Introduction
Diabetic mellitus is a worldwide common metabolic
disease. Abundant evidence indicated that diabetic mellitus was
associated not only with cardiovascular diseases, nephropathy and
retinopathy, but also with the development of numerous types of
cancer, including lung, liver, colorectal, kidney and breast
(1–4). By contrast, hyperglycemia appears to
be a protective factor for prostate cancer (5).
Glucose lowering drugs are possibly associated with
either an increased or reduced risk of cancer (6). Metformin is an commonly used oral
agent for treating patients with type 2 diabetes mellitus (T2DM)
and may be safely used in combination with other antidiabetic
agents. Two different ways by which metformin exerts
anti-neoplastic effects include: i) Activating adenosine
monophosphate-activated protein kinase (AMPK) by the liver kinase
B1; and ii) inhibition of protein synthesis. Tumor cells establish
mechanisms to downregulate AMPK, allowing them to escape its
restraining influences on growth. However, metformin can act as a
tumor growth inhibitor by upregulating AMPK and suppressing the
mammalian target of rapamycin, leading to reduced protein synthesis
in cancer cells (7–9). In addition, metformin also induces
activation of the immune system, cell arrest or apoptosis and
reduces growth factor signaling (10).
Previous studies have confirmed that metformin
inhibits tumor cell proliferation and improves the survival of
cancer patients (11–15). A meta-analysis has been performed to
evaluate the association between metformin and cancer risk in
diabetic patients, which found that metformin has a preventive
effect on cancer incidence and mortality (16). One study conducted in Taiwan found
that metformin reduced the incidences of several
gastroenterological cancers in treated diabetes (17).
Lung cancer has become one of the leading causes of
cancer-related mortality in numerous countries. Recent data have
suggested an association of metformin use with decreased lung
cancer risk of type 2 diabetic patients (18–20).
Mazzone et al (18) found
that the use of metformin is associated with a lower likelihood of
developing lung cancer in diabetic patients, which was coherent
with the study by Lai et al (20). By contrast, the study by Bodmer
et al (21) obtained the
opposite result. Another observational study by Smiechowski et
al (22) demonstrated that
metformin use was not associated with a decreased risk of lung
cancer in patients with T2DM. Thus, the results remain
controversial and the present meta-analysis was performed to
investigate the association between metformin use and lung cancer
risk of individuals with T2DM.
Materials and methods
Search strategy
A comprehensive literature search was performed for
all the studies addressing the association between metformin use
and lung cancer risk. Electronic databases searched included
Pubmed, CBM and ISI Web of Science until October 2013, without
language restrictions. The Mesh terms and/or the text words used
included ‘metformin’ or ‘biguanides’ and lung ‘cancer’ or
‘neoplasms’. In addition, the reference lists were also
inspected.
Selection criteria
The inclusion criteria of a study in the
meta-analysis included the following: i) Epidemiological studies,
which included case-control or cohort studies; ii) designed to
evaluate the association between metformin and risk of lung cancer
in diabetic patients; and iii) contained sufficient information to
allow adequate estimation of hazard ratio (HR), odds ratio (OR), or
relative risk (RR) and 95% confidence interval (95% CI) to estimate
the lung cancer risk with diabetes using metformin compared to
other antidiabetic treatments or no treatment. When two independent
control groups were set in the same case-control study, the study
was treated as two separate studies, only considering the limited
relevant studies enrolled.
Data extraction and quality
assessment
The enrolled studies were evaluated by two
independent authors (Ning Zhu and Yuanyuan Zhang). For the included
studies, these data were extracted: First author, year of
publication, country or area, study design, source of cases, number
of total participants or lung cancer cases, study time or follow-up
time, unadjusted and adjusted RR with their 95% CIs and the
confounding factors, which had been adjusted. To ascertain the
validity of the eligible studies, the quality of each study was
appraised in reference to the Newcastle-Ottawa statement (23). In this ‘star system’ scale, studies
were judged on three aspects: Selection, comparability and
exposure. For the selection and exposure categories, a maximum of
one star was awarded for each numbered item, whereas for the
comparability, a maximum of two stars was awarded. Therefore, the
quality of each study, with nine stars at most, was classified as
follows: ≤5 stars as low quality and ≥6 stars as high quality.
Disagreements were resolved by discussion between the two
investigators. When required, another investigator (Xiaodong Chen)
was consulted to resolve the dispute.
Statistical analyses
The value of RR was adopted for the cohort studies
or OR for the case-control studies to evaluate the risk estimate in
the meta-analysis. When the adjusted RRs were available, they were
used to estimate the association. Otherwise, the unadjusted RRs
were adopted. The adjusted HR was pooled using the DerSimonian and
Laird statistic. Stratified analyses were conducted according to
type of study design, type of HRs, study quality, country, duration
of treatment or control drugs. Studies were pooled and weighted
according to inverse variance using the random-effects model of
DerSimonian and Laird. P<0.05 was considered to indicate a
statistically significant difference for all the tests. Sensitivity
analysis was conducted by sequentially excluding one study with a
high weight or low quality. Finally, the publication bias was
detected using the Begg's funnel plot and Egger's regression
asymmetry test. All analyses were performed using Stata version
11.0 (StataCorp, College Station, TX, USA).
Results
Search results
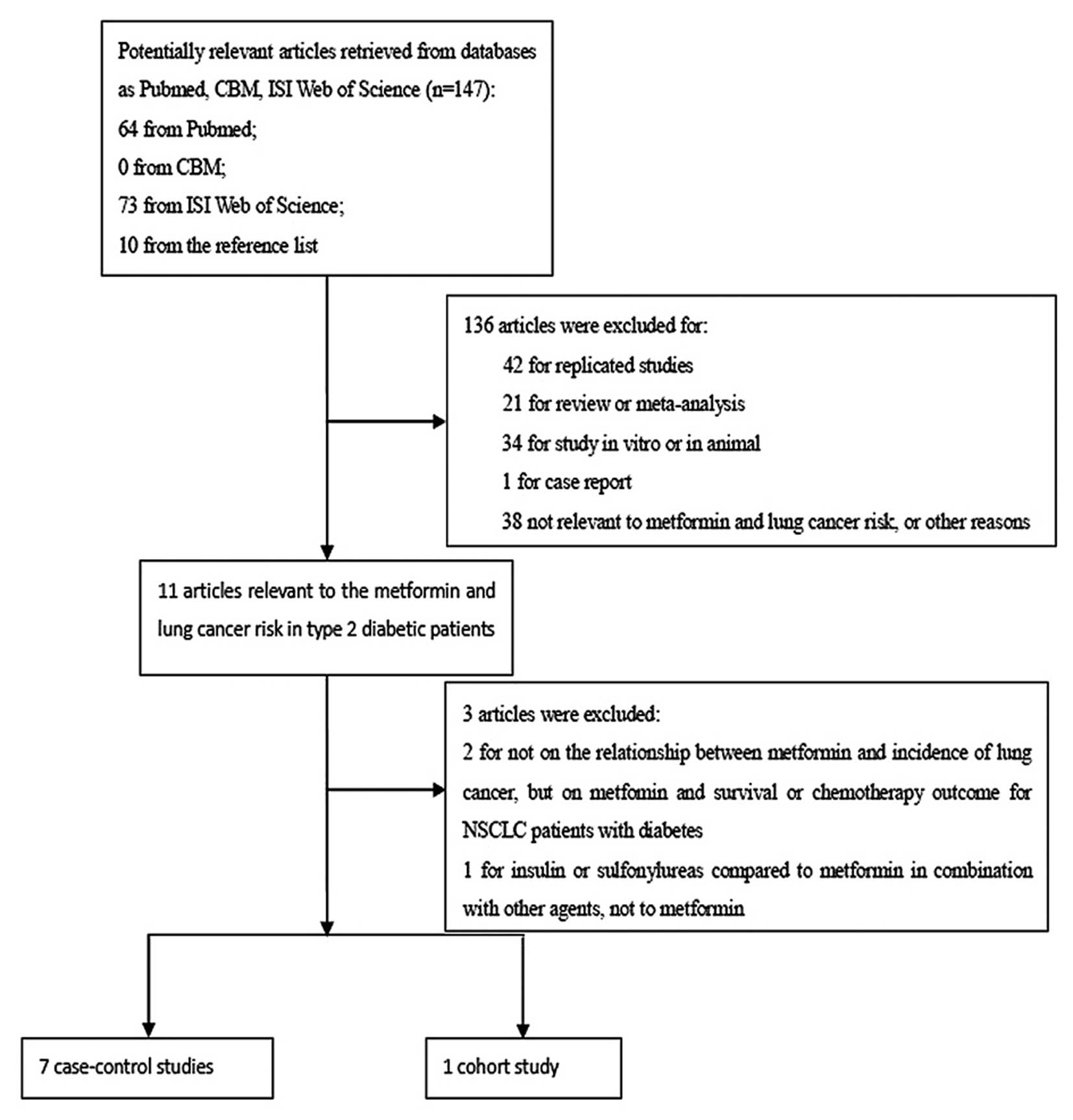
The participant flow diagram for the study inclusion
in the meta-analysis is shown in Fig.
1. Eight studies, including 17,997 lung cancer patients, were
finally enrolled into the meta-analysis, which contained seven
case-control (18–22, 24,
25) and one cohort study (26). The general information regarding the
studies, including author, year, type of study design, source of
case, duration of observation, number of cases, matched factor,
adjusted or unadjusted HR and study quality, are presented in the
Table I.
 | Table I.Baseline characteristics of the
included studies. |
Table I.
Baseline characteristics of the
included studies.
|
|
|
|
|
|
|
| Unadjusted | Adjusted |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author
(year) | Country | Study design | Duration of
observation, years | No. of metformin
users (lung cancer Source patients) | Source of case | Matched factor | HR | 95% CI | HR | 95% CI | Study |
qualitya
(Refs.) |
|---|
| Wang (2013) | Taiwan | Cohort | 1998-2009 | 37,055 (162) | National Health
Insurance datasets | Age, gender and
occupation | NA | NA | 1.11 | 0.94-1.47 | 4 | (25) |
| Smiechowski
(2013) | Canada | Nested
case-control | Mean follow-up,
5.6 | 115,923
(1,061) | UK General Practice
Research Database | Age, gender,
calender time of cohort entry, duration of follow up | 0.97 | NA | 0.94 | 0.76-1.17 | 7 | (22) |
| Lai (2012) | Taiwan | Retro-spective
case-control | 2000-2008 | 19,624 (96) | National Health
Research Institutes in Taiwan | Age, gender | 0.43 | 0.29-0.63 | 0.55 | 0.37-0.82 | 7 | (20) |
| Hsiehb (a) (2012) | Taiwan | Case-control | 2000-2008 | 3,963 (1,226) | Taiwan's National
Health Insurance Medical Claims Database | Age, gender | NA | NA | 0.95 | 0.46-1.95 | 6 | (24) |
| Hsiehb (b) (2012) | Taiwan | Case-control | 2000-2008 | 3,963 (1,226) | Taiwan's National
Health Insurance Medical Claims Database | Age, gender | NA | NA | 0.64 | 0.45-0.9 | 6 | (24) |
| (2012) control
(1,226) National Health |
| Mazzone (2012) | USA | Retro-spective
case-control | 2001-2011 | 93,939 (522) | Cleveland Clinic
Health System | Age, gender smoking
history | NA | NA | 0.48 | 0.28-0.81 | 6 | (18) |
| Ruiter (2012) | The
Netherlands | Case-control | 1998-2008 | 85,289 (1,590) | PHARMO Record
Linkage System | Age, gender,
calendar time, no. of unique drugs used and no. of
hospitalizations | NA | NA | 0.87 | 0.84-0.91 | 6 | (19) |
| Bodmer (2012) | Switzerland | Case-control | 1995-2009 | NA (13,043) | UK-based General
Practice Research Database | Age, gender,
general practice, same index date and no. of years of active
history | 1.07 | 0.87-1.31 | 1.09 | 0.85-1.38 | 6 | (21) |
| Libby (2009) | Scotland | Cohort | 1993-2003 | 4,085 (297) | Resident Population
of Tayside Health Board (Scotland, UK) | Age, gender, BMI,
HbA1c, smoking, deprivation, other drug use | 0.49 | 0.32-0.74 | 0.7 | 0.43-1.15 | 6 | (26) |
These studies were conducted in six countries, which
were Canada (22), the Netherlands
(19), Switzerland (21), Scotland (UK) (26), Taiwan (China) (20, 24,
25) and USA (18). Three studies were conducted in Asian
countries and five studies in Western countries. One case-control
study, performed in Taiwan by Hsieh et al (24), separately compared insulin or
sulfonylureas with metformin for the effect on lung cancer risk of
patients with T2DM. Considering these two independent antidiabetic
agents and the limited enrolled studies, this study was treated as
two independent case-control studies in the meta-analysis. Only two
studies evaluated the effect of metformin on the lung cancer risk
of patients with T2DM by comparing to sulfonylureas (19, 24).
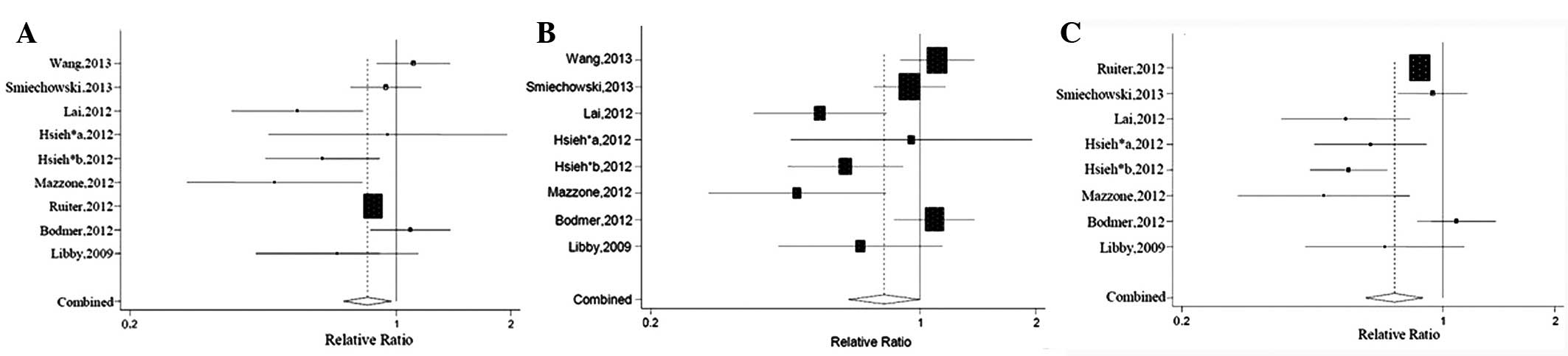
Overall analysis
The baseline characteristics of these enrolled
studies are demonstrated in Table
I. The adjusted RR was derived from all the eight studies.
Compared to other antidiabetic agents, metformin was significantly
associated with a 16% reduction of lung cancer risk in type 2
diabetic patients (RR=0.84; 95% CI, 0.73-0.97; P=0.019). As
significant heterogeneity existed among these studies
(Q-value=22.10, P=0.005), the random-effects model was used. In
order to confirm the validity and stability of the results, a study
with high weight (19) or low
quality (25) was separately
excluded in the sensitivity analysis and consistent results were
observed (RR=0.81; 95% CI, 0.65-0.99; and RR=0.74; 95% CI,
0.62-0.89). The forest plot is shown in Fig. 2.
Subgroup analysis
In order to validate the results of the overall
analysis and find the possible source of statistical heterogeneity
among studies, the subgroup analysis was performed. As mentioned
above, subgroup analysis was conducted according to several
different variables, which are shown in Table II.
 | Table II.Results of the subgroup analysis
according to different variables. |
Table II.
Results of the subgroup analysis
according to different variables.
|
|
|
| Pooled RR | Heterogeneity |
|---|
|
|
|
|
|
|
|---|
| Subgroup | No. of studies | Model | RR (95% CI) | P-value | Q-value | P-value |
|---|
| Type of design of
studies |
|
Case-control | 8 | Random | 0.85
(0.73–0.99) | 0.04 | 21.32 | 0.003 |
| Quality of enrolled
study |
|
High | 8 | Random | 0.74
(0.62–0.89) | 0.001 | 29.91 | <0.001 |
| Unadjusted or
adjusted HR |
|
Adjusted | 9 | Random | 0.84
(0.73–0.97) | 0.02 | 22.10 | 0.005 |
|
Unadjusted | 3 | Random | 0.62
(0.33–1.18) | 0.15 | 22.85 | <0.001 |
| Country |
|
Western | 5 | Random | 0.87
(0.75–1.02) | 0.09 | 9.35 | 0.053 |
|
Asian | 4 | Random | 0.78
(0.53–1.15) | 0.21 | 12.72 | 0.005 |
| Duration of
treatment |
|
Long-term usea | 4 | Random | 0.89
(0.79–1.01) | 0.36 | 11.77 | 0.008 |
| Control drugs |
| Control
groups use sulfonylureas | 2 | Fixed | 0.79
(0.83–0.9) | 0.000 | 2.98 | 0.085 |
A statistically significant reduction of the lung
cancer risk in type 2 diabetic patients using metformin was
obtained when the studies were restricted to case-control studies
or studies with high quality (RR=0.85; 95% CI, 0.73-0.99; and
RR=0.74; 95% CI, 0.62-0.89), which were consistent with the result
of the overall analysis. In the subgroup analysis regarding the
unadjusted HR (20, 21, 26),
no significant association was found between metformin use and the
lung cancer risk of patients with T2DM (RR=0.62; 95% CI,
0.33-1.18). When stratified by country, it was suggested that
metformin use was not significantly associated with the reduction
of lung cancer risk in type 2 diabetic patients in Western or Asian
countries (RR=0.87; 95% CI, 0.75-1.02; and RR=0.78; 95% CI,
0.53-1.15). Regarding long-term use of metformin, a null result was
found (RR=0.89; 95% CI, 0.79-1.01). In addition, when compared to
sulfonylureas, the use of metformin decreased the lung cancer risk
of type 2 diabetic patients (RR=0.79; 95% CI, 0.83-0.9), without
significant heterogeneity (Q-value=2.98, P=0.085).
Publication bias
As shown in Fig. 3,
the publication bias was assessed for the included studies using
the Begg's funnel plot and Egger's regression asymmetry test. No
significant risk of publication bias was observed by Begg's
(Z=-1.04, P=0.3) and Egger's test (t=-0.59, P=0.572).
Discussion
Increasing evidence has strengthened that
hyperglycemia, obesity-related insulin resistance and secondary
hyperinsulinemia are important regulators of the development of
cancer (27–30). Recent data have suggested that
insulin and insulin secretagogues (sulfonylureas and glinides) may
increase the overall cancer incidence (21, 31,
32), but insulin sensitizers
(metformin and thiazolidinedione) are associated with the reduction
of the cancer incidence (33,
34). Metformin improved insulin
resistance and alleviated the circulating insulin levels, which may
be the major reason to decrease the cancer risk in diabetic
patients. Within the past years, several studies and meta-analyses
have focused on the association between metformin and the reduction
of lung cancer risk in patients with T2DM, but the controversy
remains among the results, which raise the necessity to resolve
this dispute. One previous meta-analysis by Noto et al
(16), which included three
studies, confirmed the association between metformin use and the
reduction of the lung cancer risk among patients with T2DM
(RR=0.67, 95% CI, 0.45-0.99), which was contradictory to that of
another meta-analysis based on four observational studies (RR=0.83,
95% CI, 0.64-1.06) (6). However,
these meta-analyses may be affected by possible publication bias
due to the few studies enrolled. Therefore, the present study
selected and analyzed all the available observational studies to
reconfirm the association in the meta-analysis. Eight retrospective
studies, including seven case-control and one cohort study, were
eventually selected. The majority of them obtainied a high
assessment score according to the Newcastle-Ottawa statement, with
the exception of one study by Wang et al (25), which was due to lack of detailed
information. A significant 52% reduction was noted in one study
conducted in the USA (RR=0.48, 95% CI, 0.28-0.81) (18).
In the present meta-analysis, metformin therapy for
type 2 diabetes patients was demonstrated to decrease the lung
cancer incidence, compared to other antidiabetic agents. This
result was comparable to that of a previously published
meta-analysis by Noto et al (16). The major strength of the present
meta-analysis was the large number of studies and patients
included, which may provide the results with an improved reliance.
In addition, rigorous criteria were adopted for the study
selection. One study, which was included in the meta-analysis by
van Staa et al (35), was
excluded for comparing insulin or sulfonylureas with metformin in
combination with other agents, rather than metformin only. In the
sensitivity analysis, by separately excluding the study with a high
weight (19) or lower quality
(25), the results did not
materially change, which indicated that the result of overall
analysis was stable.
Furthermore, in order to further inspect the
stability of the results and find the possible source of
statistical heterogeneity, subgroup analysis was performed
according to several variables. Each study provided the adjusted
HR. All the studies were adjusted for age and gender, but other
potential confounders, such as smoking status, body mass index
(BMI), dosage of metformin and comorbidity, were not matched in
certain enrolled studies, which may cause the material change of
the final results. Unadjusted HR was retrieved from three studies
(20, 21, 26)
and no significant association was obtained according to the
unadjusted HR (RR=0.62; 95% CI, 0.33-1.18). The magnitude of lung
cancer risk reduction was strengthened when compared to
sulfonylureas (RR=0.79; 95% CI, 0.83-0.9), without significant
heterogeneity (Q-value=2.98, P=0.085), which may be attributed to
the decreasing levels of endogenous insulin in metformin users.
Different control drugs may partly contribute to the heterogeneity.
Four studies evaluated the effect of exposure (duration and dosage)
of metformin on the association. Lai et al (20) reported that the HR was reduced to
0.33 among patients who had used metformin for >3 years
(RR=0.33; 95% CI, 0.19-0.57), compared to non-users. However,
Smiechowski et al (22)
found that no interaction was obtained with long-term use of
metformin (RR=0.90; 95% CI, 0.67-1.22). As the dose of metformin
always increases with increasing duration of use, dose variables
can be confounded by duration. Therefore, the duration and dosage
of metformin were combined together in the subgroup analysis
restricted to long-term use. Although a null result was found
(RR=0.89; 95% CI, 0.79-1.01), this indicated that a trend toward
reduction of lung cancer risk was linked to long-term use of
metformin. Of note, no interaction was obtained in the analysis
stratified by different countries.
Several limitations of the present meta-analysis
should be addressed. First, the reliance of the results was
weakened by the drawbacks that are inherent to retrospective
observational studies, such as possible investigator and recall
biases. All the eight studies enrolled were retrospective. Details
of metformin dose, duration and other information on potential
confounders and risk factors were incomplete, some of which were
not fully adjusted in several studies. For example, tobacco use is
a well-known risk factor, but only one study evaluated the effect
of tobacco use on the lung cancer risk of diabetes patients with
metformin therapy (22). Other
potentially important clinical covariants, such as BMI, glycemic
control, smoking status and lung diseases, were not evaluated as
they were only attributed to a few associated studies. Second, the
studies were performed in different countries, using different
datasets and stratification standards, which may partly contribute
to the heterogeneity. For example, the classification of treatment
duration was not uniform. Lai et al (20) recognized >3 years as long-term
use and in another study by Smiechowski et al (22), >1,485 days was classified as
long-term use, which may induce a certain effect on the results of
the subgroup analysis. The comparator drugs, mainly including
insulin and insulin secretagogues, should also be taken into
account. The majority compared metformin to a combination of other
glucose-lowering agents, rather than a certain agent, which may
play an important effect on the association. Only two studies
compared metformin with sulfonylureas alone (19, 24)
and the heterogeneity disappeared in the subgroup analysis
comparing with sulfonylureas. Third, the evidence derived from
case-control or cohort studies is generally lower compared to from
randomized controlled studies. The present meta-analysis was
performed based on seven case-control and one cohort study, without
any randomized controlled study. OR was treated as approximate RR,
which may also influence the final results. Therefore, the results
of the meta-analysis should be reviewed with caution.
In conclusion, the present meta-analysis has
indicated that the use of metformin significantly decreased the
lung cancer risk of patients with T2DM. However, further
investigations, particularly randomized controlled trials, are
required to confirm the association.
References
|
1
|
Yerrabothala S, Shaaban H, Capo G,
Maroules M and Debari VA: The impact of diabetes mellitus on breast
cancer outcomes: a single center retrospective study. Pathol Oncol
Res. 20:209–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JY, Jeon I, Lee JM, Yoon JM and Park
SM: Diabetes mellitus as an independent risk factor for lung
cancer: a meta-analysis of observational studies. Eur J Cancer.
49:2411–2423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang JY, Chao TT, Lai CC, et al: Risk of
colorectal cancer in type 2 diabetic patients: a population-based
cohort study. Jpn J Clin Oncol. 43:258–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta SP, Mittal A, Jha DK, Pandeya DR and
Sathian B: Diabetes mellitus and renal cell carcinoma - a hospital
based study from Kathmandu Valley. Asian Pac J Cancer Prev.
13:4963–4965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Onitilo AA, Stankowski RV, Berg RL, et al:
Type 2 diabetes mellitus, glycemic control, and cancer risk. Eur J
Cancer Prev. 23:134–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franciosi M, Lucisano G, Lapice E,
Strippoli GF, Pellegrini F and Nicolucci A: Metformin therapy and
risk of cancer in patients with type 2 diabetes: systematic review.
PLoS One. 8:e715832013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin CC, Yeh HH, Huang WL, et al: Metformin
enhances cisplatin cytotoxicity by suppressing signal transducer
and activator of transcription-3 activity independently of the
liver kinase B1-AMP-activated protein kinase pathway. Am J Respir
Cell Mol Biol. 49:241–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han D, Li SJ, Zhu YT, Liu L and Li MX:
LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer.
Asian Pac J Cancer Prev. 14:4033–4039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quinn BJ, Dallos M, Kitagawa H, et al:
Inhibition of lung tumorigenesis by metformin is associated with
decreased plasma IGF-I and diminished receptor tyrosine kinase
signaling. Cancer Prev Res (Phila). 6:801–810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kourelis TV and Siegel RD: Metformin and
cancer: new applications for an old drug. Med Oncol. 29:1314–1327.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato K, Gong J, Iwama H, et al: The
antidiabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Storozhuk Y, Hopmans SN, Sanli T, et al:
Metformin inhibits growth and enhances radiation response of
non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J
Cancer. 108:2021–2032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar S, Meuter A, Thapa P, et al:
Metformin intake is associated with better survival in ovarian
cancer: a case-control study. Cancer. 119:555–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferla R, Haspinger E and Surmacz E:
Metformin inhibits leptin-induced growth and migration of
glioblastoma cells. Oncol Lett. 4:1077–1081. 2012.PubMed/NCBI
|
|
15
|
Kobayashi M, Kato K, Iwama H, et al:
Antitumor effect of metformin in esophageal cancer: In vitro study.
Int J Oncol. 42:517–524. 2013.PubMed/NCBI
|
|
16
|
Noto H, Goto A, Tsujimoto T and Noda M:
Cancer risk in diabetic patients treated with metformin: a
systematic review and meta-analysis. PLoS One. 7:e334112012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN,
Chang YH and Huang YC: Type 2 diabetes increases and metformin
reduces total, colorectal, liver and pancreatic cancer incidences
in Taiwanese: a representative population prospective cohort study
of 800,000 individuals. BMC Cancer. 11:202011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mazzone PJ, Rai H, Beukemann M, Xu M, Jain
A and Sasidhar M: The effect of metformin and thiazolidinedione use
on lung cancer in diabetics. BMC Cancer. 12:4102012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruiter R, Visser LE, van Herk-Sukel MP, et
al: Lower risk of cancer in patients on metformin in comparison
with those on sulfonylurea derivatives: results from a large
population-based follow-up study. Diabetes Care. 35:119–124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh
DP and Chen CC: Antidiabetes drugs correlate with decreased risk of
lung cancer: a population-based observation in Taiwan. Clin Lung
Cancer. 13:143–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bodmer M, Becker C, Jick SS and Meier CR:
Metformin does not alter the risk of lung cancer: a case-control
analysis. Lung Cancer. 78:133–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smiechowski BB, Azoulay L, Yin H, Pollak
MN and Suissa S: The use of metformin and the incidence of lung
cancer in patients with type 2 diabetes. Diabetes Care. 36:124–129.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wells GA, Shea B, O'Connell D, et al: The
Newcastle-Ottawa Scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed.
February 5–2013
|
|
24
|
Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH
and Tseng CH: The influence of type 2 diabetes and glucose-lowering
therapies on cancer risk in the Taiwanese. Exp Diabetes Res.
2012:4137822012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang SY, Chuang CS, Muo CH, et al:
Metformin and the incidence of cancer in patients with diabetes: a
nested case-control study. Diabetes Care. 36:e155–e156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Libby G, Donnelly LA, Donnan PT, Alessi
DR, Morris AD and Evans JM: New users of metformin are at low risk
of incident cancer: a cohort study among people with type 2
diabetes. Diabetes Care. 32:1620–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orgel E and Mittelman SD: The links
between insulin resistance, diabetes, and cancer. Curr Diab Rep.
13:213–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lemke LB, Rogers AB, Nambiar PR and Fox
JG: Obesity and non-insulin-dependent diabetes mellitus in
Swiss-Webster mice associated with late-onset hepatocellular
carcinoma. J Endocrinol. 199:21–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sugiyama T, Nakanishi M, Hoshimoto K, et
al: Severely fluctuating blood glucose levels associated with a
somatostatin-producing ovarian neuroendocrine tumor. J Clin
Endocrinol Metab. 97:3845–3850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pisani P: Hyper-insulinaemia and cancer,
meta-analyses of epidemiological studies. Arch Physiol Biochem.
114:63–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thakkar B, Aronis KN, Vamvini MT, Shields
K and Mantzoros CS: Metformin and sulfonylureas in relation to
cancer risk in type II diabetes patients: a meta-analysis using
primary data of published studies. Metabolism. 62:922–934. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang CH, Lin JW, Wu LC, Lai MS and Chuang
LM: Oral insulin secretagogues, insulin, and cancer risk in type 2
diabetes mellitus. J Clin Endocrinol Metab. 97:E1170–E1175. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kao CH, Sun LM, Chen PC, et al: A
population-based cohort study in Taiwan - use of insulin
sensitizers can decrease cancer risk in diabetic patients? Ann
Oncol. 24:523–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Colmers IN, Bowker SL and Johnson JA:
Thiazolidinedione use and cancer incidence in type 2 diabetes: a
systematic review and meta-analysis. Diabetes Metab. 38:475–484.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Staa TP, Patel D, Gallagher AM and de
Bruin ML: Glucose-lowering agents and the patterns of risk for
cancer: a study with the General Practice Research Database and
secondary care data. Diabetologia. 55:654–665. 2012. View Article : Google Scholar : PubMed/NCBI
|