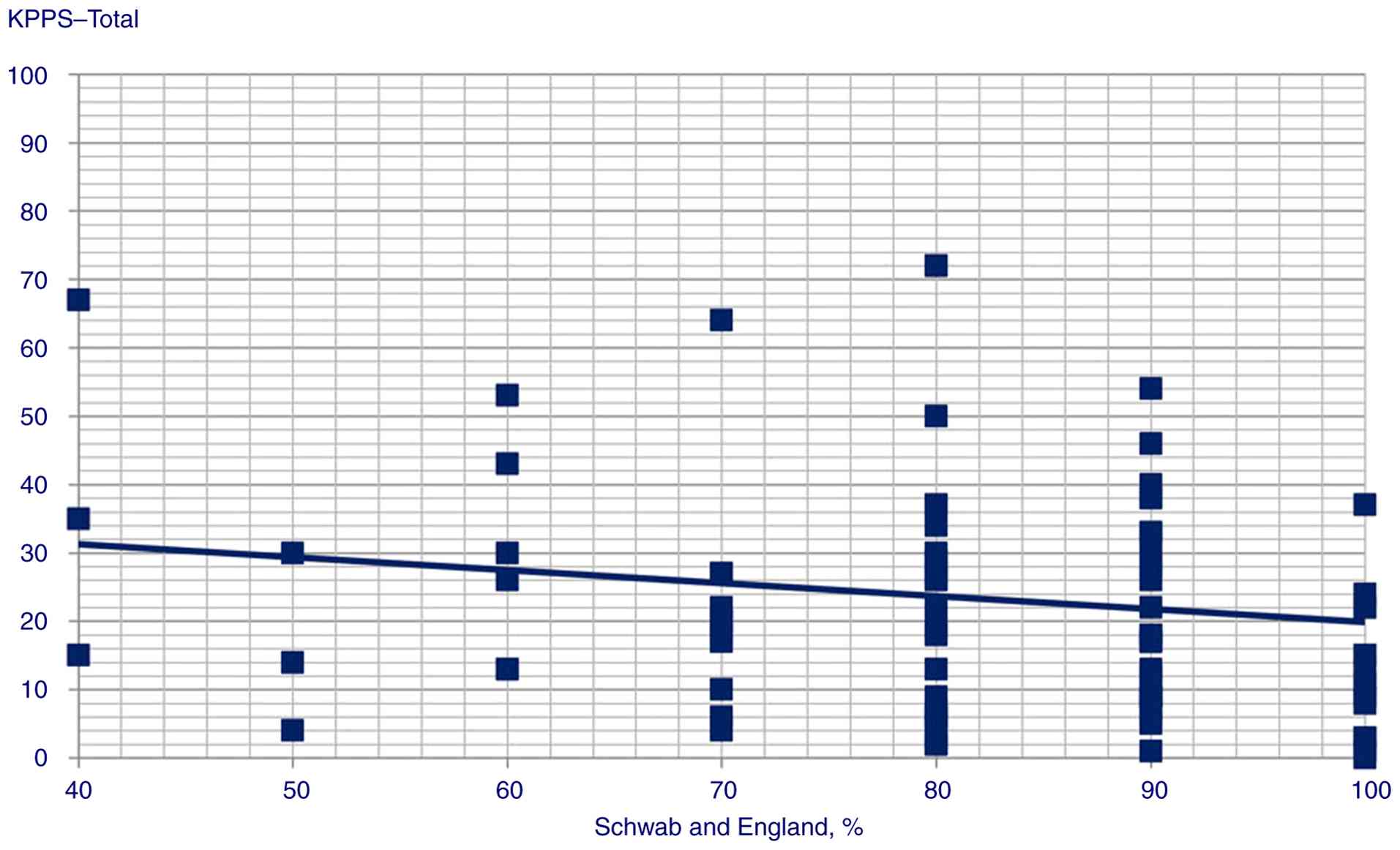
(Spearman's Rho=-0.247, P=0.013) and KPPS total scores (Spearman's
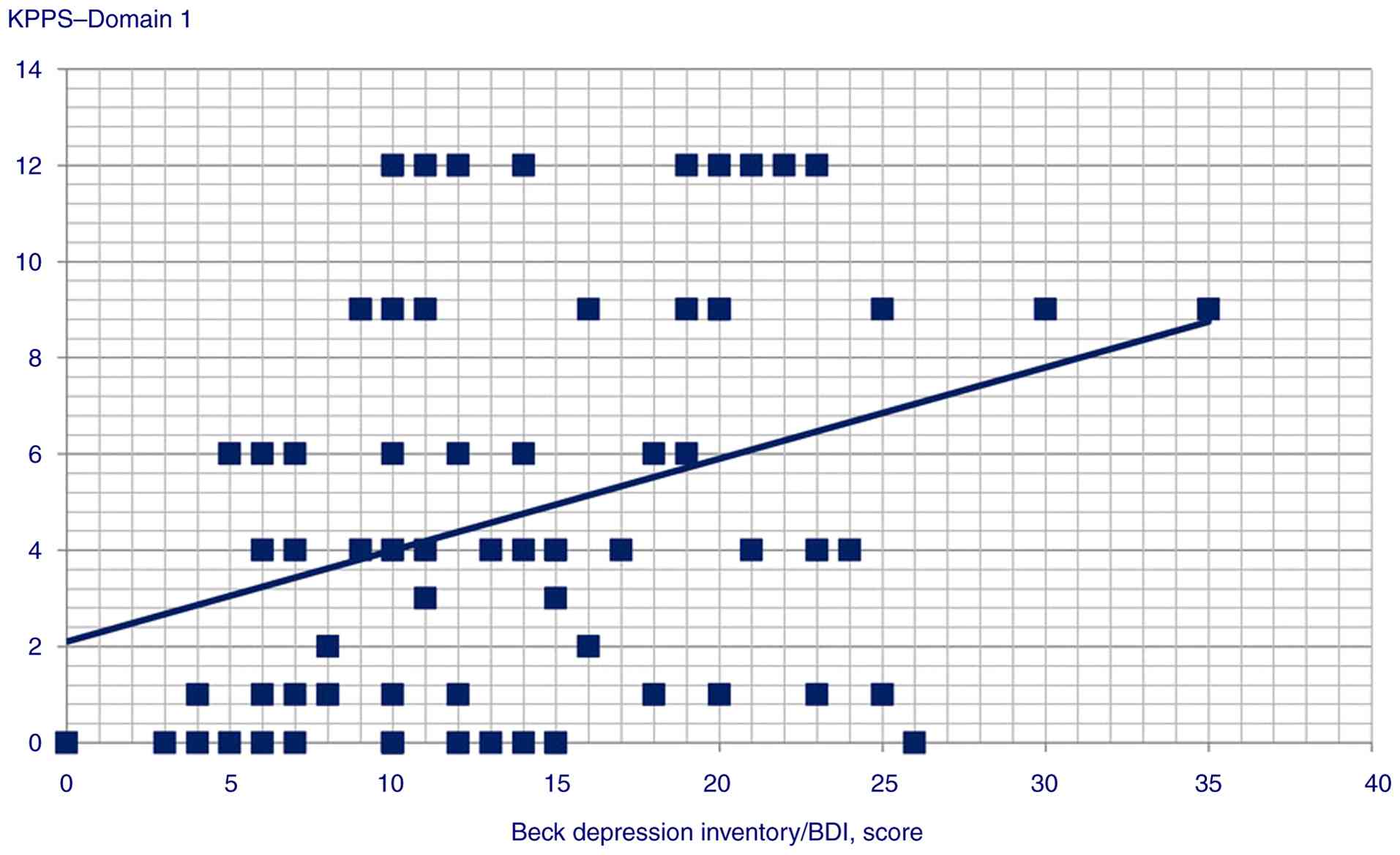
Rho=-0.233, P=0.019); iv) BDI and Domain 1 (Spearman's Rho=0.3,
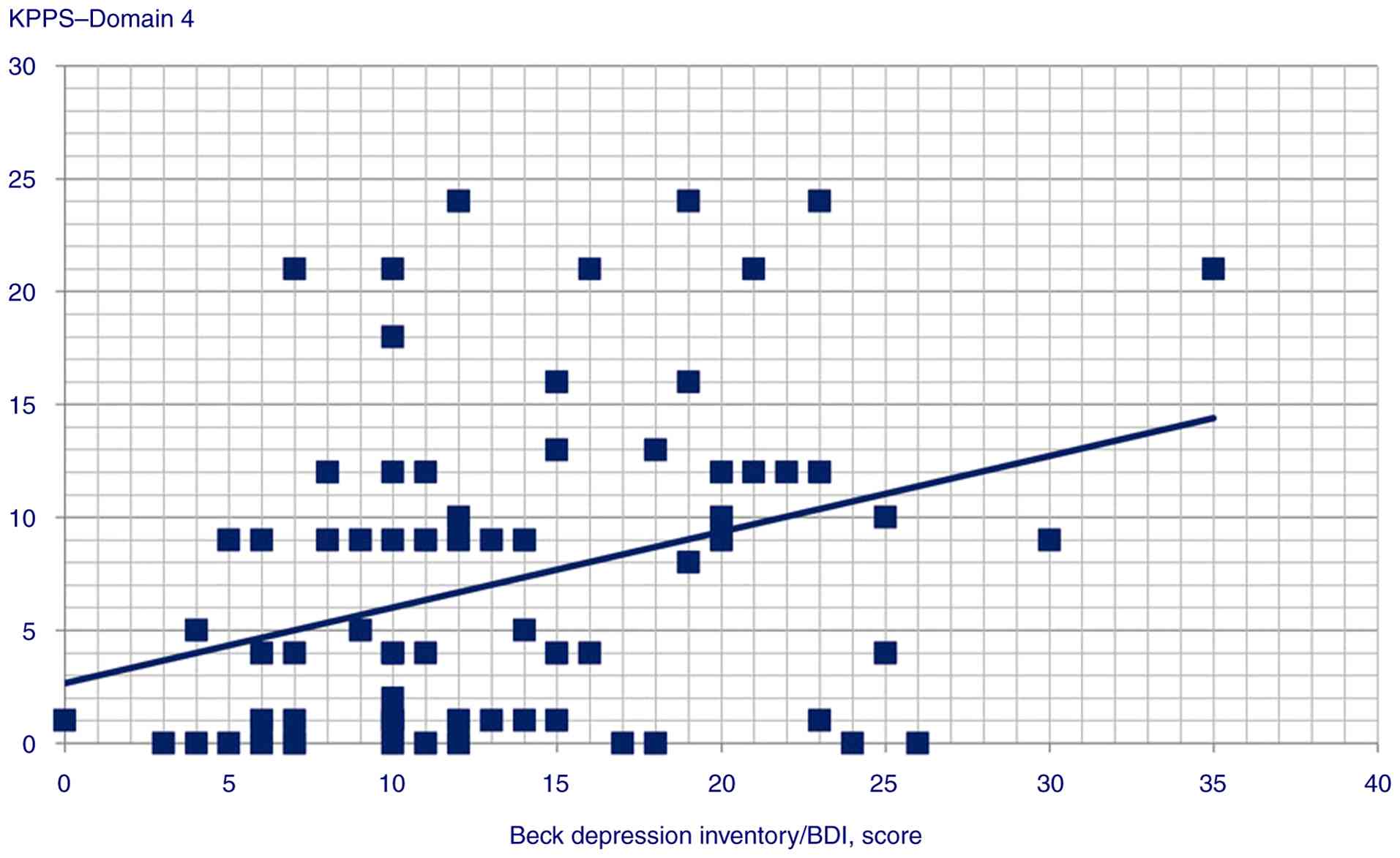
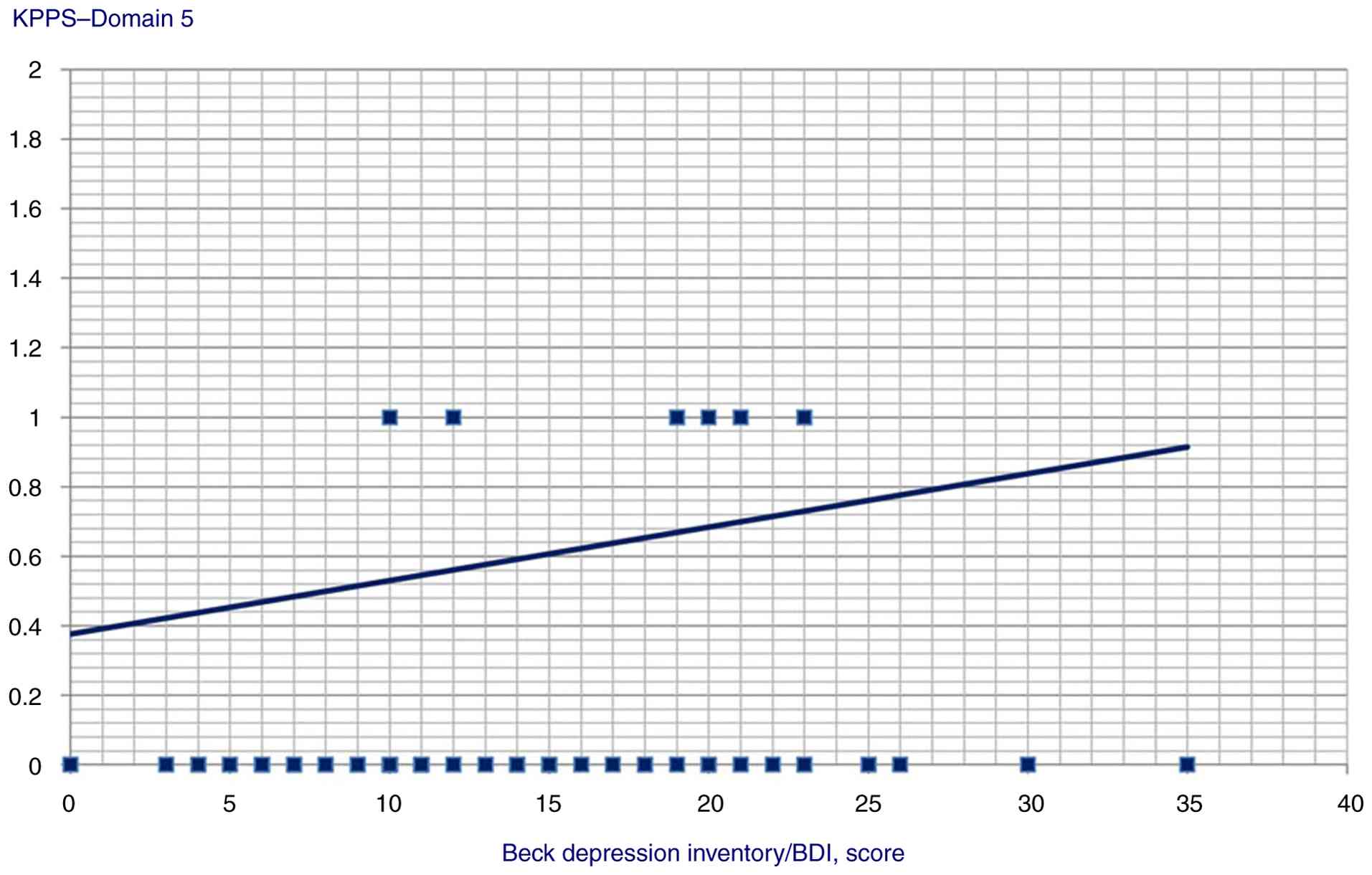
P=0.002), Domain 4 (Spearman's Rho=0.319, P=0.001), Domain 5
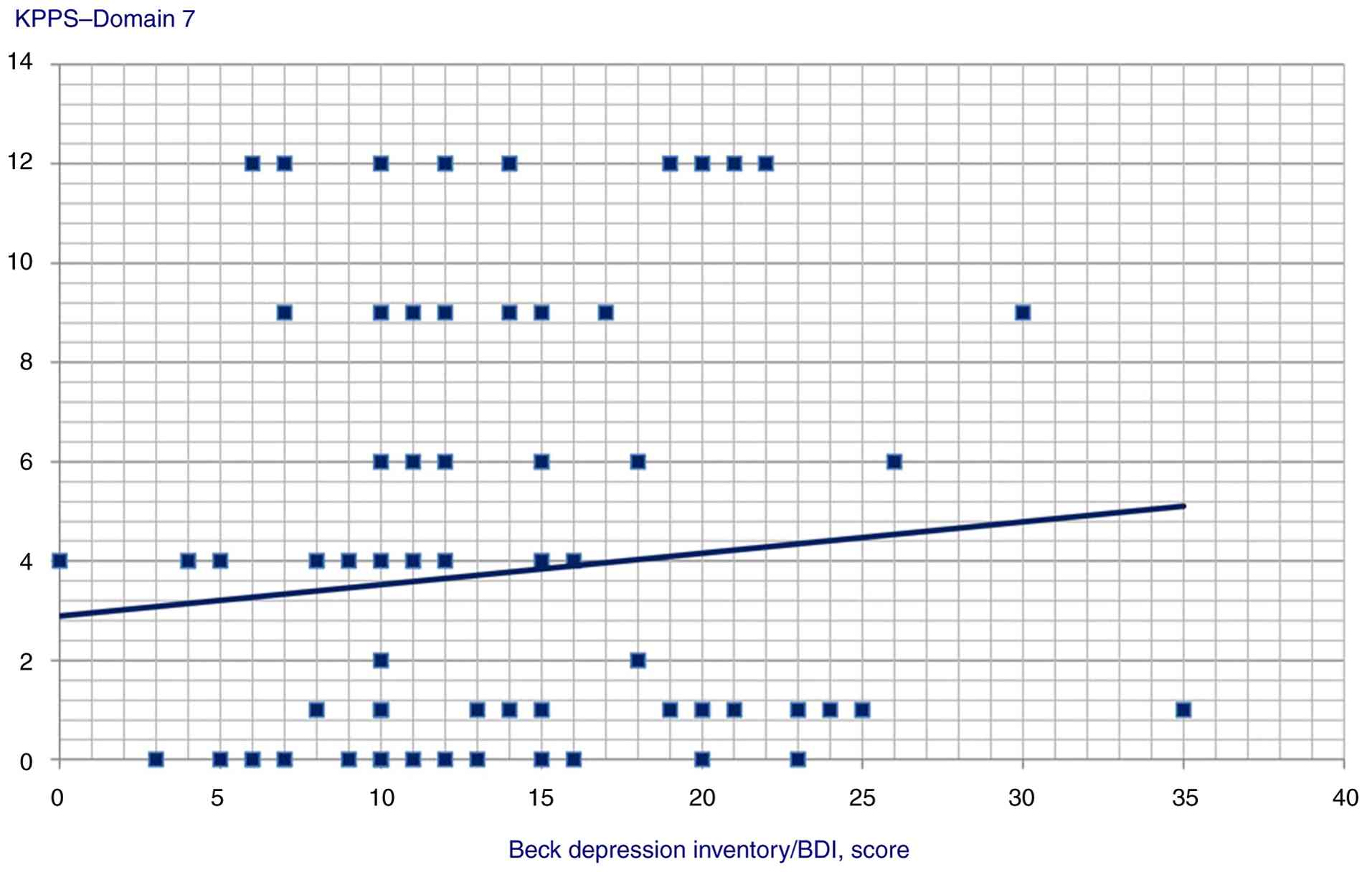
(Spearman's Rho=0.291, P=0.003), Domain 7 (Spearman's Rho=0.224,
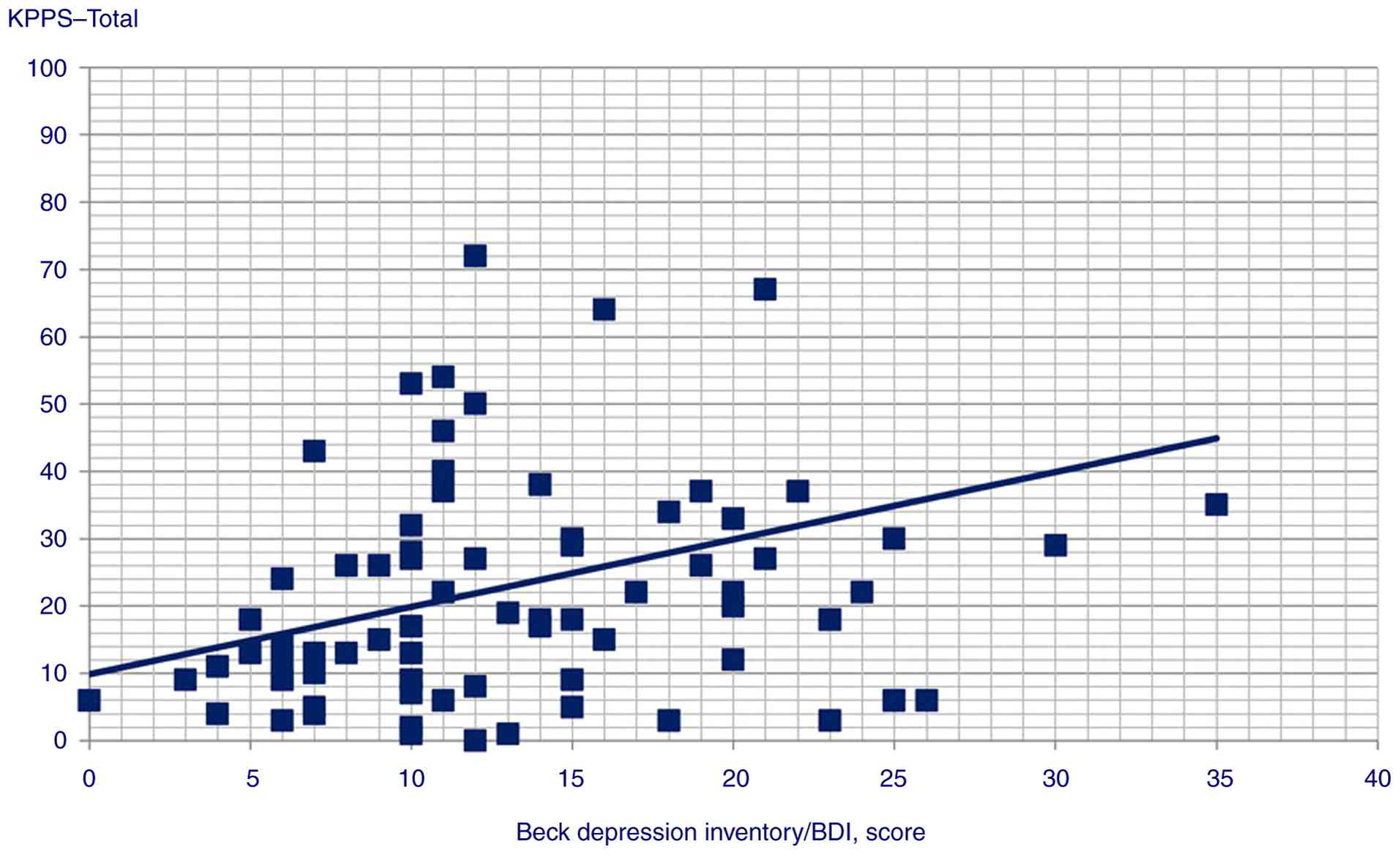
P=0.025) and KPPS total scores (Spearman's Rho=0.313, P=0.002);
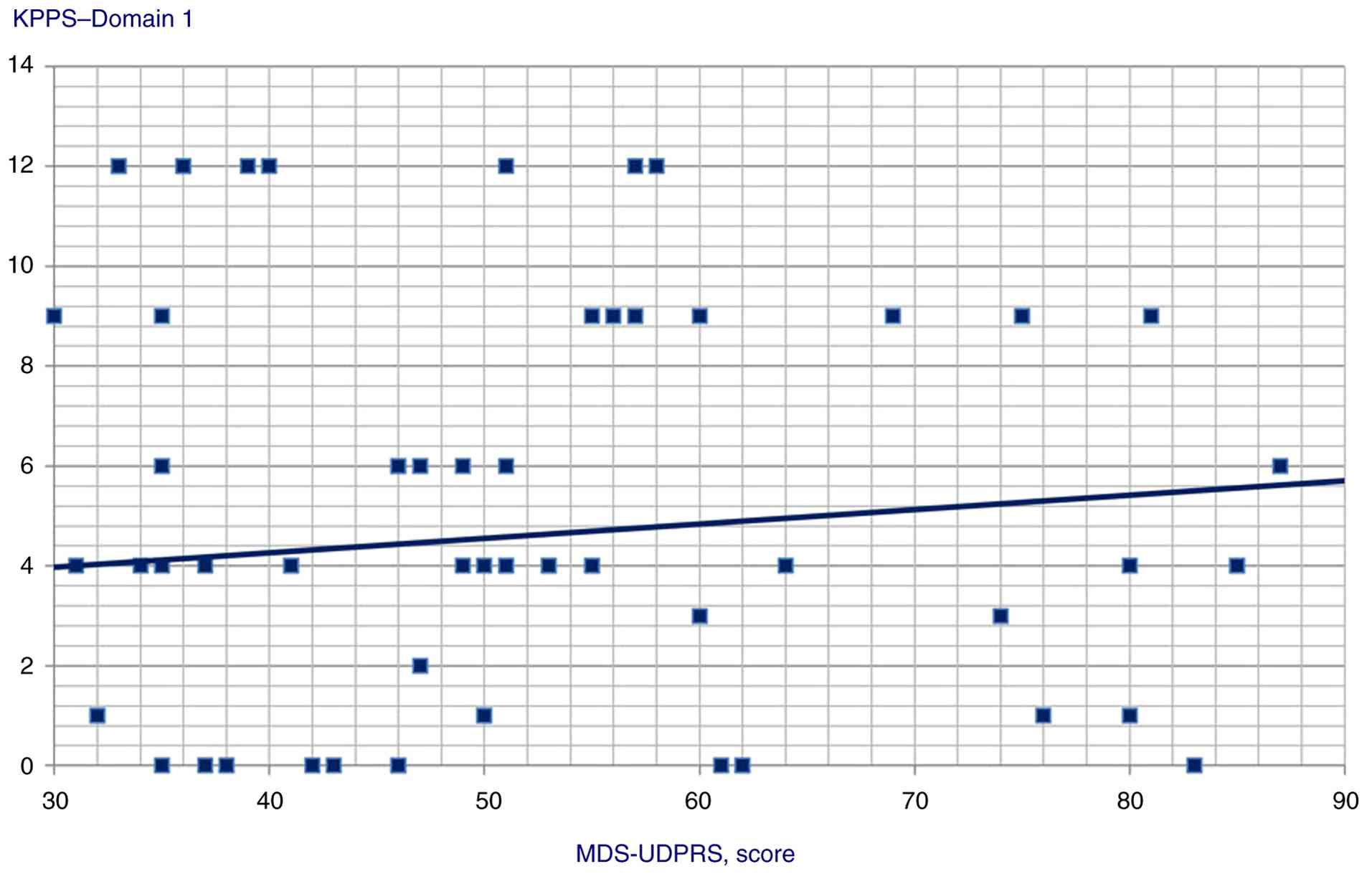
MDS-UPDRS and Domain 1 score (Spearman's Rho=0.226, P=0.024).
The correlation data between GAD score and KPPS,
MDS-UPDRS score and KPPS, and BDI and KPPS are presented in
Table II. No statistically
significant correlations were found between the length of disease,
MoCA score and KPPS total or individual domain scores.
Statistically significant correlation scatterplots are presented in
Fig. 1, 2, 3,
4, 5, 6,
7, 8, 9,
10, 11, 12,
13, 14, 15,
16 and 17.
 | Table IIResults of correlation analysis for
GAD score and KPPS, MDS-UPDRS score and KPPS and BDI and KPPS. |
Table II
Results of correlation analysis for
GAD score and KPPS, MDS-UPDRS score and KPPS and BDI and KPPS.
| A, GAD score vs.
KPPS domain and total scores |
|---|
| Domain | Pearson's r
(Spearman's Rho for Domain 1) | P-value |
|---|
| Domain 1 | 0.358 |
<0.001 |
| Domain 2 | 0.186 | 0.063 |
| Domain 3 | 0.245 | 0.014 |
| Domain 4 | 0.359 |
<0.001 |
| Domain 5 | 0.306 | 0.002 |
| Domain 6 | 0.340 |
<0.001 |
| Domain 7 | 0.170 | 0.090 |
| KPPS-total
score | 0.400 |
<0.001 |
| B, MDS-UPDRS score
vs. KPPS domain and total scores |
| Domain | Pearson's r
(Spearman's Rho for Domain 1) | P-value |
| Domain 1 | 0.226 | 0.024 |
| Domain 2 | 0.001 | 0.993 |
| Domain 3 | -0.011 | 0.917 |
| Domain 4 | 0.414 |
<0.001 |
| Domain 5 | 0.131 | 0.193 |
| Domain 6 | 0.041 | 0.683 |
| Domain 7 | 0.109 | 0.279 |
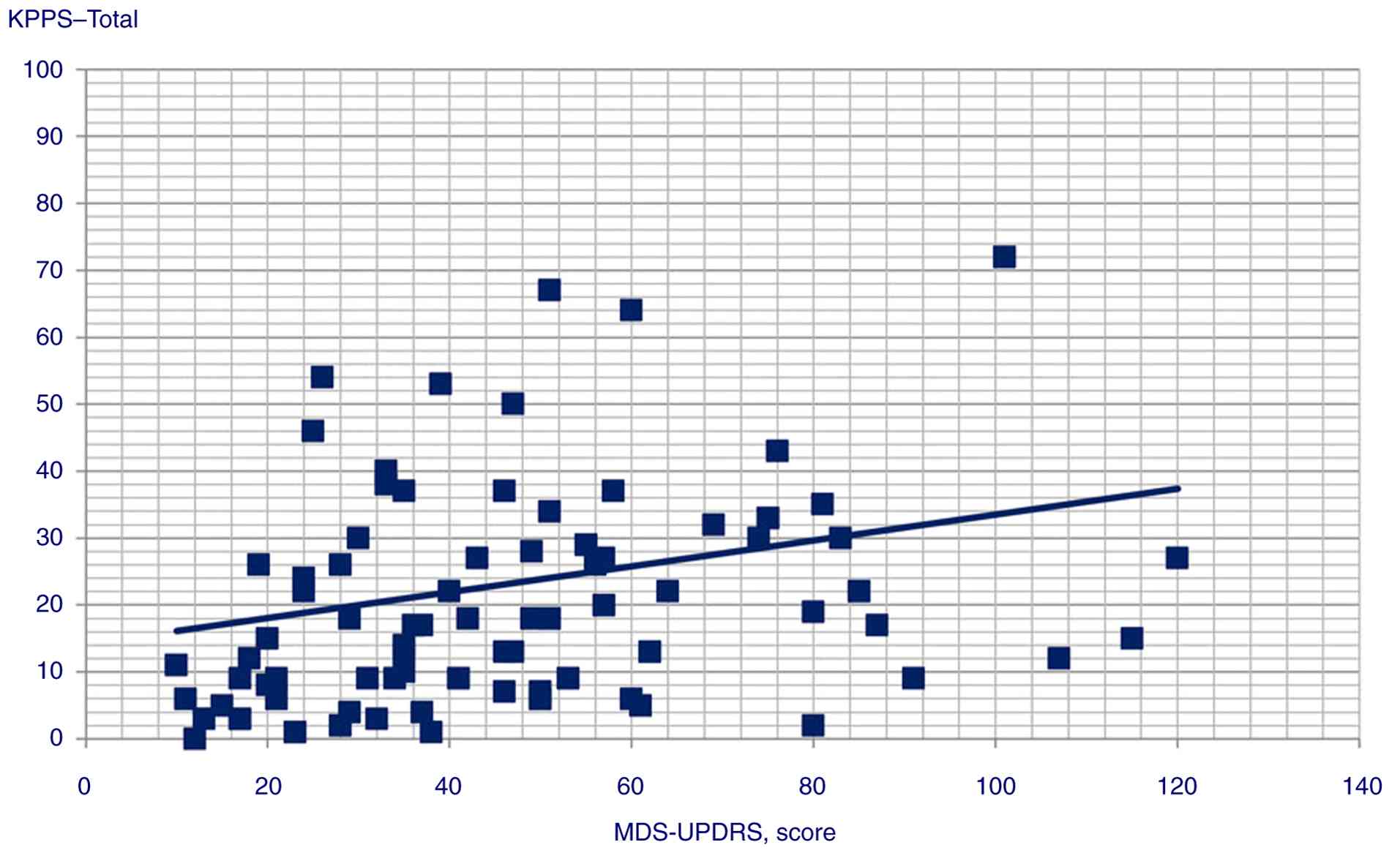
| KPPS-total
score | 0.190 | 0.059 |
| C, BDI score vs.
KPPS domain and total scores |
| Domain | Spearman's rho | P-value |
| Domain 1 | 0.300 | 0.002 |
| Domain 2 | 0.027 | 0.789 |
| Domain 3 | -0.029 | 0.771 |
| Domain 4 | 0.319 | 0.001 |
| Domain 5 | 0.291 | 0.003 |
| Domain 6 | 0.039 | 0.703 |
| Domain 7 | 0.224 | 0.025 |
| KPPS-total
score | 0.313 | 0.002 |
The majority of the correlation data were also
reflected in multiple regression models: i) The GAD score was found
to be a predictor of Domain 1 (P=0.025), Domain 3 (P=0.014), Domain
4 (P=0.003), Domain 5 (P=0.002), Domain 6 (P=0.001) and KPPS total
scores (P<0.001); ii) MDS-UPDRS score was a predictor of Domain
4 score (P<0.001); iii) Schwab and England ADL predicted-KPPS
total score (P=0.044); iv) BDI predicted Domain 1 (P<0.001)
(Table III).
 | Table IIIStatistically significant predictors
from multiple regression analysis models. |
Table III
Statistically significant predictors
from multiple regression analysis models.
| Outcome
variable | Predictor | Statistics |
|---|
| KPPS total
score | Schwab and England
score | Beta
coefficient=-0.08; t=2.05, P=0.044 |
| | GAD score | Beta
coefficient=0.39; t=4.17, P<0.001 |
| KPPS, Domain 1 | BDI score | Beta
coefficient=0.10; t=3.82, P<0.001 |
| | GAD score | Beta
coefficient=0.28; t=2.28, P=0.025 |
| KPPS, Domain 2 | GAD score | Beta
coefficient=0.28; t=2.28, P=0.025 |
| KPPS, Domain 3 | GAD score | Beta
coefficient=0.25; t=2.50, P=0.014 |
| KPPS, Domain 4 | GAD score | Beta
coefficient=0.28; t=3.06, P=0.003 |
| | MDS-UPDRS
score | Beta
coefficient=0.35; t=3.86, P<0.001 |
| KPPS, Domain 5 | GAD score | Beta
coefficient=0.31; t=3.18, P=0.002 |
| KPPS, Domain 6 | GAD score | Beta
coefficient=0.34; t=3.58, P=0.001 |
No other variables were found to be individual
predictors. When comparing men and women, the sex parameter was not
found to be a significant risk factor for responding positively to
any item (relative risk with P≥0.1 for all items, Table IV).
 | Table IVProportion of patients with positive
responses of KPPS (n=100) by sex. |
Table IV
Proportion of patients with positive
responses of KPPS (n=100) by sex.
| | Females | Males | |
|---|
| Items | No. of
patients | % | No. of
patients | % | RR (95% CI) | P-value |
|---|
| Musculoskeletal
pain | 47 | 81.0 | 29 | 69.0 | 1.35
(0.84-2.16) | 0.211 |
| Pain deep within
the body | 8 | 13.8 | 7 | 16.7 | 0.91
(0.55-1.50) | 0.704 |
| Pain related to an
internal organ (e.g. pain in the liver, stomach or
intestines-visceral pain) | 3 | 5.2 | 4 | 9.5 | 0.72
(0.30-1.73) | 0.469 |
| Dyskinetic
pain | 7 | 12.1 | 6 | 14.3 | 0.91
(0.54-1.57) | 0.755 |
| ‘Off’ period
dystonia in a specific area | 10 | 17.2 | 9 | 21.4 | 0.88
(0.56-1.41) | 0.616 |
| Generalized ‘off’
period pain | 4 | 6.9 | 7 | 16.7 | 0.60
(0.27-1.33) | 0.209 |
| Pain associated
with periodic limb movements (PLM) or an unpleasant burning
sensation in the legs that improves with movement | 24 | 41.4 | 11 | 26.2 | 1.31
(0.95-1.81) | 0.100 |
| Pain associated
with difficulty turning over in bed at night | 43 | 74.1 | 31 | 73.8 | 1.01
(0.69-1.48) | 0.971 |
| Pain when
chewing | 6 | 10.3 | 5 | 11.9 | 0.93
(0.53-1.65) | 0.812 |
| Pain due to
grinding teeth in sleeping | 0 | 0.0 | 0 | 0.0 | N/A | N/A |
| Burning mouth
syndrome | 3 | 5.2 | 2 | 4.8 | 1.03
(0.50-2.16) | 0.924 |
| Burning pain in
limbs | 26 | 44.8 | 17 | 40.5 | 1.08
(0.77-1.50) | 0.663 |
| Generalized lower
abdominal pain | 4 | 6.9 | 4 | 9.5 | 0.85
(0.42-1.74) | 0.660 |
| Sharp/stabbing pain
in the lower limbs | 43 | 74.1 | 29 | 69.0 | 1.11
(0.75-1.65) | 0.588 |
Discussion
The associations between PD-related pain and other
clinical and demographic variables have been studied extensively,
predating the development of the KPPS. However, due to the weak
nature of the correlations, demonstrating their presence has been
consistently challenging. For example, previous studies (13-15)
have found an association between depression and Parkinsonian pain,
while others (16,17) could not report any significant
correlation. One possible explanation may be that the studies that
found the association had larger sample sizes (314,450,227 vs.
117,96). Although the present study found depression to be
correlated with KPPS total and various individual domains by
Spearman's correlation analysis, depression was only found to
predict a single domain in the multiple regression model,
suggesting that even if a weak correlation may exist, a
cause-and-effect association between the two is less likely. While
the minimal prediction result of the BDI score may be explained by
the modest sample size of the present study (n=100), one notable
point brought up by in the study by Valkovic et al (18) in 2015 paints a more nuanced
picture. That study found that, even if average pain intensity is
not associated with a higher BDI score, the higher peak pain
severity and pain with periodicity are significantly correlated
with a higher depression index. This suggested that if, to quantify
PD-related pain, one uses different scales that accentuate
different facets of pain, the correlations and regression
predictions may shift from present to absent, and vice versa.
The association between MDS-UPDRS scores and
PD-related pain is also not universally agreed upon. For example,
the validation study of KPPS Bulgarian version found an association
between MDS-UPDRS part III and KPPS total score (19). However, the full MDS-UPDRS
questionnaire was not administered. On the contrary, a Japanese
validation study found a correlation with the total MDS-UPDRS
score, but not with part III specifically (20). In addition, a study from India
demonstrated that the KPPS total correlated with MDS-UPDRS parts II
and IV, but not any other parts or the total score (21). In the domain-by-domain regression
analysis performed herein, MDS-UPDRS score only predicted KPPS
Domain 4; thus, the correlation results in the studies mentioned
above may have also been influenced by the frequency of Domain 4
pain in their respective samples (19-21).
The clinical significance of this association warrants further
assessment in future studies.
If a correlation between depression and PD-related
pain is still somewhat debatable, the association between anxiety
and PD-related pain has been consistently observed. A Japanese KPPS
validation study (20) and two
other studies (22,23) have pointed to the presence of the
link also observed in the present study.
The consistency of correlations and reflection of
all correlations in the regression models in the present study
strongly suggested a cause-and-effect association between anxiety
and PD-related pain, although causal mechanisms remain to be
investigated. The correlation between pain and lower ADL scores was
also consistently shown. In their original study, Chaudhuri et
al (8) used the Scales for
Outcomes in Parkinson's disease-Motor ADL (SCOPA-Motor ADL) score,
while a Chinese validation study (22), as in the present study, used Schwab
and England ADL score, with moderate correlations found in both
instances.
Although the present study further confirmed the
absence of an association between the age of the patients and KPPS
total score, consistent with previous studies (8,13,22),
a weak association was noted for age and musculoskeletal pain
(Domain 1). This correlation was likely at least partially
confounded, considering that 58% of the present sample were female;
female patients are at a higher risk of developing osteoporosis and
the prevalence of osteoporosis increases with age (24). However, as osteoporosis data were
not collected in the present study, this remains speculative.
Disease duration has been cited as the variable
correlated with KPPS scores (8,20).
However, the Chinese validation study resulted in the correlation
being just below the significance threshold (n=89, P=0.051), and a
study from India also did not note any (21,22).
The present study found no significant association, which may be
explained by sample size differences (n=178, n=151 vs. n=89, n=119
and n=100 in the present study) or variability in diagnostic
practices across countries. Future studies with standardized
diagnostic criteria may help clarify this association.
Findings in previous studies are rather inconsistent
regarding sex. While certain studies have found a link between
female sex and higher KPPS scores (21,25),
others did not (8,22). The present study found no
significant sex-based differences in PD-related pain. A similar
disagreement in sex-based differences in pain was found in studies
that did not use KPPS (26,27).
In the present study, sex was not found to be a risk factor for
item positivity or higher domain scores. This discrepancy suggested
that sex-based differences in PD-related pain warrant further
exploration.
MoCA and KPPS total score correlation analysis were
not performed in any previous study; however, previous studies that
touched on the topic of PD-related pain and cognitive decline
reported no such association (28,29).
These results were consistent with prior findings.
Considering the above, there is a need for further
research regarding the association between clinical and demographic
variables and PD-related pain. The majority of studies on the
subject are performed in distinct, country-specific populations.
The present study added Georgia to the geographic areas covered,
and to the best of our knowledge, correlations with individual KPPS
domains were explored herein for the first time.
The adjustment for the results of multiple
regression analyses and correlations was not performed in the
present study, introducing a theoretical risk of type I error.
However, since the link between anxiety and KPPS domains, which was
the main finding of the present study, was consistent with multiple
previous studies (20,22,23),
this possibility is deemed unlikely. Still, the predictive
connection between MDS-UPDRS and KPPS Domain 4 score may be
potentially impacted, and this lack of adjustment (by Bonferroni,
Sidak or other methods) can be considered as a limitation of the
present study.
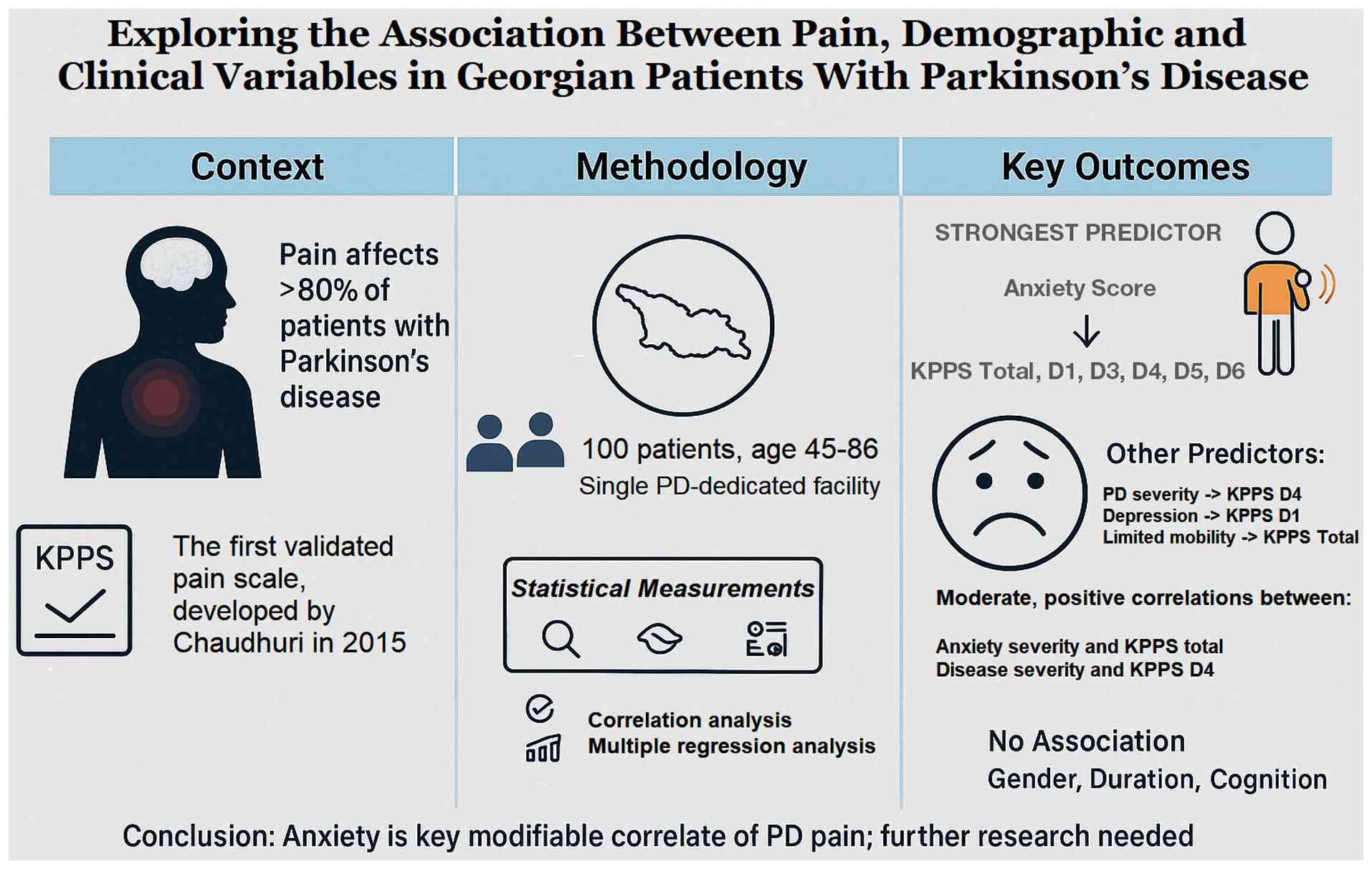
In conclusion, the present study found that higher
GAD scores were correlated with and could predict KPPS Domains 1,
3, 4, 5 and 6, and KPPS total scores, supporting the hypothesis of
a potential causal association, consistent with prior evidence. The
MDS-UPDRS score correlated with and predicted the KPPS Domain 4
score. The association between depression and pain may be subtle
and nuanced, although a direct causal link is less likely. The
positive correlation between BDI score and musculoskeletal pain in
the present study is probably confounded. No association was found
between KPPS and disease duration, MoCA score or sex. Future
studies with larger, more diverse samples are required to confirm
these findings and explore additional contributing factors. The
findings of the present study are summarized in the diagram
illustrated in Fig. 18.
Acknowledgements
The present study would not have been possible
without the support of Dr K. Ray Chaudhuri (King's College London,
Parkinson's Centre of Excellence), the developer of the KPPS, who
provided the free-of-charge license for its translation in the
Georgian language and validation in Georgia. The authors would also
like to thank Dr Nina Javakhishvili (Ilia State University,
Tbilisi, Georgia), who locally adopted and validated BDI and GAD-7
scales and offered these instrumental tools to us for use. The
authors also express their gratitude to Dr Ekaterine Mirvelashvili
(Tbilisi State Medical University, Tbilisi, Georgia) for providing
helpful insights and assistance with the statistical analysis. In
addition, the authors would like to thank Mr. Omar Selim, medical
student (Ilia State University) for assisting with data
organization, and Dr David Tananashvili (Gagua Clinic, Tbilisi,
Georgia) for assisting with statistical analysis and graphics.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are
available from the corresponding author on reasonable request.
Authors' contributions
GK wrote the first draft of the manuscript and
ensured its submission to the journal. All authors (GK, AT, MG, SS,
IK and MM) contributed to the conception and design of the study,
material preparation, data collection, analysis and interpretation.
AT contributed to the analysis of the data and draft editing. SS,
IK and MM revised the manuscript critically for important
intellectual content. All authors have read and approved the final
version of the article. GK and AT confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Ilia State University (approval no. R/244-23 Tbilisi,
Georgia). The study was conducted with the voluntary written
consent of the participants or their guardians. Research subjects
could withdraw from the study at any stage and all information
obtained was used solely for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang ZX, Dong ZH and Román GC: Early
descriptions of Parkinson disease in ancient China. Arch Neurol.
63:782–784. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Parkinson J: An essay on the shaking palsy
1817. J Neuropsychiatry Clin Neurosci. 14:223–222. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
GBD 2015 Neurological Disorders
Collaborator Group. Global, regional, and national burden of
neurological disorders during 1990-2015: A systematic analysis for
the Global Burden of Disease Study 2015. Lancet Neurol. 16:877–897.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dorsey ER and Bloem BR: The Parkinson
pandemic-a call to action. JAMA Neurol. 75:9–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
GBD 2016 Parkinson's Disease
Collaborators. Global, regional, and national burden of Parkinson's
disease, 1990-2016: A systematic analysis for the Global burden of
disease study 2016. Lancet Neurol. 17:939–953. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lee MA, Walker RW, Hildreth TJ and
Prentice WM: A survey of pain in idiopathic Parkinson's disease. J
Pain Symptom Manage. 32:462–469. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Beiske AG, Loge JH, Rønningen A and
Svensson E: Pain in Parkinson's disease: Prevalence and
characteristics. Pain. 141:173–177. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chaudhuri KR, Rizos A, Trenkwalder C,
Rascol O, Pal S, Martino D, Carroll C, Paviour D, Falup-Pecurariu
C, Kessel B, et al: King's Parkinson's disease pain scale, the
first scale for pain in PD: An international validation. Mov
Disord. 30:1623–1631. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Beck AT, Ward CH, Mendelson M, Mock J and
Erbaugh J: An inventory for measuring depression. Arch Gen
Psychiatry. 4:561–571. 1961.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Spitzer RL, Kroenke K, Williams JB and
Löwe B: A brief measure for assessing generalized anxiety disorder:
The GAD-7. Arch Intern Med. 166:1092–1097. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nasreddine ZS, Phillips NA, Bédirian V,
Charbonneau S, Whitehead V, Collin I, Cummings JL and Chertkow H:
The Montreal cognitive assessment, MoCA: A brief screening tool for
mild cognitive impairment. J Am Geriatr Soc. 53:695–699.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goetz CG, Fahn S, Martinez-Martin P, Poewe
W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B,
et al: Movement disorder Society-sponsored revision of the Unified
Parkinson's disease rating scale (MDS-UPDRS): Process, format, and
clinimetric testing plan. Mov Disord. 22:41–47. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rodríguez-Violante M, Alvarado-Bolaños A,
Cervantes-Arriaga A, Martinez-Martin P, Rizos A and Chaudhuri KR:
Clinical determinants of Parkinson's disease-associated pain using
the King's Parkinson's disease pain scale. Mov Disord Clin Pract.
4:545–551. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nègre-Pagès L, Regragui W, Bouhassira D,
Grandjean H and Rascol O: DoPaMiP Study Group. Chronic pain in
Parkinson's disease: The cross-sectional French DoPaMiP survey. Mov
Disord. 23:1361–1369. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ehrt U, Larsen JP and Aarsland D: Pain and
its relationship to depression in Parkinson disease. The Am J
Geriatr Psychiatry. 17:269–275. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tinazzi M, Del Vesco C, Fincati E,
Ottaviani S, Smania N, Moretto G, Fiaschi A, Martino D and Defazio
G: Pain and motor complications in Parkinson's disease. J Neurol
Neurosurg Psychiatry. 77:822–825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hanagasi HA, Akat S, Gurvit H, Yazici J
and Emre M: Pain is common in Parkinson's disease. Clin Neurol
Neurosurga. 113:11–13. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Valkovic P, Minar M, Singliarova H,
Harsany J, Hanakova M, Martinkova J and Benetin J: Pain in
Parkinson's disease: A cross-sectional study of its prevalence,
types, and relationship to depression and quality of life. PLoS
One. 10(e0136541)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stoyanova-Piroth G, Milanov I and
Stambolieva K: Translation, adaptation and validation of the
Bulgarian version of the King's Parkinson's disease Pain Scale. BMC
Neurol. 21(357)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kurihara K, Fujioka S, Mizutani Y,
Watanabe H, Iwaoka K, Maeda T, Seki M, Tezuka T, Nakahara J, Konno
T, et al: Validation study of the Japanese version of the King's
Parkinson's disease pain scale and the King's Parkinson's disease
pain questionnaire. Parkinsonism Relat Disord.
120(106012)2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Behari M, Srivastava A, Achtani R, Nandal
N and Dutta RB: Pain assessment in Indian Parkinson's disease
patients using King's Parkinson's disease pain scale. Ann Indian
Acad Neurol. 23:774–780. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao L, Huang W, Cai L and Peng Y: Pain
assessment in Chinese Parkinson's disease patients using King's
Parkinson's disease pain scale. J Pain Res. 15:715–722.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rana AQ, Qureshi ARM, Kachhvi HB, Rana MA
and Chou KL: Increased likelihood of anxiety and poor sleep quality
in Parkinson's disease patients with pain. J Neurol Sci.
369:212–215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fejer R and Ruhe A: What is the prevalence
of musculoskeletal problems in the elderly population in developed
countries? A systematic critical literature review. Chiropr Man
Therap. 20(31)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Silverdale MA, Kobylecki C, Kass-Iliyya L,
Martinez-Martin P, Lawton M, Cotterill S, Chaudhuri KR, Morris H,
Baig F, Williams N, et al: A detailed clinical study of pain in
1957 participants with early/moderate Parkinson's disease.
Parkinsonism Relat Disord. 56:27–32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Defazio G, Antonini A, Tinazzi M, Gigante
AF, Pietracupa S, Pellicciari R, Bloise M, Bacchin R, Marcante A,
Fabbrini G and Berardelli A: Relationship between pain and motor
and non-motor symptoms in Parkinson's disease. Eur J Neurol.
24:974–980. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Young Blood MR, Ferro MM, Munhoz RP, Teive
HA and Camargo CH: Classification and characteristics of pain
associated with Parkinson's disease. Parkinsons Dis.
2016(6067132)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kurihara K, Fujioka S, Mishima T and
Tsuboi Y: Evaluation of perception threshold and pain in patients
with Parkinson's disease using PainVision®. Front
Neurol. 14(1130986)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kurihara K, Fujioka S, Kawazoe M, Mishima
T, Ouma S and Tsuboi Y: Fluctuating pain in Parkinson's disease:
Its prevalence and impact on quality of life. eNeurologicalSci.
25(100371)2021.PubMed/NCBI View Article : Google Scholar
|