Introduction
Aging is a natural process that increases the
vulnerability of an organism to the challenges of environment and
diseases. For humans, the aging of a population implicates the
rising morbidity and mortality of age-associated disorders, such as
Alzheimer's disease, heart failure and stroke, which exert a heavy
burden on society. As is known so far, >300 theories (1) of aging have been proposed, suggesting a
complex and multifactorial process. For a long time, slowing or
delaying the aging process has attracted increasing attention in
the field of medicine research. However, there are no supplements
or products that are known to stop the aging process (2).
Chronic D-galactose administration has been
demonstrated to accelerate the aging process in rodents and
Drosophia (3,4). The animals administered with D-galactose
exhibited typical symptoms, such as cognitive dysfunction,
neurodegeneration, weakened motor function and shortened lifespan
that resemble the natural process of aging. Therefore, this model
is used widely for the study of the aging process and screening of
anti-aging drugs.
Qing'E formula (QEF) is a traditional prescription
with four ingredients, Eucommiae Cortex, Psoraleae
Fructus, Juglandis Semen and Garlic Rhizoma, in a
specified ratio according to the Chinese Pharmacopoeia (5). In China, QEF was used 1,000 years ago
from the Song dynasty (10th century CE), and it is associated with
invigorating the kidneys, replenishing bone and muscle, causing the
body to become slimmer and benefiting complexion. QEF can
clinically alleviate osteoporosis in postmenopausal woman (6), and improve menopausal symptoms (7). However, little is known regarding whether
QEF has an anti-aging effect. In the present study, the anti-aging
function of QEF was assessed on D-galactose-induced aging mice. QEF
showed marked attenuation on the impaired motor and memory of mice.
Following this, the possible underlying mechanisms were
investigated. The study may contribute to the clinical application
of QEF as a remedy for anti-aging.
Materials and methods
Materials
QEF was prepared as reported previously (8). Briefly, the four ingredients,
Eucommiae Cortex (baked with salt), Psoraleae Fructus
(baked with salt), Juglandis Semen and Garlic Rhizoma
(steamed and dried) were mixed at the ratio of 16:8:5:4 (w/w) and
pulverized to a fine powder following identification by Professor
Hong Xu, at the Ministry of Education Key Laboratory for
Standardization of Chinese Medicines, Shanghai University of
Traditional Chinese Medicine (Shanghai, China). Specimens of the
herbs were kept in the laboratory for authentication. The
representative components of QEF determined by high-pressure liquid
chromatography were as follows: Psoralen (0.0785%), isopsoralen
(0.0668%), isobavachalcone (0.098%), bavachin (0.031%), corylifol A
(0.036%), neobavaisoflavone (0.056%) and pinoresinol diglucoside
(0.024%).
Biochemical kits for measuring glutathione (GSH),
catalase (CAT), total antioxidant capacity (T-AOC), total
superoxide dismutase (T-SOD), malondialdehyde (MDA), nitric oxide
(NO), NO synthase (NOS), blood urea nitrogen (BUN), creatinine
(CREA), alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). D-galactose was obtained
from Sigma-Aldrich (St. Louis, MO, USA). All the other reagents
were of analytical grade and commercially available.
Animals and treatment
Three-month-old male mice (Kunming strain), weighing
45±5.0 g, were provided by Shanghai University of Traditional
Chinese Medicine. The animals were acclimated to the laboratory
environment for 1 week before the experiment. They were housed
under standard conditions (25°C; 12-h light and 12-h dark) with
free access to food and water. All experimental procedures were
carried out in compliance with the Chinese legislation on the use
and care of laboratory animals and were approved by the university
committees for animal experiments.
The mice were divided randomly into 6 groups (12
mice/group): The control, model, vitamin E (VE), and QEF-low,
-middle and -high groups. Mice in the control group were
intraperitoneally injected with saline, and the other mice were
administered a daily subcutaneous injection of D-galactose (100
mg/kg/day) at the neck consecutively for 8 weeks. Mice also
received intragastric gavage with different drugs suspended in 0.5%
sodium carboxymethyl cellulose. The control and model group mice
were treated with saline. For VE group mice, they were administered
with VE (100 mg/kg/day). And for QEF group mice, they were given
QEF at doses of 1.62 (QEF-L), 3.24 (QEF-M) or 6.48 (QEF-H)
g/kg/day.
Behavioral tests
Rotarod test (RRT)
RRT was carried out with a Rota-Rod Treadmill
(Mobiledatum, Inc., Shanghai, China). Subsequent to being trained
to walk steadily on a horizontally oriented rod, the mice walked on
the rod rotating at 40 rpm for a ≤600 sec on the testing day. The
trial for each mouse was repeated three times with an interval
longer >1 h. The data were averaged for statistical
analysis.
Passive avoidance test (PAT)
PAT was conducted in the shuttle boxes, as described
previously (9). Briefly, on day 1, the
mice were trained to learn to stay in the bright chamber with a
criterion of 300 sec. On day 2, the time for the passive avoidance
response of the animals was evaluated in 300 sec. The latency to
enter the dark chamber and the number of entries were recorded by a
video-tracking system (Mobiledatum, Inc.).
Biochemical analysis
Following behavioral tests, the mice were
anesthetized with isoflurane. The orbital blood of mice was
collected. Subsequently, the liver, kidney and hippocampus from the
mice were dissected on ice. All the tissue samples were snap-frozen
in liquid nitrogen and stored at −80°C until further analysis. The
activities of T-AOC, ALT, AST, CAT, T-SOD and NOS, and the levels
of GSH, MDA, NO, BUN and CREA were determined using the respective
kits, following the manufacturers' protocols.
Reverse transcription-quantitative polymerase
chain reaction (RT-qPCR)
Total RNAs from hippocampal tissues of mice were
extracted using TRIzol according to the manufacturer's protocol
(Life Technologies, Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Subsequent to eliminating the trace amount of DNA
contamination with DNase I, the RNA was reverse transcribed into
cDNA with the RevertAid First Strand cDNA Synthesis kit (Fermentas,
Thermo Fisher Scientific, Inc.). The synthesized cDNA was used as
templates for the RT-qPCR reaction using the Taqman SYBR kit (Life
Technologies, Thermo Fisher Scientific, Inc.). The primers used
[Klotho, sirtuin 1 (SIRT1), forkhead box O transcription factor 3
(FOXO3), peroxisome proliferator-activated receptor γ
coactivator-1α (PGC-1α), insulin-like growth factor-1 (IGF-1) and
peroxiredoxin-3 (PRDX-3)] are listed in Table I. Relative expression levels of the
respective genes were normalized to that of
glyceraldehyde-3-phosphate dehydrogenase within the same
sample.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| GAPDH |
ATGTGTCCGTCGTGGATCTGA |
ATGCCTGCTTCACCACCTTCT |
| Klotho |
GGGACACTTTCACCCATCACT |
ACGTTGTTGTAACTATCGCTGG |
| SIRT1 |
GCTGACGACTTCGACGACG |
TCGGTCAACAGGAGGTTGTCT |
| PGC-1α |
TATGGAGTGACATAGAGTGTGCT |
CCACTTCAATCCACCCAGAAAG |
| PRDX-3 |
TCGACGACTTTAAGGGGAAA |
CCATTCTTTCTTGGCGTGTT |
| IGF-1 |
GATGGGGAAAATCAGCAGTC |
GCTGGTAAAGGTGAGCAAGC |
| FOXO3 |
CTGGGGGAACCTGTCCTATG |
TCATTCTGAACGCGCATGAAG |
Western blotting
Hippocampal tissues were homogenized by sonication
in CelLytic™ MT mammalian tissue lysis reagent (Sigma-Aldrich)
supplemented with protease and phosphatase inhibitor cocktails.
After centrifugation at 13,523 × g at 4°C for 10 min, the
supernatant of the lysate was collected and subjected to SDS-PAGE.
Following this, the proteins were transferred onto polyvinylidene
fluoride membranes. Subsequent to blocking with 5% skimmed milk,
the membranes were incubated with primary antibodies against SIRT1
(cat. no. ab110304; 1:1,000, mouse monoclonal antibody), PGC-1α
(cat. no. ST1202; 1:1,000, mouse monoclonal antibody), KLOTHO (cat.
no. ab154163; 1:1,000, rabbit monoclonal antibody) and β-actin
(cat. no. 4967; 1:5,000, rabbit polyclonal antibody) overnight at
4°C followed by incubation with respective horseradish
peroxide-conjugated secondary antibodies. The bands were visualized
with the ECL prime kit (GE Healthcare, Chalfont, UK). Relative
quantification of the blots was conducted using ImageJ 1.46r
(National Institute of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as mean ± standard error of
the mean. The difference between groups was evaluated by one-way
analysis of variance with Dunnett's multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of QEF on behavioral
parameters
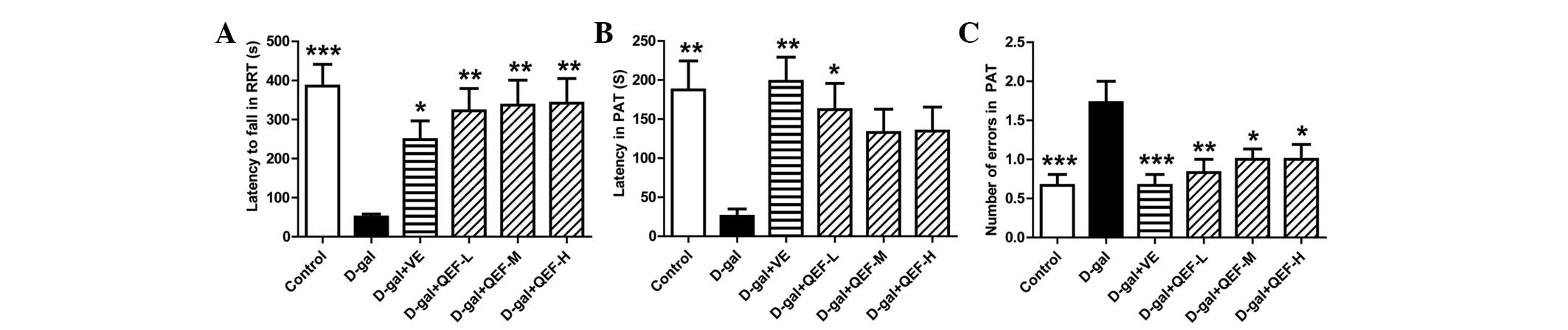
Following treatment with QEF for 8 weeks, the mice
were first subjected to RRT. As shown in Fig. 1A, D-galactose-injected mice fell
quicker from the rod in the RRT (P<0.001) in comparison with the
control group mice. Similar to VE, the positive drug, QEF at all
doses significantly prolonged the residence time of mice on the rod
(P<0.01).
In PAT, D-galactose injection impaired the memory of
mice as they exhibited shorter latency (Fig. 1B) (P<0.01) but increased entries
(Fig. 1C) (P<0.001) into the dark
chamber. Although only low dose of QEF significantly extended the
latency of D-galactose-treated mice (P<0.05), all doses of QEF
on mice markedly reduced their entries into the dark chamber.
The behavioral tests indicated that QEF treatment
could enhance motor coordination and alleviate the impairment of
memory of the aging mice.
Effect of QEF on serum parameters
Biochemical tests showed that D-galactose increased
the serum levels of BUN, CREA, AST, ALT, NO and MDA markedly
(P<0.05 or P<0.001) (Fig. 2).
QEF treatment at all doses could reverse all the aforementioned
indexes, although not in a dose-dependent manner (P<0.05,
P<0.01 or P<0.001). By contrast, VE could only decrease serum
BUN, CREA and AST levels (P<0.05 or P<0.001). The results
implicated that QEF attenuated the aging process in the blood.
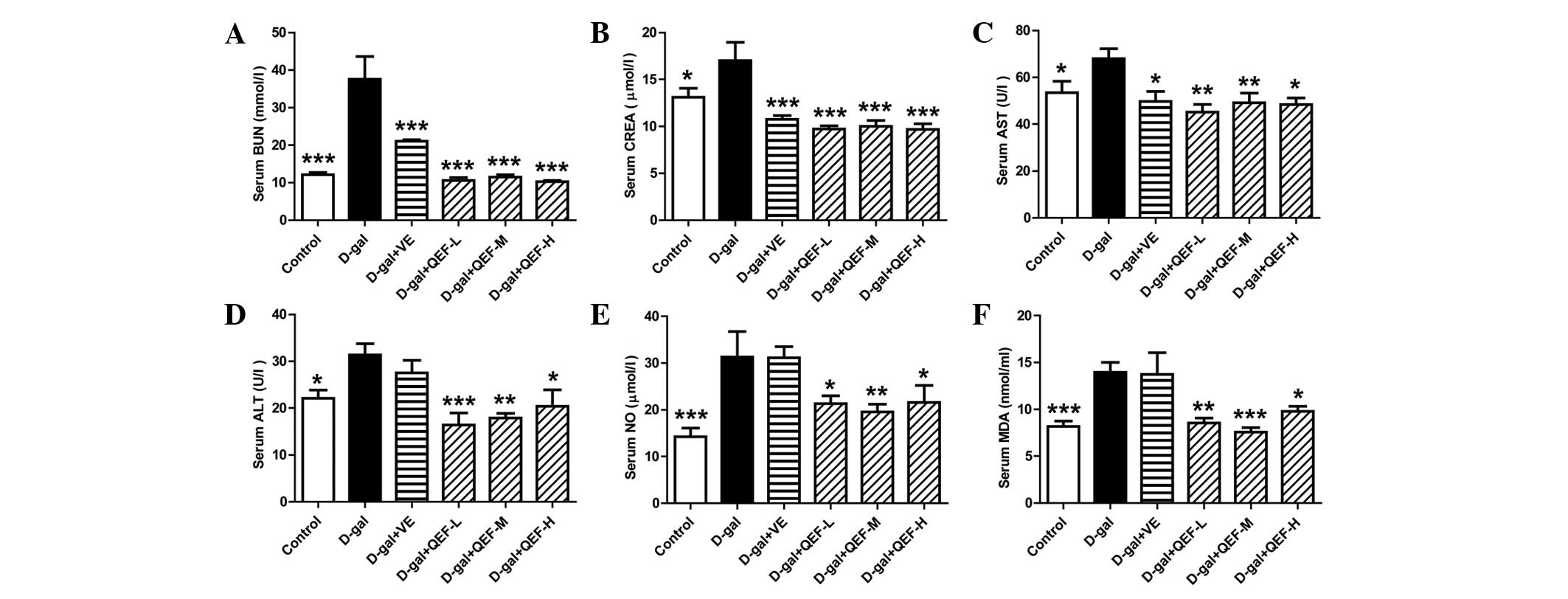 | Figure 2.Effect of QEF on the serum parameters
of D-gal-induced aging mice. (A) QEF improved the increased BUN
level. (B) QEF prevented the elevation of CREA level. (C) QEF
reduced the increased AST activity. (D) QEF deceased the increased
ALT activity. (E) QEF lessened the elevated NO level. (F) QEF
mitigated the increased serum MDA level. All data are presented as
mean ± standard error of the mean. n=10–12/group. *P<0.05,
**P<0.01, ***P<0.001 vs. D-gal group. QEF, Qing'E formula;
D-gal, D-galactose; L, low dose (1.62 g/kg/day); M, medium dose
(3.24 g/kg/day); H, high dose (6.48 g/kg/day); VE, vitamin E; BUN,
blood urea nitrogen; CREA, creatinine; AST, aspartate
aminotransferase; ALT, alanine aminotransferase; NO, nitric oxide;
NOS, NO synthase. |
Effect of QEF on liver parameters
In the mouse liver, long-term D-galactose treatment
decreased GSH level and reduced T-AOC and CAT activities (P<0.05
or P<0.001) (Fig. 3A–C). However,
it induced the elevation of NO, MDA levels and NOS activity
(P<0.05 or P<0.01) (Fig. 3D–F).
Following QEF treatment, all the biochemical parameters in liver
were improved. However, the positive control, VE, improved GSH, MDA
and NOS in the liver (P<0.05 or P<0.01). These suggested that
QEF improved the antioxidative system in liver.
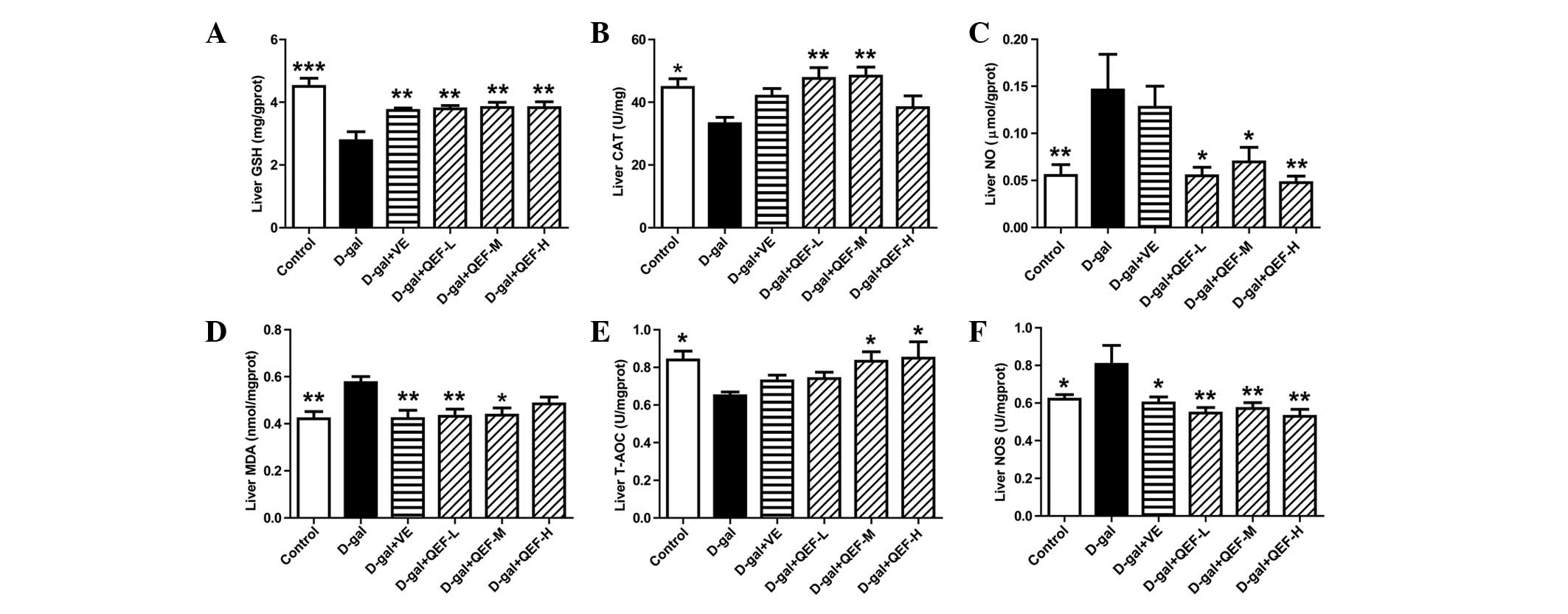 | Figure 3.Effect of QEF on liver parameters of
D-gal-induced aging mice. (A) QEF increased GSH level. (B) QEF
elevated CAT activity. (C) QEF decreased the elevated NO level. (D)
QEF reduced the production of MDA. (E) QEF enhanced T-AOC. (F) QEF
inhibited the NOS activity. All data are presented as mean ±
standard error of the mean. n=10–12/group. *P<0.05, **P<0.01,
***P<0.001 vs. D-gal group. QEF, Qing'E formula; D-gal,
D-galactose; L, low dose (1.62 g/kg/day); M, medium dose (3.24
g/kg/day); H, high dose (6.48 g/kg/day); VE, vitamin E; GSH,
glutathione; T-AOC, total antioxidant capacity; CAT, catalase; NO,
nitric oxide; MDA, malondialdehyde; NOS, NO synthase. |
Effect of QEF on the hippocampal
parameters
In the hippocampus, D-galactose did not show
significant changes in mRNA expression levels of Klotho, SIRT1,
FOXO3, PGC-1α, IGF-1 and PRDX-3 (Fig.
4), the genes that are well known to be actively involved in
aging process. However, QEF treatment for 8 weeks could elicit
significant upregulation of these genes (P<0.05, P<0.01 or
P<0.001), which appeared to be in a dose-dependent manner. By
contrast, VE treatment only enhanced the gene expression of Klotho
(P<0.01). Consistent with its effect on mRNA expression, QEF
treatment induced significant expression of Klotho and SIRT1 at
protein levels (P<0.05, P<0.01 or P<0.001) (Fig. 5A–C). In terms of PGC-1α, D-galactose
induced marked elevation of the protein (P<0.001), the effect of
which could be counteracted by QEF treatment (P<0.01 or
P<0.001) (Fig. 5D). For VE, it
increased Klotho and SIRT1 levels, but reduced the PGC-1α level
(P<0.05, P<0.01 or P<0.001). These results implicated that
QEF administration improved the aging process in hippocampus.
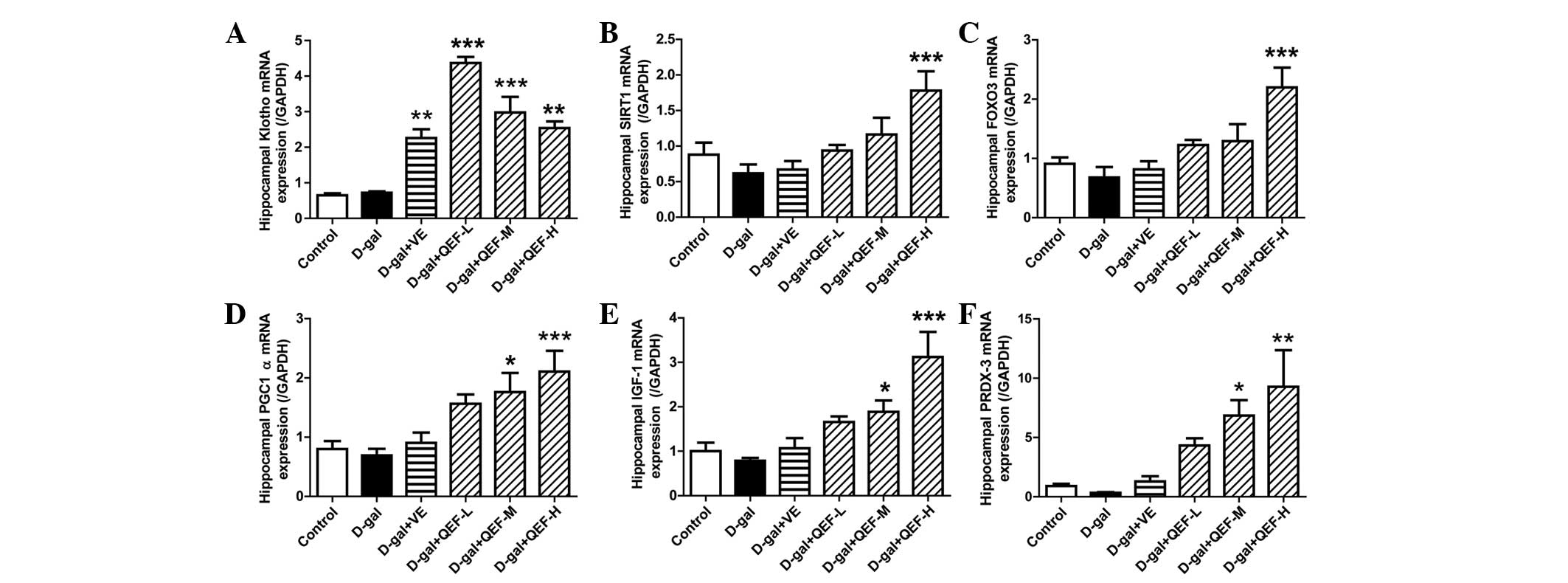 | Figure 4.Effect of QEF on hippocampal gene
expression levels of D-gal-induced aging mice. QEF increased (A)
Klotho, (B) SIRT1, (C) FOXO3, (D) PGC-1α, (E) IGF-1 and (F) PRDX-3
mRNA expression levels. All data are presented as mean ± standard
error of the mean. n=4–5/group. *P<0.05, **P<0.01,
***P<0.001 vs. D-gal group. QEF, Qing'E formula; D-gal,
D-galactose; L, low dose (1.62 g/kg/day); M, medium dose (3.24
g/kg/day); H, high dose (6.48 g/kg/day); VE, vitamin E; SIRT1,
sirtuin 1; FOXO3, forkhead box O transcription factor 3; PGC-1α,
peroxisome proliferator-activated receptor γ coactivator-1α; IGF-1,
insulin-like growth factor-1; PRDX-3, peroxiredoxin-3. |
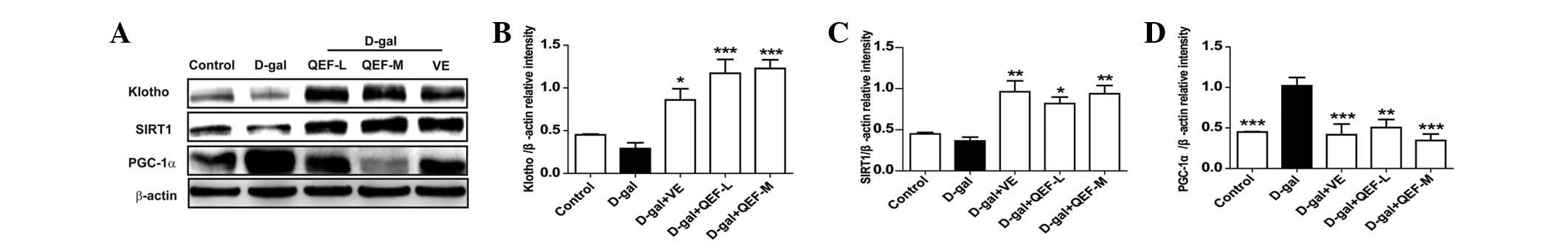 | Figure 5.Effect of QEF on hippocampal protein
expressions of D-gal-induced aging mice. (A) Western blot analysis.
Gray intensity analysis of (B) Klotho, (C) SIRT1 and (D) PGC-1α.
All data are presented as mean ± standard error of the mean.
n=4/group. *P<0.05, **P<0.01, ***P<0.001 vs. D-gal group.
QEF, Qing'E formula; D-gal, D-galactose; L, low dose (1.62
g/kg/day); M, medium dose (3.24 g/kg/day); H, high dose (6.48
g/kg/day); VE, vitamin E; SIRT1, sirtuin 1; PGC-1α, peroxisome
proliferator-activated receptor γ coactivator-1α. |
Discussion
In the present study, the effect of QEF on the
D-galactose-induced aging mice was evaluated. The results showed
that QEF improved motor coordination and memory impairment in aging
mice, indicating its potential application as an anti-aging
drug.
As aging occurs, the body loses muscle component,
resulting in a loss of strength and more easily fatigued muscle
(10), which is accompanied with
declined learning and memory capability. In the present study, the
motor coordination of mice reflecting one aspect of the strength of
muscle was evaluated by RRT (11),
while the memory of mice was assessed with PAT (12). In the experiments, D-galactose-treated
mice showed typical aging symptoms, as evidenced by reduced
endurance in RRT and impaired memory in PAT. By contrast, QEF
treatment could reverse the deteriorated behavior of mice,
suggesting its alleviative effect on motor and memory of aging
mice.
The free radical theory is one of the most popular
aging theories. According to the theory, accumulated free radicals,
such as reactive oxygen species (ROS), damage lipids, DNA, proteins
and tissues in organisms (13).
Enzymatic antioxidants, such as superoxide dismutase, CAT and GSH
peroxidase, and non-enzymatic antioxidants, such as VE and GSH, can
neutralize ROS and prevent against further injury. In the present
study, QEF administration enhanced antioxidants, including GSH,
T-AOC and CAT, in the serum or liver of D-galactose-induced aging
mice. As a result, MDA, the product of lipid peroxidation and
marker for oxidative stress (14), was
reduced. These results demonstrated the regulatory effect of QEF on
the unbalanced oxidants in aging mice.
Klotho, or its secreted form, α Klotho, has been
demonstrated to function as an anti-aging and organ protection
factor by inhibiting signaling of multiple growth factors, such as
insulin, IGF-1 and TGF-β (15–17). SIRT1 is a key protein that controls the
aging process by the regulation of energy metabolism (18), and has been shown to benefit the health
of aging mice (19). FOXO3 is a potent
transcriptional activator that triggers the expression levels of
numerous genes involved in cell cycle arrest, DNA repair, hypoxia
and apoptosis (20). In humans,
single-nucleotide polymorphisms of FOXO3 have been shown to
be closely associated with longevity (21) and have an important role in
ameliorating senescence and aging (22). PRDX-3 is mainly responsible for the
detoxification of 90% of the hydrogen peroxide in the mitochondria
(23). By contrast, low IGF-1
signaling is closely associated with improved longevity (24). As aforementioned, QEF improved impaired
memory of D-galactose-treated mice, therefore, the expression
levels of these genes or their products in hippocampus were
investigated. The present results suggested that QEF alleviated
memory impairment was associated with balancing the expression
levels of longevity-related genes at mRNA or protein levels.
Blood BUN and CREA are the common indicators for
kidney health, while ALT and AST are the common parameters for the
evaluation of liver health. In the present study, chronic
D-galactose administration was shown to increase all the serum
parameters significantly. By contrast, QEF treatment could
efficiently prevent against the elevation of BUN, CREA, ALT and
AST, implicating a protective effect on the liver and kidney.
However, its mechanism remains to be elucidated at the current
stage and requires further investigation.
In conclusion, QEF counteracted the accelerated
aging process induced by D-galactose in mice, which may be due to
its effects on enhancing antioxidants, protecting renal and hepatic
health, and balancing hippocampal expression levels of the
longevity-related genes.
Acknowledgements
The present study was supported by Shanghai Eastern
Scholar Program (grant no. 2013-59), Shanghai Three-Year Plan in
Advancing Traditional Medicine (grant no. ZYSNXD-GH-FFYJ) and
Shanghai E-research Institute of Bioactive Constituent in TCM
plan.
Glossary
Abbreviations
Abbreviations:
|
QEF
|
Qing'E formula
|
|
D-gal
|
D-galactose
|
|
GSH
|
glutathione
|
|
CAT
|
catalase
|
|
T-AOC
|
total antioxidant capacity
|
|
MDA
|
malondialdehyde
|
|
NO
|
nitric oxide
|
|
NOS
|
nitric oxide synthase
|
|
BUN
|
urea nitrogen
|
|
CREA
|
creatinine
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
SIRT1
|
sirtuin 1
|
|
FOXO3
|
forkhead box O transcription factor
3
|
|
PGC-1α
|
peroxisome proliferator-activated
receptor γ coactivator-1α
|
|
IGF-1
|
insulin-like growth factor-1
|
|
PRDX-3
|
peroxiredoxin-3
|
References
|
1.
|
Jin K: Modern biological theories of
aging. Aging Dis. 1:72–74. 2010.PubMed/NCBI
|
|
2.
|
Lipsky MS and King M: Biological theories
of aging. Dis Mon. 61:460–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ho SC, Liu JH and Wu RY: Establishment of
the mimetic aging effect in mice caused by D-galactose.
Biogerontology. 4:15–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Cui X, Wang L, Zuo P, Han Z, Fang Z, Li W
and Liu J: D-galactose-caused life shortening in Drosophila
melanogaster and Musca domestica is associated with
oxidative stress. Biogerontology. 5:317–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
National Pharmacopoeia Committee: Chinese
Pharmacopoeia. Beijing: Chemical Industry Press. 2010.
|
|
6.
|
Yang YP, Shuai B, Shen L, Xu XJ, Ma C and
Lv L: Effect of Qing'e formula on circulating sclerostin levels in
patients with postmenopausal osteoporosis. J Huazhong Univ Sci
Technolog Med Sci. 35:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Xia Y, Zhao Y, Ren M, Zhang J, Wang Y,
Chang Y, Fu S, Fan G, Zhu Y, Huang Y, et al: A randomized
double-blind placebo-controlled trial of a Chinese herbal medicine
preparation (Jiawei Qing'e Fang) for hot flashes and quality of
life in perimenopausal women. Menopause. 19:234–244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Xu Y, Zhang ZJ, Geng F, Su SB, White KN,
Bligh SW, Branford-White CJ and Wang ZT: Treatment with Qing'E, a
kidney-invigorating Chinese herbal formula, antagonizes the
estrogen decline in ovariectomized mice. Rejuvenation Res.
13:479–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Huang F, Wang T, Lan Y, Yang L, Pan W, Zhu
Y, Lv B, Wei Y, Shi H, Wu H, et al: Deletion of mouse FXR gene
disturbs multiple neurotransmitter systems and alters
neurobehavior. Front Behav Neurosci. 9:702015. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kim S, Kang IH, Nam JB, Cho Y, Chung DY,
Kim SH, Kim JS, Cho YD, Hong EK, Sohn NW, et al: Ameliorating the
effect of astragaloside IV on learning and memory deficit after
chronic cerebral hypoperfusion in rats. Molecules. 20:1904–1921.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jansen EM and Low WC: Long-term effects of
neonatal ischemic-hypoxic brain injury on sensorimotor and
locomotor tasks in rats. Behav Brain Res. 78:189–194. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Karimi S, Hejazian SH, Alikhani V and
Hosseini M: The effects of tamoxifen on spatial and nonspatial
learning and memory impairments induced by scopolamine and the
brain tissues oxidative damage in ovariectomized rats. Adv Biomed
Res. 4:1962015.PubMed/NCBI
|
|
13.
|
Harman D: The free radical theory of
aging. Antioxid Redox Signal. 5:557–561. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Uzar E, Koyuncuoglu HR, Uz E, Yilmaz HR,
Kutluhan S, Kilbas S and Gultekin F: The activities of antioxidant
enzymes and the level of malondialdehyde in cerebellum of rats
subjected to methotrexate: Protective effect of caffeic acid
phenethyl ester. Mol Cell Biochem. 291:63–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kurosu H, Yamamoto M, Clark JD, Pastor JV,
Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M,
Kawaguchi H, et al: Suppression of aging in mice by the hormone
Klotho. Science. 309:1829–1833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yahata K, Mori K, Arai H, Koide S, Ogawa
Y, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, Nabeshima Y, et al:
Molecular cloning and expression of a novel klotho-related protein.
J Mol Med Berl. 78:389–394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fon Tacer K, Bookout AL, Ding X, Kurosu H,
John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, et
al: Research resource: Comprehensive expression atlas of the
fibroblast growth factor system in adult mouse. Mol Endocrinol.
24:2050–2064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wu H, Wang H, Zhang W, Wei X, Zhao J, Yan
P and Liu C: rhEPO affects apoptosis in hippocampus of aging rats
by upregulating SIRT1. Int J Clin Exp Pathol. 8:6870–6880.
2015.PubMed/NCBI
|
|
19.
|
Crandall JP and Barzilai N: Exploring the
promise of resveratrol: Where do we go from here? Diabetes.
62:1022–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Renault VM, Thekkat PU, Hoang KL, White
JL, Brady CA, Kenzelmann Broz D, Venturelli OS, Johnson TM, Oskoui
PR, Xuan Z, et al: The pro-longevity gene FoxO3 is a direct target
of the p53 tumor suppressor. Oncogene. 30:3207–3221. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Anselmi CV, Malovini A, Roncarati R,
Novelli V, Villa F, Condorelli G, Bellazzi R and Puca AA:
Association of the FOXO3A locus with extreme longevity in a
southern Italian centenarian study. Rejuvenation Res. 12:95–104.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Xiong S, Patrushev N, Forouzandeh F,
Hilenski L and Alexander RW: PGC-1α Modulates Telomere Function and
DNA Damage in Protecting against Aging-Related Chronic Diseases.
Cell Reports. 12:1391–1399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Cox AG, Winterbourn CC and Hampton MB:
Mitochondrial peroxiredoxin involvement in antioxidant defence and
redox signalling. Biochem J. 425:313–325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Huffman DM, Farias Quipildor G, Mao K,
Zhang X, Wan J, Apontes P, Cohen P and Barzilai N: Central
insulin-like growth factor-1 (IGF-1) restores whole-body insulin
action in a model of age-related insulin resistance and IGF-1
decline. Aging Cell. 15:181–186. 2016. View Article : Google Scholar : PubMed/NCBI
|