Introduction
Recently, a growing body of literature has focused
on reshaping immunomodulation by blocking the abnormal upregulation
of immune checkpoint proteins, including programmed cell death
protein 1 (PD-1), programmed death ligand 1 (PD-L1), PD-L2 and
cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), to prevent
tumours from co-opting immune resistance and to enhance antitumour
specificity (1). Owing to the
introduction to clinical practice of immune checkpoint inhibitors
(ICIs), a spectrum of monoclonal antibodies (mAbs) intervening in
the interactions of immune checkpoints, high efficacy has been
achieved in the treatment of malignant tumours, especially those
refractory to conventional therapies, which enriches the
therapeutic arsenal (2).
However, clinicians have been confronted with a set
of immune-related adverse events (irAEs) upon the use of ICIs,
albeit at the same time as promising antitumour activity (2,3). The
irAEs caused by ICIs are experienced by 20-85% patients, and can
affect almost all organs, with cutaneous irAEs being the most
frequent toxicities, affecting 7-68% of patients receiving ICIs,
followed by gastrointestinal/hepatic irAEs (7-50%), endocrine irAEs
(1-23.7%) and pulmonary irAEs (0-9%) (2,4-6).
Other rare irAEs affect systems such as the cardiovascular,
neurological, ocular, rheumatological, renal and haematological
systems (2). It is worth noting
that some irAEs are life threatening, albeit at a relatively low
incidence rate. According to a previous meta-analysis, the
incidence rates of irAE-associated fatality were 0.36% for PD-1
inhibitors), 0.38% for PD-L1 inhibitors and 1.08% for CTLA-4
inhibitors (7). Within the spectrum
of fatal irAEs, myocarditis accounted for the highest fatality rate
(39.7-46.0%), albeit at an incidence of 1.14% (7-9).
The incidence of all-grade and high-grade ICI-related pneumonitis
also increased significantly compared with that of the controls
(RR, 4.70 and 3.33, respectively) (10). Other severe irAEs with high fatality
rates include hepatitis, myositis, nephritis, and neurological and
hematological toxicities (10-17%) (7). The expression of PD-1 has been found
on a broad range of immune cells, including T lymphocytes, B
lymphocytes, monocytes, macrophages, dendritic cells and natural
killer cells, and is upregulated in tumour infiltrating T
lymphocytes (1). The binding of
PD-1 with ligands (mainly PD-L1) leads to immunotolerance and
tumour escape by inhibiting effector T cells and promoting
regulatory T cells (1). Monoclonal
antibodies (mAbs) intervening in the interactions within PD-1/PD-L1
pathway can augment endogenous antitumour immunoreactions, modulate
components in the tumour microenvironment and restore balanced
immune homeostasis (1,2). Camrelizumab (SHR-1210) is a humanized
high-affinity IgG4 mAb against PD-1 (11,12).
Since receiving its first approval in China for relapsed or
refractory classical Hodgkin's lymphoma in May 2019, SHR-1210 has
shown tremendous efficacy in various cancer types (11,13),
including advanced hepatocellular carcinoma (14,15),
advanced gastric carcinoma (15),
advanced esophageal carcinoma (16)
or advanced esophagogastric junction carcinoma (15), metastatic colorectal cancer
(17), nasopharyngeal carcinoma
(18), advanced osteosarcoma
(19), advanced non-squamous
non-small cell lung cancer (20),
advanced or recurrent cervical cancer (21,22),
and advanced triple-negative breast cancer (23). However, SHR-1210 is associated with
a unique AE that has a high incidence rate of 67-97%, namely,
reactive cutaneous capillary endothelial proliferations (RCCEPs)
(24-26).
While RCCEPs related to the treatment of SHR-1210 have been widely
reported in the head, neck, trunk and extremities according to
previous studies (24,27-29),
to the best of our knowledge, ocular RCCEPs have not been reported.
The present study describes a rare case of ocular RCCEPs and
reviews the current methods to alleviate and treat this AE.
Case report
A 44-year-old woman was diagnosed with cervical
carcinoma at Peking University First Hospital (Beijing, China) in
April 2021. The pathological results revealed stage IIa2 well- to
moderately differentiated squamous cell carcinoma of the cervix
following a total hysterectomy plus bilateral adnexectomy and
pelvic lymphadenectomy. The patient received total three cycles of
neoadjuvant chemotherapy with the platinum-doublet chemotherapy
(intravenous paclitaxel at a dose of 300 mg and intravenous
cisplatin at a dose of 120 mg each time) 1 month before the sugery,
1 week after the sugery and 1 month after the sugery, respectively.
After 20 days, the patient began to receive radiotherapy (50 Gy in
25 fractions delivered within 5 weeks). In addition to the
traditional neoadjuvant chemotherapy and radiotherapy, the patient
underwent an immunohistochemical examination of PD-L1 expression,
which showed a combined positive score (CPS) (30,31) of
35. The CPS was used to assess the PD-L1 expression in tumour
cells, which was defined as the sum of the total number of
PD-L1-postive cells (tumour cells, lymphocytes and macrophages)
divided by the total number of tumour cells, multiplied by 100
(30,31). Specimens with CPS of 1 or higher
were considered to be PD-L1 postive (30,31).
The patient was subsequently treated with 200 mg SHR-1210
intravenous transfusion once every 3 weeks.
The patient developed sporadic dome-shaped or
papillary red lesions 1-3 mm in size on the eyelid margin, forehead
and scalp, without pruritus or pain, 1 week after the first cycle
of SHR-1210. The patient received another injection of 200 mg
SHR-1210 20 days later. During this time period, the lesions
increased in both size and number. When the patient presented to
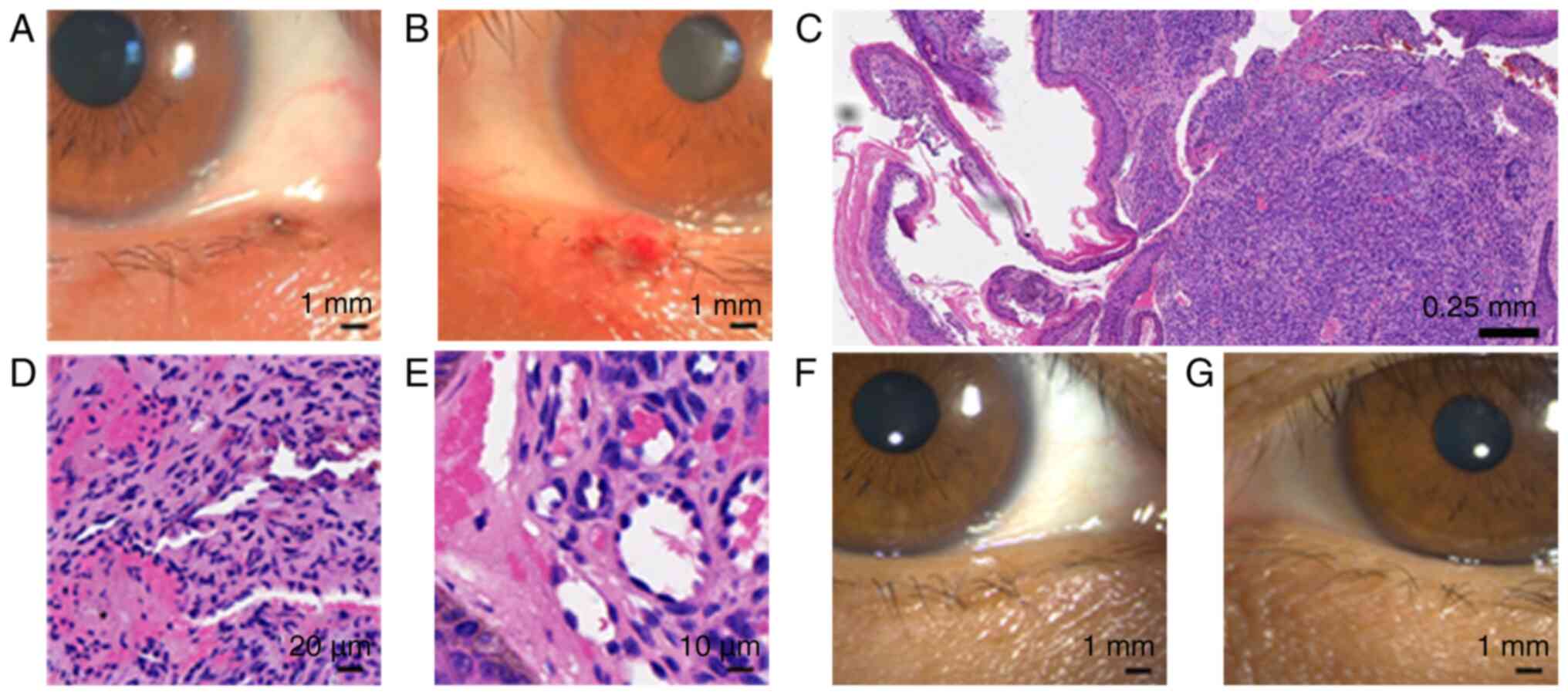
the Department of Ophthalmology 1 month later, three papillary,
smooth, red lesions were present on the lower eyelid and lateral
canthus of the right eye, and the lower eyelid of the left eye,
with sizes of 1x3, 1x3 and 5x3.5 mm respectively (Fig. 1A-C). There were domed, smooth, red
sporadic lesions on the forehead (Fig.
1D and E). The patient
complained of eye irritation and foreign body sensation. Several
lesions on the scalp were associated with ulceration, bleeding and
pigment deposition (Fig. 1F). No
obvious abnormalities, except lesions, were observed in terms of
visual acuity, intraocular pressure, anterior segment under slit
lamp, optical coherence tomography angiography (Fig. S1) and fundus photography. The
lesions on the eyelid margin were successfully resected (Fig. 2A and B). Histological analysis of the ocular
lesions showed a dense proliferation of briskly growing fusiform
and ovoid cells, forming several spaces, which is a typical feature
for RCCEPs (Fig. 2C-E). The patient
has not received SHR-1210 therapy since then, as all the antitumour
treatments for the patient had been successfully completed. The
other RCCEPs on the scalp and forehead disappeared ~5 months from
the occurrence, which is consistent with the spontaneous regression
median time of 221 days (range, 14-448 days) for this condition
(24). No recurrence of RCCEPs was
found during the 1-week, 6-month and 1-year follow-up examinations
in the Department of Ophthalmology (Fig. 2F and G). The patient undergoes regular check-ups
at the Department of Obstetrics and Gynecology every 2-3 months and
has received imageological examinations regularly, which have shown
a good prognosis without recurrence.
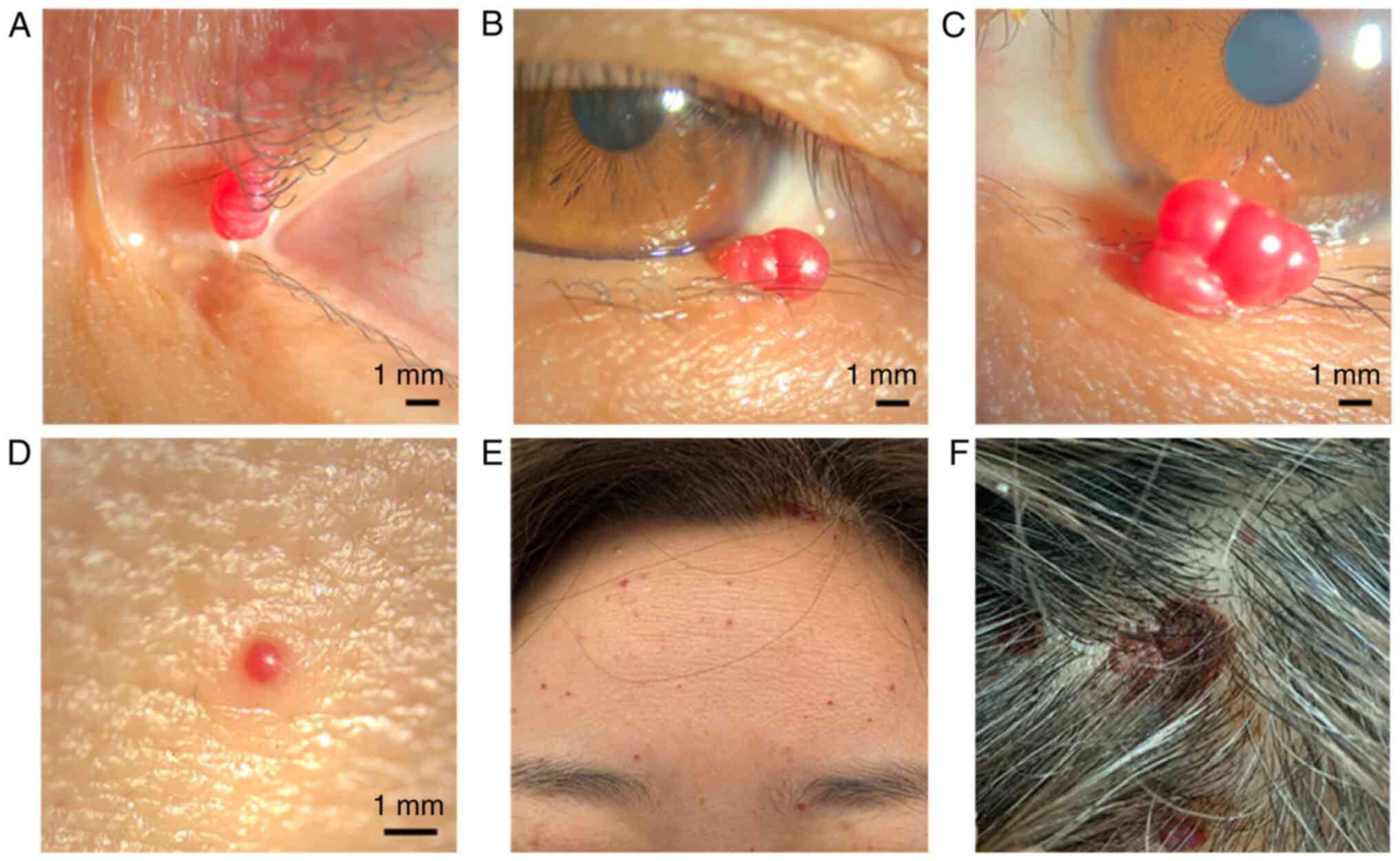 | Figure 1Morphology of the RCCEPs. (A) A
papillary, smooth, red lesion located on the lateral canthus of the
right eye, measuring 1x3 mm. (B) Another papillary, smooth, red
lesion located on the lower eyelid of the right eye, measuring 1x3
mm. (C) A papillary, smooth, red lesion on the lower eyelid of the
left eye, measuring 5x3.5 mm. (D) A domed, smooth, red lesion
located on the forehead, measuring <1 mm. (E) Domed, smooth, red
sporadic lesions distributed on the forehead. (F) A lesion on the
scalp that exhibited ulceration, bleeding and pigment deposition
[(A-C) x10 magnification; (D) x16 magnification]. |
Methods
Immunohistochemistry
Immunohistochemistry was performed using
formalin-fixed paraffin-embedded specimens. The lesions were fixed
with 10% neutral buffered formalin (room temperature for 24 h),
dehydrated with an alcohol gradient, infiltrated and embedded in
paraffin. Sections were cut to a 4-µm thickness. After incubating
with 3% bovine serum albumin for 1 h at room temperature, the
sections were stained with anti-PD-L1 rabbit monoclonal primary
antibody (dilution 1:200; clone SP263 cat. no.
741-4905/07419821001; Roche Tissue Diagnostics) at 4˚C overnight.
The sections were washed and covered with enzyme conjugated goat
anti-rabbit IgG (ready-to-use; cat. no. PV6001; Origene
Technologies, Inc.) for 1 h at room temperature, followed by
visualization with a diaminobenzidine kit (cat. no. ZLI-9017;
Origene Technologies, Inc.). The stained sections were evaluated by
light microscopy (Olympus BX41; Olympus Corporation).
Histology. The formalin-fixed
paraffin-embedded specimens were created from resected lesions that
were immediately fixed with 10% neutral buffered formalin (room
temperature for 24 h), dehydrated with an alcohol gradient,
infiltrated and embedded in paraffin. Sections were cut to a 4-µm
thickness, dewaxed, and stained in hematoxylin for 3 min (room
temperature) and in eosin for 3 min (room temperature). The stained
sections were evaluated by light microscopy (Olympus BX41).
Discussion
SHR-1210 is a humanized high affinity anti-PD-1 IgG4
mAb that blocks the interaction between PD-1 and its ligands, PD-L1
and PD-L2 (11,12); it received its first approval in
China in May 2019 for relapsed or refractory classical Hodgkin's
lymphoma, and has received approval for a number of other tumours
since then (11).
Although they have promising antitumour activity,
ICIs have a wide spectrum of irAEs involving almost all organ
systems, with the dermatological AEs (DAEs) being the most common
toxicities (3). However, the AEs of
SHR-1210 are of different types than other ICIs such as
pembrolizumab and nivolumab, for which rash, pruritus and vitiligo
are the most frequently reported DAEs (3,32),
while SHR-1210 has unique RCCEPs (24). Previous clinical trials revealed
that treatment-related AEs occurred in 83.3-97.2% of patients
receiving treatment with SHR-1210 (11,12,25).
Although dermatological toxicities still account for most AEs after
SHR-1210 treatment, RCCEPs seem to be novel DAEs absent in other
ICIs and are the most common AEs of SHR-1210 (24,25).
In a phase I clinical study of SHR-1210, Chen et al
(24) systematically enrolled 98
patients with advanced solid tumours to examine DAEs after
treatment with SHR-1210, and observed that RCCEPs occurred most
frequently on cutaneous/mucosal surfaces (85.7% of patients),
followed by rash (29.6%) and pruritus (16.3%). Mo et al
(12) enrolled 36 patients with
advanced solid tumours to assess the safety of SHR-1210 and found
that RCCEPs (83.3%), pruritus (33.3%) and fatigue (30.6%) were the
top three treatment-related AEs. Another phase I clinical trial of
SHR-1210 in patients with advanced oesophageal squamous cell
carcinoma revealed that the occurrence of RCCEPs was 76.7%
(25).
The onset time of RCCEPs after the initial use of
SHR-1210 was dose-dependent, with a median time of 20 days for all
doses and a median time of 18.5 days for the 200 mg dose (24). In the case of the present patient,
the onset time of RCCEPs was 7 days with a dose of 200 mg.
According to a previous clinical trial, the RCCEPs were found as
multiple, diffuse red papules or macules with clear boundaries,
which gradually enlarged during treatment, accompanied by recurrent
haemorrhage without pain or pruritus, and mostly regressed
spontaneously with a median time of 211 days (24). Although RCCEPs are widely
distributed over the skin and mucosa, the head and neck, trunk and
extremities are the most commonly affected areas, with the mucosa,
sclera, gingiva, nasal cavity, buccal mucosa, lip and tongue
reported in a few cases (24,27,33,34).
Although RCCEPs are usually small lesions with a maximum diameter
of 2 mm, as reported by previous clinical trials (24,29),
one case report showed that a RCCEP located on the inner thigh in a
patient with esophageal squamous cell carcinoma treated with
SHR-1210 was ~40 mm (24), and
another case report showed that an oral RCCEP on the gingiva in a
patient with non-small cell lung cancer treated with SHR-1210 was
15x7 mm (33). To the best of our
knowledge, no cases of ocular RCCEPs have been reported in previous
studies. Based on the pathogenesis of RCCEPs, it is feasible for
these lesions to grow in the eye region but with low incidence.
In the present case, the RCCEPs occurred at the
junctional margin of the skin and mucosa. However, an examination
of the palpebral conjunctiva, bulbar conjunctiva and retinal
vessels (Fig. S1) showed no
vascular-related abnormalities. Therefore, we hypothesized that the
occurrence of RCCEPs is mostly associated with the vascular network
in epithelial tissue. As an emerging phenomenon, there is little
experience with the treatment of RCCEPs in the eye. Based on our
previous experience of the treatment of RCCEPs, resections of the
RCCEPs were chosen for the protruding palpebral lesions. The
follow-up observations at 1 year post-resection showed no
recurrence or lasting visual signs of the lesions.
Although the precise mechanism of SHR-1210-related
RCCEPs remains unclear, it has been found that SHR-1210 may mediate
activation of vascular endothelial cells through binding to
vascular endothelial growth factor receptor 2 (VEGFR2) (35). Under most circumstances, RCCEPs will
regress spontaneously after SHR-1210 treatment, with a median time
of 211 days (24). However, prompt
treatment of RCCEPs is sitll required in some cases, including
those with a high risk of haemorrhage (plump or vulnerable lesions)
(24), when there is difficulty in
distinguishing a metastatic tumour from an RCCEP (36) or in cases with obvious clinical
manifestations, such as the eye irritation in this patient. The
treatments for RCCEPs are predominantly focused on two aspects:
Medication and surgery.
Since the pathogenesis of RCCEPs has links with the
upregulation of the VEGF signalling pathway, finding the
antiangiogenesis target among the VEGF/VEGFRs is a promising
prospect. Apatinib, a novel anti-angiogenic molecule, is a
selective VEGFR2 tyrosine kinase inhibitor that can inhibit
angiogenesis, as well as induce autophagy and apoptosis of tumour
cells (37). A new spectrum of
seminal studies has demonstrated that the combination of ICIs and
antiangiogenic agents could improve efficacies and reduce
toxicities (15,26,28,38).
The combination of SHR-1210 with apatinib exhibited synergistic
activities, with sensitive antitumour activity and manageable
safety profiles (14,15,26,38).
In clinical studies of SHR-1210 plus apatinib in solid tumours, the
incidence of RCCEP ranged from 11.9-29.5% (14,15,26),
which was significantly lower than the occurrence rate of 67-97%
for SHR-1210 monotherapy (24-26).
Significant regression of RCCEPs was reported in several cases
after the initiation of apatinib (36,39).
Furthermore, unlike other antiangiogenesis agents such as
bevacizumab, which can increase anastomotic leakage and wound
healing complications, apatinib is free of this problem (38). Taken together, the feasibility of
the combination therapy of apatinib plus SHR-1210 to reduce
prevalent irAEs such as RCCEPs and to improve efficacy deserves
deeper interrogation, in order to guide further clinical practices.
Curative surgical resection is another optional therapy. In some
conditions, it is difficult to differentiate the tomour-like RCCEPs
from suspect metastatic lesions, which increases the demands of
lesion excision to obtain definitive pathological findings to avoid
delaying antitumour treatment (36). Other surgical indications include
lesions with an unusual distribution, such as in the present case
where the appearance and normal blinking were affected, as well as
in lesions with a high risk of bleeding. The present case
highlights the significance of the prompt diagnosis and treatment
of irAEs. Although RCCEPs are usually slight-risk toxicities that
pose no threat to the continuity of treatment, lesions with
distributions that cause disturbances in normal life require proper
treatment, such as surgical excision.
Supplementary Material
OCTA results. (A-D) The OCTA of the
right eye: (A) The superficial vascular complex, (B) the deep
vascular complex, (C) the avascular layer and (D) the laminae
choriocapillaris, which were distributed normally, without
malformation, hemangioma, neovascularization and non-perfusion
areas. (E-H) The OCTA of the left eye: (E) The superficial vascular
complex, (F) the deep vascular complex, (G) the avascular layer and
(H) the laminae choriocapillaris, which were distributed normally,
without malformation, hemangioma, neovascularization and
non-perfusion areas. OCTA, optical coherence tomography
angiography.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by National High Level Hospital
Clinical Research Funding: Interdepartmental Clinical Research
Project of Peking University First Hospital (grant no.
2022CR24).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
XZ, XY and YW all provided care to the patient,
advised on patient treatment and analyzed patient results. XZ
drafted the manuscript. XY was responsible for study
conceptualization and supervision. YW performed critical revision
of manuscript. All authors approved the final version of this
manuscript. YW is responsible for the overall content as guarantor.
XZ, XY and YW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The requirement for approval was waived by the
institutional review board owing to the anonymized and
retrospective nature of the report.
Consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang G, Liu S, Dong J, Xi X, Kong R, Li W
and Du Q: PD-1 inhibitor-based adverse events in solid tumors: A
retrospective real-world study. Front Pharmacol.
13(974376)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Villadolid J and Amin A: Immune checkpoint
inhibitors in clinical practice: Update on management of
immune-related toxicities. Transl Lung Cancer Res. 4:560–575.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Das S and Johnson DB: Immune-related
adverse events and anti-tumor efficacy of immune checkpoint
inhibitors. J Immunother Cancer. 7(306)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Moslehi JJ, Salem JE, Sosman JA,
Lebrun-Vignes B and Johnson DB: Increased reporting of fatal immune
checkpoint inhibitor-associated myocarditis. Lancet.
391(933)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mahmood SS, Fradley MG, Cohen JV, Nohria
A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R,
Chen CL, Gupta D, et al: Myocarditis in patients treated with
immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–1764.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ma K, Lu Y, Jiang S, Tang J, Li X and
Zhang Y: The relative risk and incidence of immune checkpoint
inhibitors related pneumonitis in patients with advanced cancer: A
meta-analysis. Front Pharmacol. 9(1430)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Markham A and Keam SJ: Camrelizumab: First
global approval. Drugs. 79:1355–1361. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mo H, Huang J, Xu J, Chen X, Wu D, Qu D,
Wang X, Lan B, Wang X, Xu J, et al: Safety, anti-tumour activity,
and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody
in advanced solid tumours: A dose-escalation, phase 1 study. Br J
Cancer. 119:538–545. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Wang C, Li X, Dong L, Yang Q, Chen
M, Shi F, Brock M, Liu M, Mei Q, et al: Improved clinical outcome
in a randomized phase II study of anti-PD-1 camrelizumab plus
decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother
Cancer. 9(e002347)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J,
Shao G, Zhang Y, Xu L, Yin T, et al: Camrelizumab in combination
with apatinib in patients with advanced hepatocellular carcinoma
(RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer
Res. 27:1003–1011. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu
R, Zhang G, Zhao C, Zhang Y, Chen C, et al: Anti-PD-1 antibody
SHR-1210 combined with apatinib for advanced hepatocellular
carcinoma, gastric, or esophagogastric junction cancer: An
open-label, dose escalation and expansion study. lin Cancer Res.
25:515–523. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ren C, Mai ZJ, Jin Y, He MM, Wang ZQ, Luo
HY, Zhang DS, Wu CY, Wang F and Xu RH: Anti-PD-1 antibody SHR-1210
plus apatinib for metastatic colorectal cancer: A prospective,
single-arm, open-label, phase II trial. Am J Cancer Res.
10:2946–2954. 2020.PubMed/NCBI
|
|
18
|
Fang W, Yang Y, Ma Y, Hong S, Lin L, He X,
Xiong J, Li P, Zhao H, Huang Y, et al: Camrelizumab (SHR-1210)
alone or in combination with gemcitabine plus cisplatin for
nasopharyngeal carcinoma: Results from two single-arm, phase 1
trials. Lancet Oncol. 19:1338–1350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xie L, Xu J, Sun X, Guo W, Gu J, Liu K,
Zheng B, Ren T, Huang Y, Tang X, et al: Apatinib plus camrelizumab
(anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO)
progressing after chemotherapy: A single-arm, open-label, phase 2
trial. J Immunother Cancer. 8(e000798)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lan C, Shen J, Wang Y, Li J, Liu Z, He M,
Cao X, Ling J, Huang J, Zheng M, et al: Camrelizumab plus apatinib
in patients with advanced cervical cancer (CLAP): A multicenter,
open-label, single-arm, phase II trial. J Clin Oncol. 38:4095–4106.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cohen AC, Roane BM and Leath CA III: Novel
therapeutics for recurrent cervical cancer: Moving towards
personalized therapy. Drugs. 80:217–227. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu J, Wang Y, Tian Z, Lin Y, Li H, Zhu Z,
Liu Q, Su S, Zeng Y, Jia W, et al: Multicenter phase II trial of
camrelizumab combined with apatinib and eribulin in heavily
pretreated patients with advanced triple-negative breast cancer.
Nat Commun. 13(3011)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen X, Ma L, Wang X, Mo H, Wu D, Lan B,
Qu D, Zhang H, Huang J and Xu B: Reactive capillary hemangiomas: A
novel dermatologic toxicity following anti-PD-1 treatment with
SHR-1210. Cancer Biol Med. 16:173–181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang J, Xu B, Mo H, Zhang W, Chen X, Wu
D, Qu D, Wang X, Lan B, Yang B, et al: Safety, activity, and
biomarkers of SHR-1210, an Anti-PD-1 antibody, for patients with
advanced esophageal carcinoma. Clin Cancer Res. 24:1296–1304.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fan Y, Zhao J, Wang Q, Huang D, Li X, Chen
J, Fang Y, Duan J, Zhou C, Hu Y, et al: Camrelizumab plus apatinib
in extensive-stage SCLC (PASSION): A multicenter, two-stage, phase
2 trial. J Thorac Oncol. 16:299–309. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Teng Y, Guo R, Sun J, Jiang Y and Liu Y:
Reactive capillary hemangiomas induced by camrelizumab (SHR-1210),
an anti-PD-1 agent. Acta Oncol. 58:388–389. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu B and Sun HC: Camrelizumab: An
investigational agent for hepatocellular carcinoma. Expert Opin
Investig Drugs. 31:337–346. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen
Z, Chai X, Xiong J, Bai Y, Yang L, et al: Reactive cutaneous
capillary endothelial proliferation in advanced hepatocellular
carcinoma patients treated with camrelizumab: Data derived from a
multicenter phase 2 trial. J Hematol Oncol. 13(47)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li K, Chen J, Hu Y, Wang YZ, Shen Y, Chen
G, Peng W, Fang Z, Xia B, Chen X, et al: Neoadjuvant chemotherapy
plus camrelizumab for locally advanced cervical cancer (NACI
study): A multicentre, single-arm, phase 2 trial. Lancet Oncol.
25:76–85. 2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen L, Lucas E, Zhang X, Liu Q, Zhuang Y,
Lin W, Chen H and Zhou F: Programmed death-ligand 1 expression in
human papillomavirus-independent cervical adenocarcinoma and its
prognostic significance. Histopathology. 80:338–347.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Belum VR, Benhuri B, Postow MA, Hellmann
MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD
and Lacouture ME: Characterisation and management of dermatologic
adverse events to agents targeting the PD-1 receptor. Eur J Cancer.
60:12–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou J, Mao Q, Li Y, Li Z, He H, Chen Q
and Liu C: Oral reactive capillary hemangiomas induced by SHR-1210
in the treatment of non-small cell lung cancer: A case report and
literature review. BMC Oral Health. 21(559)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yu Q and Wang WX: Camrelizumab (SHR-1210)
leading to reactive capillary hemangioma in the gingiva: A case
report. World J Clin Cases. 8:624–629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Finlay WJJ, Coleman JE, Edwards JS and
Johnson KS: Anti-PD1 ‘SHR-1210’ aberrantly targets pro-angiogenic
receptors and this polyspecificity can be ablated by paratope
refinement. MAbs. 11:26–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu J, Cao G, Zhang G, Liu S and Shi D:
Nasal alar metastasis of advanced hepatocellular carcinoma
misdiagnosed as reactive cutaneous capillary endothelial
proliferation in a patient treated with camrelizumab and apatinib:
A case report. J Gastrointest Oncol. 14:1643–1649. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xie C, Zhou X, Liang C, Li X, Ge M, Chen
Y, Yin J, Zhu J and Zhong C: Apatinib triggers autophagic and
apoptotic cell death via VEGFR2/STAT3/PD-L1 and ROS/Nrf2/p62
signaling in lung cancer. J Exp Clin Cancer Res.
40(266)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi
D, Yu D, Gao P, Chen C, et al: Neoadjuvant therapy with immune
checkpoint blockade, antiangiogenesis, and chemotherapy for locally
advanced gastric cancer. Nat Commun. 14(8)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang J, Li S, Zhang L and Zhang X: A
combination of anti-PD-1 therapy and apatinib successfully treated
a patient with EGFR mutation-negative advanced lung adenocarcinoma:
A case report. J Cancer Res Ther. 19:141–143. 2023.PubMed/NCBI View Article : Google Scholar
|