Introduction
Ginsenosides are the main active components of
ginseng and have various pharmaceutical activities, such as
antitumor, anti-oxidant and neuroprotective activities (1–4).
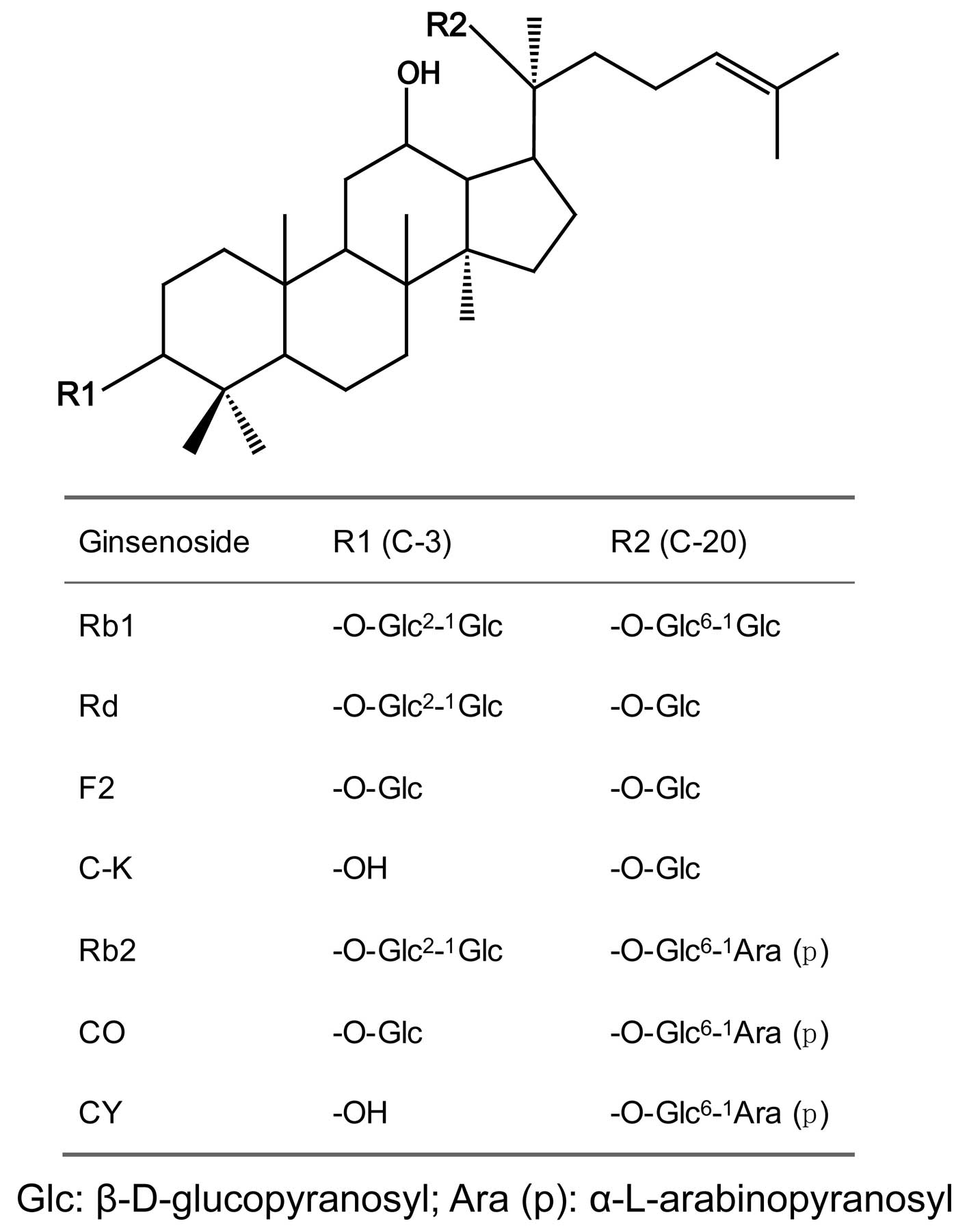
Protopanaxadiol ginsenosides Rb1 and Rb2 are abundantly found in
ginseng. Protopanaxadiol ginsenosides Rd, F2 and compound K (C-K)
are the biotransformation products of Rb1, while CO, CY and C-K are
the biotransformation products of Rb2. It has been demonstrated
that certain biotransformation products of Rb1 and Rb2 have
significant pharmacological activities, such as C-K possessing
antiallergic and anti-inflammatory activities (5–8). All
these protopanaxadiol ginsenosides have the same aglycone
(protopanaxadiol); however, the sugar chains are different
(Fig. 1). The structure-activity
relationship of ginsenosides has been investigated, but not fully
elucidated (9,10). Ginsenosides with fewer sugar
residues appear to be more active. In this study, we investigated
the activities of seven protopanaxadiol ginsenosides on the
inhibition of proliferation of the HCT-116 and HT-29 human
colorectal cancer lines. The results may provide information on the
antiproliferative activity-structure relationship of
ginsenosides.
Materials and methods
Materials
Standard ginsenosides were purchased from Chengdu
Mansite Biotechnology Co., Ltd. (Chengdu, China). The ginsenosides
Rb1 and Rb2 and their biotransformation products were prepared as
described in our previous study (11). The metabolites were identified by
thin layer chromatography, high-performance liquid chromatography
and 13C-NMR spectrometry. Dulbecco’s modified Eagle’s
medium/Nutrient Mixture F-12 (DMEM/F12), Iscove’s modified
Dulbecco’s medium (IMDM) and fetal bovine serum (FBS) were
purchased from Gibco-BRL (Carlsbad, CA, USA).
Penicillin/streptomycin were purchased from the Tianjin Hao Yang
biological manufacture Co., Ltd. (Tianjin, China).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
was purchased from Sigma (St. Louis, MO, USA). The plates used in
this study were purchased from Nalge Nunc International (Rochester,
NY, USA). Chemicals used were of analytical grade or higher.
Cell culture
The HCT-116 and HT-29 human colorectal cancer cell
lines were obtained from the American Type Culture Collection
(Manassas, VA, USA). HCT-116 cells were grown in IMDM with 10%
heat-inactivated FBS and 100 U/ml penicillin and streptomycin.
HT-29 cells were maintained in DMEM/F12 containing 10%
heat-inactivated FBS. The cells were maintained in a humidified
chamber of 95% air and 5% CO2 at 37°C.
MTT assay
Cells were seeded at a density of 1×104
cells/well in 96-well plates. After 24 h, the cells were treated
with each ginsenoside at different concentrations (50, 100, 150,
200 and 250 μM) for 72 h. Control cells were treated
similarly without the addition of ginsenosides. Subsequently, the
media were removed and MTT solution (0.5 mg/ml) was added to each
well. The plate was incubated in a humidified atmosphere at 37°C
for 4 h and the media were carefully aspirated. Dimethyl sulfoxide
(100 μl) was added and the absorbance was measured at 570 nm
by a microplate reader (Bio-Rad, Hercules, CA, USA). The
experiments were performed in triplicate.
Statistical analysis
The results were expressed as mean ± standard
deviation. Data were analyzed by SPSS software version 17.0 (SPSS
Inc., Chicago, IL, USA). Statistical significance was compared
between the treatment and the control groups by one-way analysis of
variance. The differences were considered statistically significant
when P<0.05 and P<0.01 (Fig.
2). The experiments were performed in triplicate.
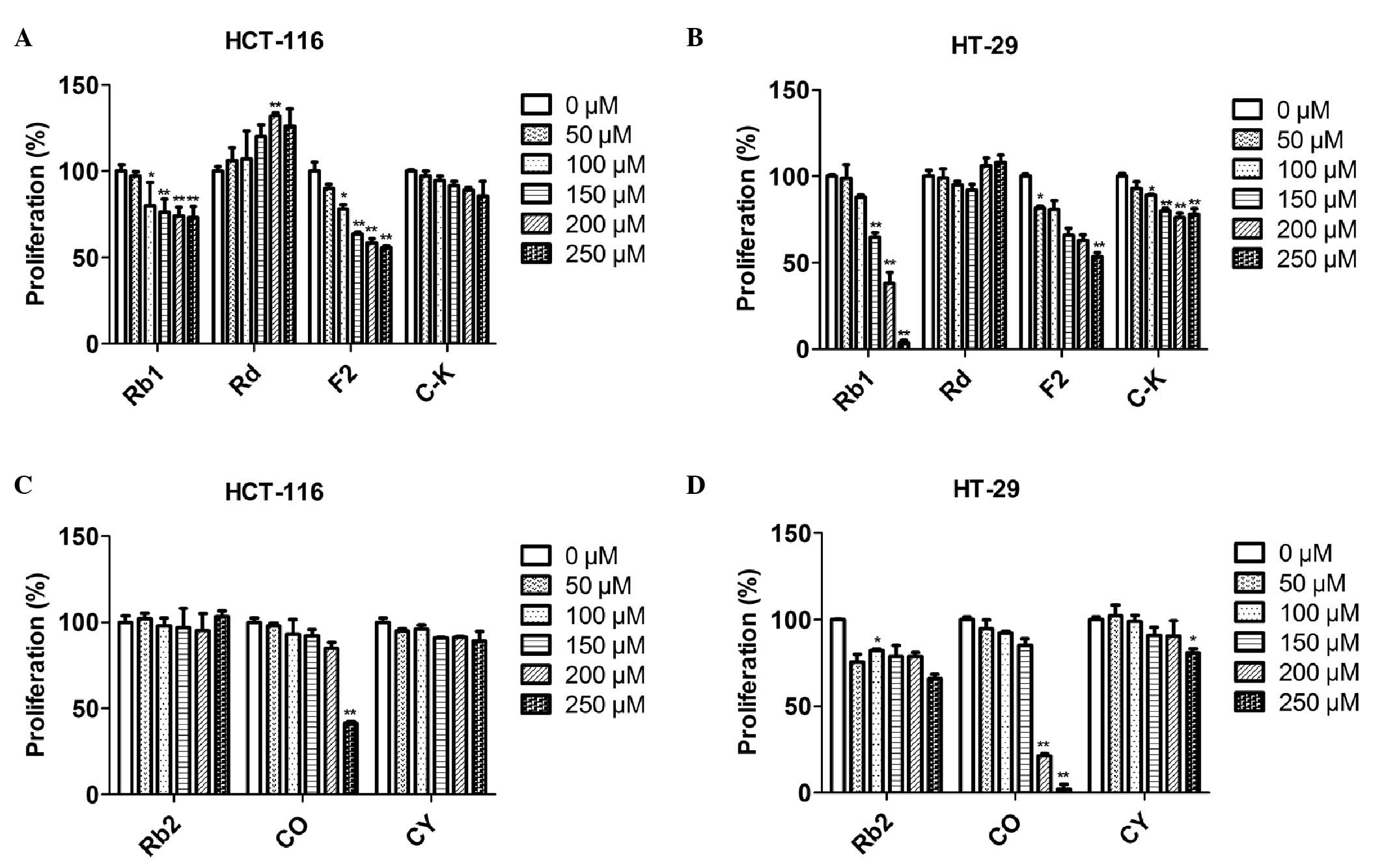 | Figure 2.Effects of ginsenosides on HCT-116 and
HT-29 cell proliferation. Cells were treated with seven types of
ginsenosides at various concentrations (0, 50, 100, 150, 200 and
250 μM) for 72 h. The antiproliferative effects of the
ginsenosides were determined by the MTT assay. (A) Effects of Rb1,
Rd, F2 and C-K on HCT-116 cell proliferation. (B) Effects of Rb1,
Rd, F2 and C-K on HT-29 cell proliferation. (C) Effects of Rb2, CO
and CY on HCT-116 cell proliferation (D) Effects of Rb2, CO and CY
on HT-29 cell proliferation. Error bars and all data are expressed
as mean ± standard error in triplicate. *P<0.05,
**P<0.01. |
Results
Effects of Rb1 and its biotransformation
products on cell proliferation
Ginsenoside Rb1 and its biotransformation products
Rd, F2 and C-K were assessed for their in vitro
antiproliferative activity on two human colon cancer cell lines.
The MTT assay results demonstrated that Rb1 exhibited no
antiproliferative activity on HCT-116 cells at a low concentration
(50 μM). Starting from a dose of 100 μM, Rb1
exhibited a marginal dose-dependent inhibitory effect. At a dose of
250 μM, the inhibitory rate of Rb1 was 27.0% (Fig. 2A). The HT-29 cell line was more
sensitive to Rb1. Treated with 100 μM or higher
concentration of Rb1, the HT-29 cell growth was inhibited in a
dose-dependent manner. At the highest dose of 250 μM, the
inhibitory rate was 96.4% (Fig.
2B). Ginsenoside Rd significantly increased HCT-116 cell
proliferation, whereas it exerted no obvious effect on HT-29 cell
growth. Ginsenoside F2 exhibited a moderate antiproliferative
activity on HCT-116 cells, with an inhibitory rate of 44.2% at 250
μM of F2. A similar inhibitory effect was observed on HT-29
cells, with an inhibitory rate of 46.4% at 250 μM.
Ginsenoside C-K exerted almost no antiproliferative activity on
HCT-116 cells and a marginal inhibitory effect on HT-29 cells, with
an inhibitory rate of 22.0% at the highest dose of 250
μM.
Effects of Rb2 and its biotransformation
products on cell proliferation
Ginsenoside Rb2 and its metabolites CO and CY were
assessed for their in vitro antiproliferative activity. As
shown in Fig. 2C and D, Rb2 exerted
no antiproliferative effect on HCT-116 cells, even at the highest
concentration of 250 μM. Rb2 exhibited a marginal inhibitory
activity on HT-29 cells. The antiproliferative effect of Rb2 was
not significantly altered at low concentrations (50–200 μM).
Treatment at the highest dose of 250 μM achieved an
inhibitory rate of 34.1%. Ginsenoside CO, the deglycosylation
product of Rb2, exerted no inhibitory effect on HCT-116 cell
proliferation at low concentrations (50–200 μM), whereas it
exerted a significant antiproliferative effect at the highest dose
of 250 μM, with an inhibitory rate of 58.7%. HT-29 cells
were more sensitive to ginsenoside CO compared to HCT-116 cells. At
low doses (50–150 μM), there was no antiproliferative effect
on HT-29 cells. However, following treatment with 200 and 250
μM of CO, the inhibitory rate was 78.7 and 98.0%,
respectively. The CY hydrolytic product of Rb2 exerted no
inhibitory effect on HCT-116 cell proliferation. Similar results
were observed on HT-29 cells at the range of 50–200 μM. CY
exhibited a marginal antiproliferative effect on HT-29 cells, with
an inhibitory rate of 19.1% only at the highest dose of 250
μM. C-K, with one sugar residue at C-20 of protopanaxadiol,
is also a hydrolytic product of Rb2 and it exerted no inhibitory
effect on HCT-116 or HT-29 cell growth (Fig. 2A and B).
Discussion
Ginsenosides are the major active components of
ginseng. It was reported that certain protopanaxadiol ginsenosides,
such as Rb1 and Rb2, are transformed into rare bioactive
ginsenosides through the hydrolysis of sugar residues, which is
usually enabled by glycosidases (11). In this study, ginsenoside Rb1 and
Rb2 and their biotransformation products Rd, F2, C-K (from Rb1), CO
and CY (from Rb2), were selected for assessment of their
antiproliferative activity on human colon cancer cells. It was
observed that the seven ginsenosides possessed the same aglycone
but different sugar residues. Therefore, the association between
the sugar residues in protopanaxadiol ginsenosides and their
antiproliferative activity was elucidated in this study.
Our results demonstrated that the number of sugar
residues significantly affected the inhibitory activity. As shown
in Fig. 1, ginsenoside Rb1 and Rb2
have four sugar residues, Rd and CO have three, F2 and CY have two
and C-K has one. Taking Rb1 and its deglycosylation products as an
example, when the sugar residues decreased from four to one, the
anti-proliferative activity was significantly altered. Similar
effects were observed on HCT-116 and HT-29 cells. Moreover, Rb2 and
its metabolites also exhibited different inhibitory effects on the
two human colon cancer cells tested. Ginsenoside F2, found in
ginseng in minute amounts, was produced by deglycosylation of Rb1
with the catalyzing action of glycosidase. Ginsenoside F2 exerted a
more potent inhibitory effect compared to that of Rb1. Similar to
F2, the minor ginsenoside CO, which was produced by deglycosylation
of Rb2, exerted more potent inhibitory effects compared to those of
Rb2. These results indicated that the number of sugar residues may
affect the antiproliferative activity of ginsenosides. However, the
detailed mechanism needs to be further investigated.
In addition, our results demonstrated that the
antiproliferative activity of ginsenosides was also correlated with
the type of sugar residue. Ginsenoside Rb1 and Rb2 have a similar
structure, with two sugar residues substituted at C-3 and C-20,
respectively. The only difference between them is the disaccharide
substituted at C-20 [glucose-β-(1→6)-glucose- for Rb1 and arabinose
(p)-α-(1→6)-glucose-β- for Rb2]. It was observed that ginsenosides
at the same dose but with different terminal sugar residues
exhibited different antiproliferative activities. Ginsenoside Rb1
exerted a significant inhibitory effect on HT-29 cell growth in a
dose-dependent manner. However, the antiproliferative activity of
Rb2 was significantly lower compared to that of Rb1, whereas no
dose-dependent inhibitory effect was exerted on HT-29 cells by Rb2.
These results indicate that the terminal sugar residue at the C-20
site may affect the inhibitory effects of ginsenosides to a certain
extent.
In conclusion, we investigated the inhibitory
effects of seven protopanaxadiol ginsenosides on HCT-116 and HT-29
human colorectal cancer cell proliferation. Our results indicate
that the number and type of sugar residues may affect the
antiproliferative activity of ginsenosides, which may provide
useful information on the association between ginsenoside structure
and antiproliferative activity.
Acknowledgements
This study was supported by the
Fundamental Research Funds for the Central Universities.
References
|
1.
|
Saw CL, Yang AY, Cheng DC, et al:
Pharmacodynamics of ginsenosides: antioxidant activities,
activation of Nrf2, and potential synergistic effects of
combinations. Chem Res Toxicol. 25:1574–1580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hashimoto R, Yu J, Koizumi H, Ouchi Y and
Okabe T: Ginsenoside Rb1 prevents MPP(+)-induced apoptosis in PC12
cells by stimulating estrogen receptors with consequent activation
of ERK1/2, Akt and inhibition of SAPK/JNK, p38 MAPK. Evid Based
Complement Alternat Med. 2012:6937172012.PubMed/NCBI
|
|
3.
|
Lee JS, Song JH, Sohn NW and Shin JW:
Inhibitory effects of ginsenoside Rb1 on neuroinflammation
following systemic lipopolysaccharide treatment in mice. Phytother
Res. Oct 8–2012.(Epub ahead of print).
|
|
4.
|
Huang X, Liu X and Deng C: Effects of the
combination of active component extracts from Astragalus
membranaceus and Panax notoginseng on apoptosis, reactive
oxygen species and mitochondrial membrane potential of PC12 cells
with oxidative injury. J Chin Integr Med. 10:1127–1134. 2012.(In
Chinese).
|
|
5.
|
Lee HU, Bae EA, Han MJ, Kim NJ and Kim DH:
Hepatoprotective effect of ginsenoside Rb1 and compound K on
tert-butyl hydro-peroxide-induced liver injury. Liver Int.
25:1069–1073. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhou W, Feng MQ, Li JY and Zhou P: Studies
on the preparation, crystal structure and bioactivity of
ginsenoside compound K. J Asian Nat Prod Res. 8:519–527. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Choi K, Kim M, Ryu J and Choi C:
Ginsenosides compound K and Rh(2) inhibit tumor necrosis
factor-alpha-induced activation of the NF-kappaB and JNK pathways
in human astroglial cells. Neurosci Lett. 421:37–41. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zhou W, Feng M, Li X, et al: X-ray
structure investigation of
(20S)-20-O-beta-D-glucopyranosyl-protopanaxadiol and antitumor
effect on Lewis lung carcinoma in vivo. Chem Biodivers. 6:380–388.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kim JH, Hong YH, Lee JH, et al: A role for
the carbohydrate portion of ginsenoside Rg3 in
Na+channel inhibition. Mol Cells. 19:137–142.
2005.PubMed/NCBI
|
|
10.
|
Chu SF and Zhang JT: New achievements in
ginseng research and its future prospects. Chin J Integr Med.
15:403–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gao J, Xu W, Fang Q, et al: Efficient
biotransformation for preparation of pharmaceutically active
ginsenoside compound K by Penicillium oxalicum sp. 68. Ann
Microbiol. 63:139–149. 2013. View Article : Google Scholar
|