Introduction
Toxoplasma gondii (T. gondii) is a
protozoan parasite that infects approximately 1/3 of the human
population worldwide (1). Anticancer
activities of this parasite have been demonstrated in previous
studies (2-4).
Notably, it has been observed that non-replicating T. gondii
reverses tumor associated immunosuppression (5). Antitumor effects of T. gondii
antigens in a murine sarcoma 180 tumor model have also been
demonstrated (4). In another study,
Balb/c inbred mice were injected with T. gondii antigens and
then challenged with WEHI-164 fibrosarcoma cells, and significant
inhibition of tumor growth was observed (6). In other work Choo et al (2) injected mice with T. gondii
antigens and observed that malignant glioma growth was inhibited
significantly in comparison with a control group. Kim et al
(3) challenged two groups of mice
with Lewis lung carcinoma, and infected one with T. gondii
as an experimental group. They observed that survival rate was
significantly increased in the parasite-injected mice.
Additionally, angiogenesis in the experimental group was notably
inhibited (3). In other work, it has
been demonstrated that intradermal injection of T. gondii
stimulated a potent antitumor immune response in vivo
(7). In this regard it has also been
shown that T. gondii is able to generate therapeutic
antitumor immunity against ovarian cancer (8). Besides T. gondii, anticancer
activities of other parasites including Trypanpsoma cruzi
(9), malaria parasite (10) and hydatid cyst [larval stage of
Echinococcus granulosus (E. granulosus)] (11-13)
have also been reported. The mechanisms underlying the anticancer
effects of parasites are not well defined. However, it has been
suggested that the immune response raised by the parasites may
nonspecifically affect the growth of cancer. For instance, it has
been demonstrated that Trypanosoma cruzi (T. cruzi)
is able to elicit a protective immune response against colon and
mammary cancers (9). The existence of
antibody against T. cruzi in mouse was associated with
inhibition of tumor growth in vivo (14). In another study, it was demonstrated
that cell-mediated immunity had a role in antitumor activity of
T. cruzi (15). In this
regard, the presence of common antigens between cancers and certain
parasites has been reported (16-18).
To identify some of the mechanisms of the anticancer activities of
parasites, in the present work a reaction panel of anti-parasite
antisera with the surface of mouse melanoma and breast cancer cell
lines was investigated.
Materials and methods
Antigen preparation
T. gondii purified tachyzoites were purchased
from the Pasture Institute, Tehran, Iran. The tachyzoites were
sonicated in PBS, centrifuged for 2 min at 600 x g at room
temperature, and the supernatant containing T. gondii
antigen was maintained at -20˚C. Trichomonas vaginalis
(T. vaginalis) parasites maintained in liquid nitrogen from
the Department of Parasitology, Isfahan University of Medical
Sciences (Isfahan, Iran) were cultured in TYIS medium (produced
in-house) and the antigen prepared as described in our previous
work (19). Briefly, the parasites
were harvested from culture medium, washed three times and
sonicated, and T. vaginalis crude antigen was kept at -20˚C.
Sheep lungs or livers infected with hydatid cysts were obtained
from slaughtered sheep in Fasaran slaughter house in Isfahan, Iran.
Hydatid cyst fluid was then aspirated and examined under a light
microscope. Fluid exhibiting the presence of protoscolices was then
centrifuged for 2 min at 600 x g and the supernatant containing
hydatid cyst fluid antigen was stored at -20˚C. The packed
protoscolices were also sonicated in PBS, centrifuged for 2 min at
600 x g and the supernatant stored as protoscolices antigen.
Cell culture
Mouse melanoma (B16F10) and breast (4T1) cancer cell
lines were purchased from the Pasture Institute. The cells were
cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) with a humidity of 70% and 5.0% CO2 as
previously mentioned (20). Normal
lymphocytes were isolated from normal mouse spleens and prepared
for further experimentation.
Animal experiments
Rabbits and mice were used in the current study,
which were purchased from the Pasture Institute. A total of 6
female Balb/c, inbred, 2-month-old mice were used to prepare spleen
cells. For this purpose, the mice were euthanized by
intraperitoneal injection of 250 mg/kg body weight pentobarbital
(Sigma, 3636 under sterile conditions and their spleens removed.
Subsequently, the spleen cells were extracted, counted and their
viability checked by using trypan blue staining. To prepare cells,
spleens were removed from scarified mice and transferred to a Petri
Dish containing Isotonic saline. Spleens were then minced using a
Scalpel blade and the mixture was passed through a four-layered
gauze to remove large debris. Isotonic saline containing cells were
then washed twice with isotonic saline and centrifuged at 600 x g
for 2 min at room temperature. Subsequently, cells were further
suspended in isotonic saline. To stain cells with Τrypan blue
(Merck, 50 µl prepared cells was mixed with 50 µl Trypan blue stain
at room temperature. Following 15 min, one drop of mixed cells with
trypan blue was applied to a Neubauer slide (HBG, Germany) and
counted using light microscope (magnification, x400).
Four month-old male rabbits were used
to raise antibodies against parasites antigens
The animals were maintained in an appropriate animal
research facility and mice kept in groups of six per cage (rabbits
one/cage) and fed with clean food and water. The rabbits were
housed at 20-25˚C with a humidity of 70-80% with a 12 h light/dark
cycle. The animal research protocols of the present study were
approved by Isfahan University of Medical Sciences Ethics Committee
with approval number IR.mui.REC.1394.824.
Preparation of rabbit antisera
Volumes of 1 ml containing 2 mg of the different
parasite antigens and sonicated breast and melanoma cancer cells
were emulsified in the same volume (1 ml for each antigen) of
Freund's adjuvant (Sigma-Aldrich; Merck KGaA) at room temperature
and each antigen injected subcutaneously into a male 4-month-old,
white New Zealand rabbit (n=5 in total). Injections were repeated
fortnightly. In all rabbits, complete Freund's adjuvant was used
for the first injections and incomplete Freund's adjuvant for the
boosters. Following the third booster, 1 ml blood samples from the
ear vein of each rabbit were collected. To prepare sera, the blood
samples were centrifuged at 3,000 x g for 5 min at room
temperature. The sera were then checked for the presence of
specific antibodies using home-made ELISA tests as described in our
previous study (20). In those
rabbits with a high titer (optical density >1.5) of antibodies,
the last booster was injected into each rabbit and then blood
samples were collected and the sera kept at -20˚C until use. For
the injection of rabbits or bleeding no anesthesia method was used.
However, following final bleeding the rabbits were euthanized by
intravenous injection of 150 mg/kg pentobarbital.
For ELISA, 96 wells plates were coated with the
antigens (T. gondii, T. vaginalis or hydatid cyst
protoscolices crude antigens) diluted 1:20 with carbonate buffer.
Following overnight incubation at 37˚C, the plates were blocked
with 1% bovine albumin (Merck KGaA) and then washed with sodium
chloride buffer containing 0.05% Tween-20, and then diluted (1:100)
antisera were added and incubated at 37˚C for 1 h. The plates were
then washed and a secondary antibody (A6154; Sigma-Aldrich was
added and incubated at 37˚C for 1 h. Finally, following washing,
the plates were incubated with the chromogenic substrate and the
optical density of wells was read at 450 nm using an ELISA reader
(Kbiokit-ELx800).
Reaction of antisera with the cancer
cell lines
Mouse cancer cells were harvested from culture
medium and normal lymphocytes obtained from normal mouse spleens.
All the cells were washed with PBS and incubated for 1hour at 37˚C
with different antisera (1:100) which raised in rabbits against
different mentioned antigens, namely anti-T. gondii,
anti-T. vaginalis, anti-hydatid cyst fluid,
anti-protoscolices antigens, anti-melanoma cells, anti-breast
cancer cells or normal rabbit serum primary antibodies. After 1 h,
the cells were washed with sodium chloride buffer containing 0.05%
Tween-20, and secondary antibodies conjugated with fluorescein
isothiocyanate (Donkey anti-rabbit IgG BioLegend, Inc., San Diego,
CA, USA; 406403; 1:1,000) were applied in the dark at 4˚C for 1 h.
Following the last washing of the test tubes, cells were analyzed
using a flow cytometer. BD Cell Quest Pro software version 6 (BD
Biosciences, San Jose, CA, USA) was used for analysis.
Statistical analysis
The experiments were repeated three times and data
are presented as the mean ± standard deviation. A linear mixed
model test was used for statistical analysis. In comparison of
means, P<0.05 was considered to indicate significance.
Results
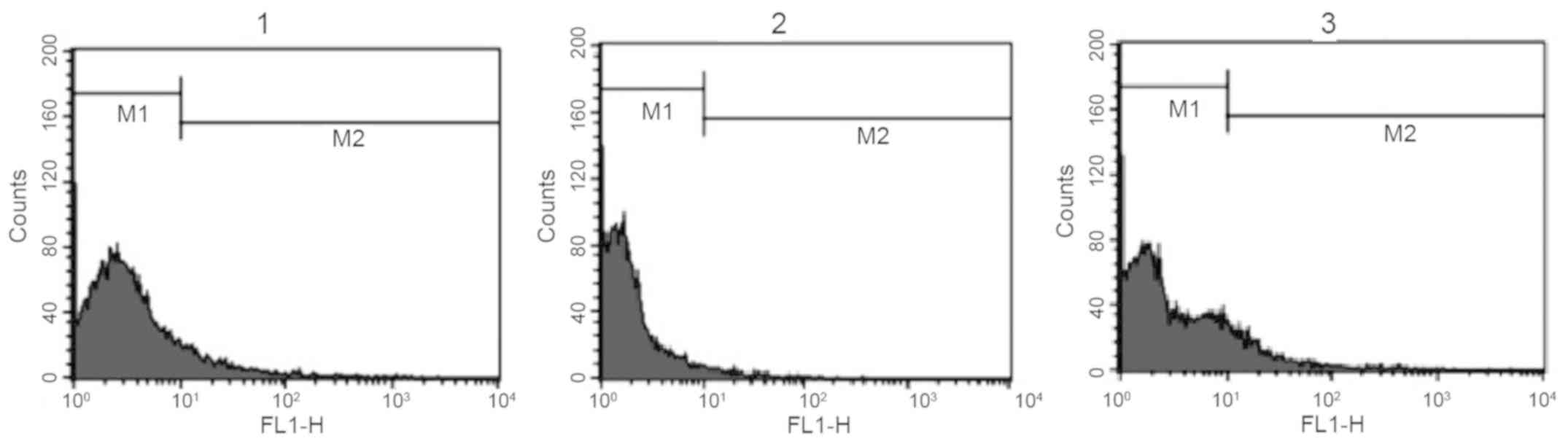
Reaction of anti-Toxoplasma antibodies
with surface of B16F10 melanoma cells
The reaction of antiserum against T. gondii
and normal rabbit serum with the surface of mouse melanoma cells
was investigated. Anti-T. gondii antiserum exhibited a
marked reaction with melanoma cells, while the reaction of normal
rabbit serum was weak. In this experiment, anti-melanoma cell
antiserum was used as a positive control and normal rabbit serum as
a negative control (Fig. 1 and
Table I). Analysis of experimental
repeats indicated that the difference in M2 (reactive) cells
between the anti-T. gondii antiserum and normal rabbit serum
groups was statistically significant (P<0.05).
 | Table I.Results of flow cytometry analysis
(M2 cell percentage of total cells) indicating the level of
reaction of different antisera with the surface of mouse melanoma
cancer cells (B16F10) and mouse normal spleen cells. |
Table I.
Results of flow cytometry analysis
(M2 cell percentage of total cells) indicating the level of
reaction of different antisera with the surface of mouse melanoma
cancer cells (B16F10) and mouse normal spleen cells.
| | Anti-melanoma
(B16F10) antiserum | Anti-Toxoplasma
gondii antiserum | Normal rabbit
serum | Background
fluorescence |
|---|
| B16F10 | 10.28±0.49 |
11.14±1.48a | 1.91±0.31 | 1.11 |
| Spleen cell | - | 9.32±0.21 | 7.79±0.79 | 3.18 |
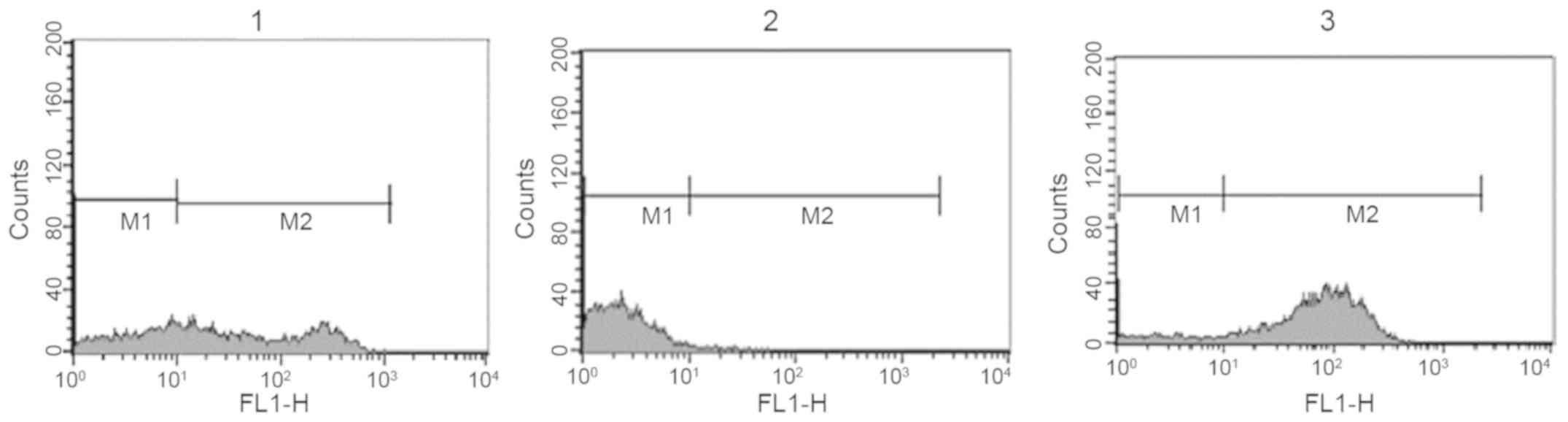
Reaction of anti-Toxoplasma antibodies
with surface of 4T1 breast cancer cells
The reaction of antisera against T. gondii
and normal rabbit serum with the surface of mouse breast cancer
cells was investigated. Again, T. gondii antiserum exhibited
a strong reaction with breast cancer cells while the reaction of
normal rabbit serum was weak. Anti-breast cancer cell antisera were
used as a positive control and normal rabbit serum as a negative
control (Fig. 2 and Table II). When the experiment was repeated,
the difference in M2 cells between the anti-T. gondii
antiserum and normal rabbit serum groups was statistically
significant (P<0.05).
 | Table II.Results of flow cytometry analysis
(M2 cell percentage of total cells) indicating the level of
reaction of different antisera with the surface of mouse breast
cancer cells (4T1) and mouse normal spleen cells. |
Table II.
Results of flow cytometry analysis
(M2 cell percentage of total cells) indicating the level of
reaction of different antisera with the surface of mouse breast
cancer cells (4T1) and mouse normal spleen cells.
| | Anti-4T1
antiserum | Anti-Toxoplasma
gondii antiserum | Normal rabbit
serum | Background
fluorescence |
|---|
| 4T1 | 62.14±7.97 |
81.77±22.2a | 2.06±0.69 | 0.17 |
| Spleen cells | - | 7.79±0.79 | 9.32±0.21 | 3.18 |
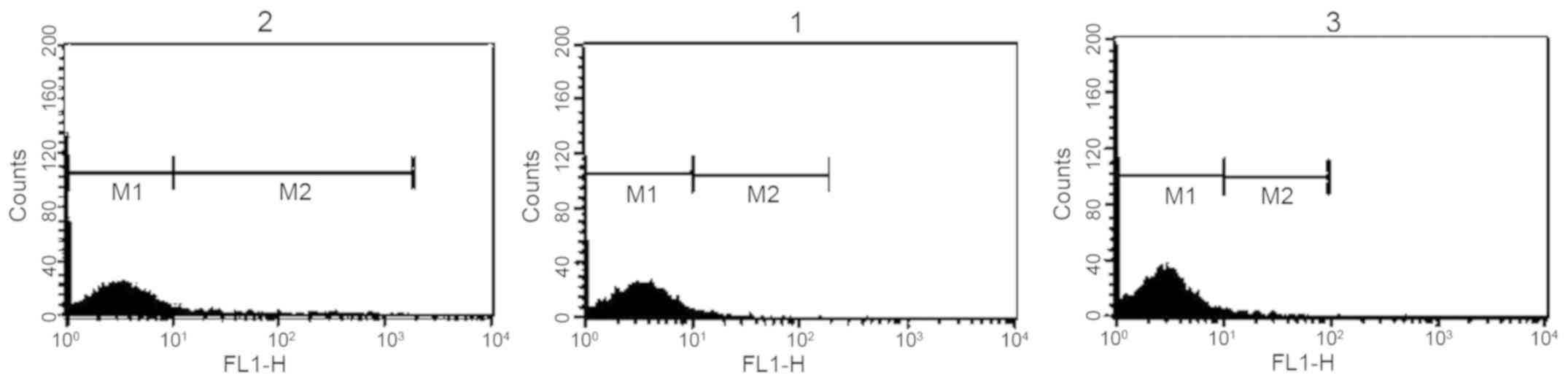
Reaction of anti-Toxoplasma antibodies
with surface of mouse normal spleen lymphocytes
The reaction of anti-T. gondii antiserum and
normal rabbit serum with the surface of mouse normal spleen
lymphocytes was investigated. The results revealed that the
reaction of anti-Toxoplasma antibodies with melanoma cells
was greater than the reaction with mouse normal lymphocytes.
However, the reaction of normal rabbit serum with mouse normal
lymphocytes was greater than the reaction with melanoma cells
(Fig. 3 and Tables I and II).
Reaction of anti-T
vaginalis and anti-protoscolices antibodies
with surface of melanoma, breast cancer or mouse normal spleen
lymphocytes. To determine if antisera against different parasites
exhibited a reaction similar to that of anti-T. gondii
antiserum, the reactions of antisera against T. vaginalis
and hydatid cyst crude protoscolices antigen with the surface of
breast cancer cells, melanoma cells and normal mouse spleen
lymphocytes were investigated. The results revealed no
statistically significant differences in the reactions of the sera
with cancer cells compared with spleen lymphocytes (Table III).
 | Table III.Results of flow cytometry analysis
(M2 cell percentage of total cells) indicating reaction of antisera
against Trichomonas vaginalis and hydatid cyst crude protoscolices
antigen with the surface of breast cancer cells, melanoma cancer
cells or normal mouse spleen lymphocytes. |
Table III.
Results of flow cytometry analysis
(M2 cell percentage of total cells) indicating reaction of antisera
against Trichomonas vaginalis and hydatid cyst crude protoscolices
antigen with the surface of breast cancer cells, melanoma cancer
cells or normal mouse spleen lymphocytes.
| | Anti-Trichomonas
vaginalis antiserum | Anti-hydatid cyst
protoscolices antiserum | Background
florescence intensity |
|---|
| Melanoma cancer
cells | 3.74±0.74 | 2.11±0.35 | 1.11±0.14 |
| Breast cancer
cells | 5.75±1.23 | 3.64±0.82 | 0.17±0.09 |
| Normal mouse spleen
lymphocytes | 11.23±2.39 | 9.43±1.97 | 3.18±0.68 |
Discussion
Results of the present work indicated that
anti-T. gondii antiserum selectively reacts with the surface
of murine melanoma and breast cancer cells and not with normal
mouse spleen lymphocytes. Additionally, the results revealed that
this selective reactivity did not occur with other anti-parasitic
antisera including anti-T. vaginalis or anti-hydatid cyst
protoscolices antigen. This is a notable finding as through
selective attachment of anti-T. gondii antibodies to cancer
cells, these antibodies may by valuable carriers of cancer
immunotherapeutics. In this regard, Salanti et al (21) demonstrated that a glycosaminoglycan
protein of Plasmodium falciparum selectively attached to cancer
cells, and they proposed that this protein may be a viable
candidate for cancer immunotherapy.
Reaction of anti-Toxoplasma antibodies with
the surface of breast and melanoma cancer cells implies that there
are shared epitopes on the surface of cancer cells and
Toxoplasma antigens. In this context, existence of common
antigens between certain parasites and cancers has been documented
(16,17). The majority of these antigens are
specific mucin glycoproteins, which have important roles in the
metastasis of cancer cells (22) and
in the interaction of parasites and their hosts. For instance,
expression of tumor-associated antigen in adult and larval stages
of E. granulosus has been observed. Additionally,
cross-reaction of the sera of cancer patients with 40 and 27 kDa
bands of hydatid cyst antigens has been demonstrated (18,23).
The effect elicited by cross-reaction of antibodies
raised against Toxoplasma with epitopes on the surface of
cancer cells remained undetermined in the present study. However,
the attachment of the antibody to the cancer cells surface may
exert some lethal effects on the cancer cells. For instance, it has
been shown that Trypanosoma cruzi antigens stimulated
protective immunity against colon and mammary cancers (9). Alternatively, this cross-reaction may
render the cancer cells vulnerable to the complement system. In
this regard it has been reported that complexes of antigen-antibody
are able to activate the complement system (24).
In previous years there has been considerable focus
on enhancing antibody activity through conjugation with cytotoxic
drugs (25-28).
Thus, with further work it may be possible to conjugate humanized
purified anti-Toxoplasma monoclonal antibodies to a
cytotoxic drug to improve its suitability for site-selective drug
delivery. Such anti-Toxoplasma monoclonal antibodies may
have the advantage of being effective against different cancers. It
has been demonstrated that drug targeting may improve the efficacy
of therapy and reduce side effects associated with drugs (29). Immunohistochemistry is now recommended
to investigate the reaction of anti-Toxoplasma antibodies
with human breast cancer tissues.
One of the main problems in the treatment of cancer
is that most of the drugs used to target cancer cells are also
cytotoxic to normal tissues. Attempts have been made to overcome
this problem by coupling anticancer drugs to antibodies which have
some degree of specificity for cancer antigens. In this regard
there are numerous patents regarding use of antibodies for drug
delivery. For instance, in a patented method by Sahin et al
(30), monoclonal antibodies against
claudin-18 were used for the treatment of different cancers. Patil
et al (31) also conjugated a
polymalic acid platform to a monoclonal antibody to enable specific
drug delivery to treat different cancers. Furthermore, conjugation
of an antibody with a cytotoxin or an enzyme for the selective
treatment of cancers has been proposed (32).
Through further study it may be possible to
conjugate humanized purified anti-Toxoplasma monoclonal
antibodies to a drug for selective treatment of certain cancers.
Tumor-associated antigens (TAAs) are expressed by tumor cells and
can be recognized by the immune system. Most known TAAs have been
found to be expressed by melanoma cancer cells, and few TAAs have
been recognized in other tumors (33). TTAs exhibit poor immunogenicity, which
results in lack of a sufficient immune response to control cancer
growth (34,35). It has been demonstrated that
vaccination with TAA peptides generally fails to induce a specific
immune response in mice (36).
Immunization with parasite-derived antigens, which have shared
epitopes with TTAs, may overcome this disadvantage and induce a
high degree of protective immunity. In this regard, it has been
observed that for virally induced tumors, prophylactic vaccination
with synthetic peptides was effective in animal models (37,38),
whereas for non-virally induced tumors this vaccination was less
effective (39,40). Thus, induction of a strong immune
response may be one of the critical advantages of immunotherapy
with parasitic antigens.
In conclusion, in the present study it was indicated
that anti-Toxoplasma antiserum selectively reacts with the
surface of mouse cancer cells but not with normal mouse spleen
lymphocytes. With further work these selectively binding antibodies
may be a useful tool in cancer immunotherapy.
Acknowledgements
The present study was prepared from thesis by a PhD
student (Mrs Mahshid Shakibapour) and an MSc student (Miss
Fereshteh Mohamadi) from Isfahan University of Medical
Sciences.
Funding
The present work was supported by a grant (grant no.
3953009) from Isfahan University of Medical Sciences (Isfahan,
Iran).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FM and MS performed the experiments. SS performed
the experiments and wrote the first draft of the manuscript. AA
consulted immunohistochemical procedures. ST assisted with
experiments. HD supervised experiments and prepared final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
John DT, Petri WA and Markell EK: Voge M.
Markell and Voge's Medical Parasitology. 9th eddition. Elsevier
Health Sciences, St. Louis. 2006.
|
|
2
|
Choo JD, Lee JS, Kang JS, Lee HS, Yeom JY
and Lee YH: Inhibitory effects of Toxoplasma antigen on
proliferation and invasion of human glioma cells. J Korean
Neurosurg Soc. 37:129–136. 2005.
|
|
3
|
Kim JO, Jung SS, Kim SY, Kim TY, Shin DW,
Lee JH and Lee YH: Inhibition of Lewis lung carcinoma growth by
Toxoplasma gondii through induction of Th1 immune responses
and inhibition of angiogenesis. J Korean Med Sci. 22:S38–S46.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pyo KH, Jung BK, Chai JY and Shin EH:
Suppressed CD31 expression in sarcoma-180 tumors after injection
with Toxoplasma gondii lysate antigen in BALB/c mice. Korean
J Parasitol. 48:171–174. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fox BA, Sanders KL and Bzik DJ:
Non-replicating Toxoplasma gondii reverses tumor-associated
immunosuppression. Oncoimmunology. 2(e26296)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Darani HY, Shirzad H, Mansoori F,
Zabardast N and Mahmoodzadeh M: Effects of Toxoplasma gondii
and Toxocara canis antigens on WEHI-164 fibrosarcoma growth in a
mouse model. Korean J Parasitol. 47:175–177. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baird JR, Byrne KT, Lizotte PH,
Toraya-Brown S, Scarlett UK, Alexander MP, Sheen MR, Fox BA, Bzik
DJ, Bosenberg M, et al: Immune-mediated regression of established
B16F10 melanoma by intratumoral injection of attenuated
Toxoplasma gondii protects against rechallenge. J Immunol.
190:469–478. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baird JR, Fox BA, Sanders KL, Lizotte PH,
Cubillos-Ruiz JR, Scarlett UK, Rutkowski MR, Conejo-Garcia JR,
Fiering S and Bzik DJ: Avirulent Toxoplasma gondii generates
therapeutic antitumor immunity by reversing immunosuppression in
the ovarian cancer microenvironment. Cancer Res. 73:3842–3851.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ubillos L, Freire T, Berriel E, Chiribao
ML, Chiale C, Festari MF, Medeiros A, Mazal D, Rondán M,
Bollati-Fogolín M, et al: Trypanosoma cruzi extracts elicit
protective immune response against chemically induced colon and
mammary cancers. Int J Cancer. 138:1719–1731. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen L, He Z, Qin L, Li Q, Shi X, Zhao S,
Chen L, Zhong N and Chen X: Antitumor effect of malaria parasite
infection in a murine Lewis lung cancer model through induction of
innate and adaptive immunity. PLoS One. 6(e24407)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chookami MB, Sharafi SM, Sefiddashti RR,
Jafari R, Bahadoran M, Pestechian N and Yousofi Darani H: Effect of
two hydatid cyst antigens on the growth of melanoma cancer in
C57/black mice. J Parasit Dis. 40:1170–1173. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Berriel E, Russo S, Monin L, Festari MF,
Berois N, Fernández G, Freire T and Osinaga E: Antitumor activity
of human hydatid cyst fluid in a murine model of colon cancer.
ScientificWorldJournal 230176. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karadayi S, Arslan S, Sumer Z, Turan M,
Sumer H and Karadayi K: Does hydatid disease have protective
effects against lung cancer? Mol Biol Rep. 40:4701–4704.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kallinikova VD, Batmonkh Ts, Kosobokova
EN, Pakhorukova LV, Ogloblina TA, Kravtsov EG, Karpenko LP and
Matekin PV: Antibodies against Trypanosoma cruzi in intact
mice and their oncoprotective effect. Med Parazitol (Mosk).
1:11–15. 2008.(In Russian). PubMed/NCBI
|
|
15
|
Zenina AV, Kravtsov EG, Tsetsegsaikhan B,
Yashina NV, Dalin MV, Karpenko LP, Sheklakova LA and Kallinikova V:
The study of immunological component in antitumor effect of
Trypanosoma cruzi. Bull Exp Biol Med. 145:352–354.
2008.PubMed/NCBI
|
|
16
|
Ubillos L, Medeiros A, Cancela M,
Casaravilla C, Saldaña J, Domínguez L, Carmona C, Le Pendu J and
Osinaga E: Characterization of the carcinoma-associated Tk antigen
in helminth parasites. Exp Parasitol. 116:129–136. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Thors C, Jansson B, Helin H and Linder E:
Thomsen-Friedenreich oncofetal antigen in Schistosoma mansoni:
Localization and immunogenicity in experimental mouse infection.
Parasitology. 132:73–81. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Daneshpour S, Bahadoran M, Hejazi SH,
Eskandarian AA, Mahmoudzadeh M and Darani HY: Common antigens
between hydatid cyst and cancers. Adv Biomed Res.
5(9)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Darani HY, Ahmadi F, Zabardast N, Yousefi
HA and Shirzad H: Development of a latex agglutination test as a
simple and rapid method for diagnosis of Trichomonas
vaginalis infection. Avicenna J Med Biotechnol. 2:63–66.
2010.PubMed/NCBI
|
|
20
|
Sharafi SM, Shirzad H, Khanahmad H, Ataei
B and Darani HY: Monoclonal antibodies production against a 40 KDa
band of hydatid cyst fluid. Recent Pat Biotechnol. 12:57–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Salanti A, Clausen TM, Agerbæk MØ, Al
Nakouzi N, Dahlbäck M, Oo HZ, Lee S, Gustavsson T, Rich JR, Hedberg
BJ, et al: Targeting human cancer by a glycosaminoglycan binding
malaria protein. Cancer Cell. 28:500–514. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Baldus SE, Engelmann K and Hanisch FG:
MUC1 and the MUCs: A family of human mucins with impact in cancer
biology. Crit Rev Clin Lab Sci. 41:189–231. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sharafi SM, Rafiei R, Rafiei R, Hadipour
M, Shirzad H, Khanahmad H and Darani HY: A Nonglycosylated 27 kDa
molecule as common antigen between human breast cancer and
Echinococcus granulosus hydatid cyst wall. Adv Breast Cancer
Res. 5:90–95. 2016. View Article : Google Scholar
|
|
24
|
Porter R and Reid K: Activation of the
complement system by antibody-antigen complexes: the classical
pathway. Advances in protein chemistry. Adv Protein Chem. 33:1–71.
1979.
|
|
25
|
Chari RV: Targeted cancer therapy:
Conferring specificity to cytotoxic drugs. Acc Chem Res. 41:98–107.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Damle NK: Tumour-targeted chemotherapy
with immunoconjugates of calicheamicin. Expert Opin Biol Ther.
4:1445–1452. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kovtun YV and Goldmacher VS: Cell killing
by antibody-drug conjugates. Cancer Lett. 255:232–240.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu AM and Senter PD: Arming antibodies:
Prospects and challenges for immunoconjugates. Nat Biotechnol.
23:1137–1146. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Trail PA, King HD and Dubowchik GM:
Monoclonal antibody drug immunoconjugates for targeted treatment of
cancer. Cancer Immunol Immunother. 52:328–337. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sahin U, Türeci Ö, Usener D, Fritz S,
Uherek C, Brandenburg G, Geppert HG, Schroeder Anja K and Thiel P:
Monoclonal antibodies against claudin-18 for treatment of cancer.
Google Patents EP 2311879 A2. Filed November 24 2005; issued April
20. 2011.
|
|
31
|
Patil R, Holler E, Black KL and Ljubimova
JY: Drug delivery of Temozolomide for systemic based treatment of
cancer. Google Patents US8785371B2. Filed December 10, 2009; issued
November 28. 2017.
|
|
32
|
Lallatin NC: Monoclonal antibodies to
human thymidine kinase to treat cancer. Google Patents. 2013.
|
|
33
|
Parmiani G, Castelli C, Dalerba P,
Mortarini R, Rivoltini L, Marincola FM and Anichini A: Cancer
immunotherapy with peptide-based vaccines: What have we achieved?
Where are we going? J Natl Cancer Inst. 94:805–818. 2002.PubMed/NCBI
|
|
34
|
Marchand M, van Baren N, Weynants P,
Brichard V, Dréno B, Tessier MH, Rankin E, Parmiani G, Arienti F,
Humblet Y, et al: Tumor regressions observed in patients with
metastatic melanoma treated with an antigenic peptide encoded by
gene MAGE-3 and presented by HLA-A1. Int J Cancer. 80:219–230.
1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Weber JS, Hua FL, Spears L, Marty V,
Kuniyoshi C and Celis E: A phase I trial of an HLA-A1 restricted
MAGE-3 epitope peptide with incomplete Freund's adjuvant in
patients with resected high-risk melanoma. J Immunother.
22:431–440. 1999.PubMed/NCBI
|
|
36
|
Valmori D, Fonteneau J-F, Lizana CM,
Gervois N, Liénard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini
JC and Romero P: Enhanced generation of specific tumor-reactive CTL
in vitro by selected Melan-A/MART-1 immunodominant peptide
analogues. J Immunol. 160:1750–1758. 1998.PubMed/NCBI
|
|
37
|
Schulz M, Zinkernagel RM and Hengartner H:
Peptide-induced antiviral protection by cytotoxic T cells. Proc
Natl Acad Sci USA. 88:991–993. 1991.PubMed/NCBI
|
|
38
|
Kast WM, Roux L, Curren J, Blom HJ,
Voordouw AC, Meloen RH, Kolakofsky D and Melief CJ: Protection
against lethal Sendai virus infection by in vivo priming of
virus-specific cytotoxic T lymphocytes with a free synthetic
peptide. Proc Natl Acad Sci USA. 88:2283–2287. 1991.PubMed/NCBI
|
|
39
|
Mandelboim O, Vadai E, Fridkin M,
Katz-Hillel A, Feldman M, Berke G and Eisenbach L: Regression of
established murine carcinoma metastases following vaccination with
tumour-associated antigen peptides. Nat Med. 1:1179–1183.
1995.PubMed/NCBI
|
|
40
|
Noguchi Y, Chen YT and Old LJ: A mouse
mutant p53 product recognized by CD4+ and
CD8+ T cells. Proc Natl Acad Sci USA. 91:3171–3175.
1994.PubMed/NCBI
|