Introduction
Cancer has become the major cause of mortality in
certain countries in the 21st century (1). There are three models for cancer
heterogeneity. Tumor heterogeneity is attained through genetic or
epigenetic modifications. The stochastic model, as the first model,
indicates that all tumor cells potentially are capable of
self-renewal or differentiation, and are tumorigenic (2). In the second model, the hierarchical
model, which is also known as the cancer stem cell (CSC) model of
tumor growth, the ability of self-renew is considered for only a
subset of tumor cells; the CSCs. These cells generate committed
progenitor cells with limited proliferative potential, which
ultimately lead to terminal differentiation (3–5). The third
model is known as the complex model and this model suggests that
epigenetic changes can potentially influence the tumor cell
phenotype and function due to micro-environmental factors, thereby
influencing tumor heterogeneity (2).
Recently, a new CSC theory, known as tumor stem
cells or tumor-initiating cells, has emerged. A CSC was precisely
defined by the American Association for Cancer Research in 2006 as
a cell within a tumor that has positive susceptibility to
self-renew and to reason the heterogeneous progeny of cancer cells
that are contained within the tumor (6). The CSC model was previously described for
hematological malignancies in 1997 (7).
Leucine-rich repeat-containing G protein-coupled
receptor 5 (LGR5) is considered an intestinal stem cell marker
(8). LGR5 has a transducer role in the
Wnt signaling pathway (9,10). This signaling pathway is well known to
be involved in the embryogenesis and carcinogenesis process
(11). Also recognized as GPR49, LGR5
is a member of the G protein-coupled receptors, the largest family
of cell-surface molecules involved in a signaling pathway. The size
of the LGR5 gene is ~144 kb and it is located at position 12q22-q23
of chromosome 12. The LGR5 protein has seven transmembrane domains.
Experimental findings showed that this protein in the mature form
contains <17 leucine-rich repeats, each composed of 24 amino
acids (2). The ligand for LGR5 is
R-spondin, and following ligand-receptor binding, it forms a
protein complex with frizzled lipoprotein receptor-related proteins
5 and 6. Subsequently, this complex positively regulates the Wnt
signaling pathway (12,13).
Despite the controversies regarding the CSC model,
CSC markers have the potential to provide a basis for new
innovative targeted therapy for origins of cancer (14,15) and
selecting the best cell line for LGR5-related studies. This may
help in obtaining more accurate results. Therefore, the aim of the
present study was to compare the LGR5 expression in different
cancer and normal cell lines by western blot analysis.
Materials and methods
Cells and cell culture
Eight commonly used cell lines, including COS-7
(fibroblast-like kidney cells), NIH3T3 (mouse embryonic fibroblast
cell line), HEK293 (human embryonic kidney cells), VERO
(fibroblast-like kidney cell from African green monkey), HeLa
(human epithelial carcinoma cell line), BHK (baby hamster kidney
fibroblasts), HepG2 (human hepatocellular liver carcinoma cell
line) and AGS (human gastric adenocarcinoma) were used in the
study. All the cell lines were purchased from the National Cell
Bank of the Iran Pasture Institute (Tehran, Iran). RPMI-1640
supplemented with 10% fetal calf serum, 100 IU/ml penicillin, and
10 µg/ml streptomycin (PAA Laboratories GmbH, Pasching, Austria)
was utilized as the medium. The cells were cultured in humidified
conditions at 37°C and in 5% CO2.
Following the exponential phase of growth, the cells
were washed twice by ice-cold phosphate buffered saline (PBS), and
adherent cells were scraped off from the flask by a cell scraper.
Following this, all cells were resuspended in 1 ml of
radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), which included 2 mM
phenylmethylsulfonyl fluoride, 10 µl of protease inhibitor cocktail
and 1 mM sodium orthovanadate (Santa Cruz Biotechnology, Inc.).
After centrifugation at 10,000 × g at 4°C for 20 min, cell debris
was removed and the supernatant was used for western blotting. The
Bradford assay protocol was utilized in order to determine the
protein concentrations (16).
Western blotting
Equal amounts of total protein from each cell line
were separated on a 12.5% discontinuous sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis in a
Mini-PROTEAN® Tetra Handcast System (Bio-Rad, Hercules,
CA, USA) for 90 min at 120 V. Subsequently, the separated proteins
were transferred to a polyvinylidene difluoride membrane (Santa
Cruz Biotechnology, Inc.) in a tank-transfer system (Bio-Rad) at
100 V for 60 min in the presence of 0.1% SDS. Following this, the
membrane was blocked with 5% skimmed dry milk in Tris-buffered
saline (pH 7.6) with 0.1% Tween-20 for 1 h at room temperature. The
membrane was incubated using LGR5 mouse monoclonal antibody clone
2A2 (OriGene Technologies, Rockville, MD, USA; cat. no. TA503316),
which was used as a specific primary antibody (diluted to
1:2,000).
The blots were washed three times in PBS-Tween and
goat anti-mouse IgG-HRP (Santa Cruz Biotechnology, Inc.; cat. no.
SC-2005) secondary antibody was used for visualizing the
antibody-antigen complex. The blots were developed with a
Supersignal West Pico Chemiluminescent Substrate kit (Pierce,
Rockford, IL, USA) for 5 min and images were captured by a Gbox
device (Syngene, Cambridge, UK). To correct for protein loading and
transfer efficiency, β-actin was used as the reference proteins for
normalization in western blotting. By comparing with known protein
size markers, the molecular weights were determined. Western blot
band densities were quantitated by the GeneTools software
(Syngene).
Results
Protein expression of LGR5 in the
different cell lines
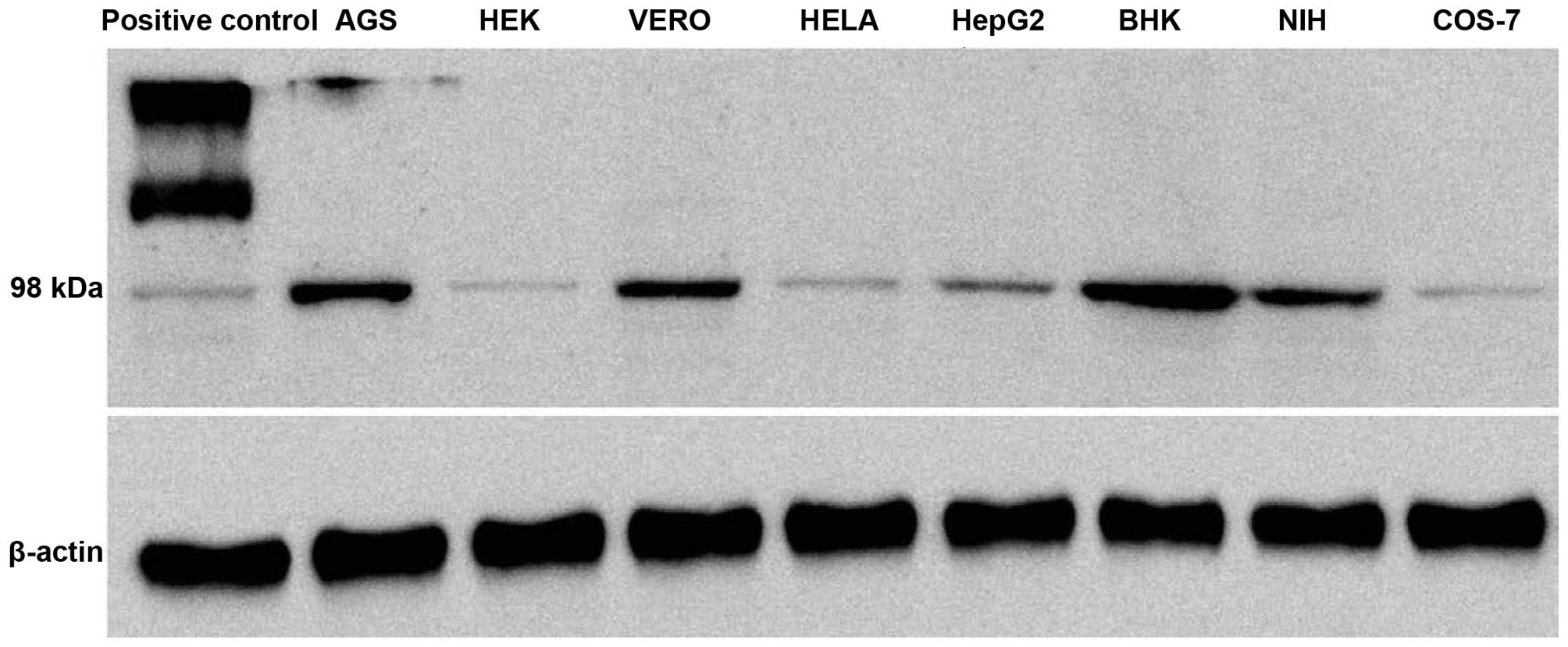
The expression pattern of the LGR5 levels in certain
commonly used laboratory cell lines, which were AGS, HeLa, HEK293,
HepG2, BHK, VERO, COS-7 and NIH3T3, was assessed. Western blotting
on the total cell lysate was carried out to determine whether LGR5
was expressed at protein levels in these cells. All the cell lines
tested showed a detectable amount of LGR5 expression; however, the
level of expression differed in these cells. Expression of the LGR5
protein in the different cell lines in comparison with β-actin is
shown in Fig. 1. A high level of LGR5
expression was detected in the AGS, BHK, VERO and NIH cell lines,
while expression was barely detected in the other tested cell
lines.
Relative expression of the LGR5
protein
To estimate the relative expression of LGR5 in the
tested cell lines, the band density for each cell line was
determined using densitometry (Table
I). The results of the normalized band densities showed that,
as expected, the ASG cells expressed a higher level of LGR5
compared to the other tested cell lines. The BHK cells showed a
higher level of LGR5 expression compared to the AGS cells. AGS
cells are gastrointestinal cancer cells that are known to have an
extremely high level of LGR5 expression, while BHK is an
immortalized normal kidney cell of hamsters. Different amounts of
LGR5 expression levels were also detected in two kidney-derived
cells from monkey; VERO and COS-7 cells. VERO cells had a higher
expression of LGR5 in comparison to COS-7 cells, which may reflect
their distinct cell lineage in the kidney.
 | Table I.Densitometry results of leucine-rich
repeat-containing G protein-coupled receptor 5 expression in
different cell lines. |
Table I.
Densitometry results of leucine-rich
repeat-containing G protein-coupled receptor 5 expression in
different cell lines.
| Type of cell
line | Cell line name | Densitometry
result |
|---|
| Cancer | AGS | 20.81 |
| Normal | HEK-292 | 1.34 |
| Normal | VERO | 18.39 |
| Cancer | HeLa | 2.96 |
| Cancer | HepG2 | 6.37 |
| Normal | BHK | 30.83 |
| Normal | NIH3T3 | 17.20 |
| Normal | COS-7 | 2.10 |
Discussion
CSCs have been a milestone in the investigations on
cancer studies as they provide a noteworthy cellular mechanism to
account for the therapeutic resistance and silent behavior
exhibited by numerous tumors (17).
LGR5 is an important target of the Wnt/β-catenin signaling pathway,
which was first identified as an intestinal stem cells marker
(10). The present study used western
blotting to assess the expression levels of LGR5 in eight commonly
used laboratory cell lines, which were AGS, HEK, VERO, HeLa, HepG2,
BHK, NIH3T3 and COS-7. The results showed that the expression of
the LGR5 protein in the BHK and AGS cell lines were higher compared
to the other cells. The HEK-292 and COS-7 cell lines expressed
lower levels of LGR5 compared with other cells. Furthermore, the
LGR5 expression in cancer cell lines was higher compared to the
normal cells.
He et al (18)
demonstrated various levels of LGR5 expression in five colorectal
cancer cell lines by quantitative RT-PCR. The study reported high
LGR5 expression levels in SW620, Caco-2 and SW480 cells, and low
levels in LoVo and HCT116 (18).
Another study carried out by Ku et al (19) focused on the establishment of 13 human
colorectal carcinoma cell lines. The CSC biomarker cluster of
differentiation 133 (CD133) was expressed in 12 of the cell lines,
while the biomarkers CD44 and LGR5 were expressed in all 13 cell
lines (19). In conclusion, the
present findings suggest that the expression levels of LGR5 varied
in different cell lines, and there were high expression levels of
LGR5 in BHK and AGS for the normal and cancer cell lines,
respectively. Therefore, these two cells lines are the best options
for in vitro cancer studies.
Acknowledgements
The present study was part of a PhD thesis by Dr
Reza Alizadeh-Navaei and was supported by a grant from Mazandaran
University of Medical Sciences, Iran (no. 92-64).
References
|
1
|
Vries RG, Huch M and Clevers H: Stem cells
and cancer of the stomach and intestine. Mol Oncol. 4:373–384.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakata S, Phillips E and Goidts V:
Emerging role for leucine-rich repeat-containing G-protein-coupled
receptors LGR5 and LGR4 in cancer stem cells. Cancer Manag Res.
6:171–180. 2014.PubMed/NCBI
|
|
3
|
Natarajan TG, Ganesan N and Fitzgerald KT:
Cancer stem cells and markers: New model of tumorigenesis with
therapeutic implications. Cancer Biomark. 9:65–99. 2010.PubMed/NCBI
|
|
4
|
Pietras A: Cancer stem cells in tumor
heterogeneity. Adv Cancer Res. 112:255–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nosrati A, Naghshvar F and Khanari S:
Cancer stem cell markers CD44, CD133 in primary gastric
adenocarcinoma. Int J Mol Cell Med. 3:279–286. 2014.PubMed/NCBI
|
|
6
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanders MA and Majumdar AP: Colon cancer
stem cells: Implications in carcinogenesis. Front Biosci (Landmark
Ed). 16:1651–1662. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Xue X, Jiang M, Guo X, Li P, Liu F,
Yuan B, Shen Y, Guo X, Zhi Q, et al: LGR5, a relevant marker of
cancer stem cells, indicates a poor prognosis in colorectal cancer
patients: A meta-analysis. Clin Res Hepatol Gastroenterol.
39:267–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pinson KI, Brennan J, Monkley S, Avery BJ
and Skarnes WC: An LDL-receptor-related protein mediates Wnt
signalling in mice. Nature. 407:535–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van der Flier LG, Sabates-Bellver J, Oving
I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S,
Van de Wetering M, Marra G, et al: The Intestinal Wnt/TCF
Signature. Gastroenterology. 132:628–632. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samaei NM, Yazdani Y, Alizadeh-Navaei R,
Azadeh H and Farazmandfar T: Promoter methylation analysis of
WNT/β-catenin pathway regulators and its association with
expression of DNMT1 enzyme in colorectal cancer. J Biomed Sci.
21:732014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schuijers J and Clevers H: Adult mammalian
stem cells: The role of Wnt, Lgr5 and R-spondins. EMBO J.
31:2685–2696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar KK, Burgess AW and Gulbis JM:
Structure and function of LGR5: an enigmatic G-protein coupled
receptor marking stem cells. Protein Sci. 23:551–565. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Connor ML, Xiang D, Shigdar S, Macdonald
J, Li Y, Wang T, Pu C, Wang Z, Qiao L and Duan W: Cancer stem
cells: A contentious hypothesis now moving forward. Cancer Lett.
344:180–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi S, Han L, Gong T, Zhang Z and Sun X:
Systemic delivery of microRNA-34a for cancer stem cell therapy.
Angew Chem Int Ed Engl. 52:3901–3905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitamura H, Okudela K, Yazawa T, Sato H
and Shimoyamada H: Cancer stem cell: Implications in cancer biology
and therapy with special reference to lung cancer. Lung Cancer.
66:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He S, Zhou H, Zhu X, et al: Expression of
Lgr5, a marker of intestinal stem cells, in colorectal cancer and
its clinicopathological significance. Biomed Pharmacother.
68:507–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ku JL, Shin YK, Kim DW, Kim KH, Choi JS,
Hong SH, Jeon YK, Kim SH, Kim HS, Park JH, et al: Establishment and
characterization of 13 human colorectal carcinoma cell lines:
Mutations of genes and expressions of drug-sensitivity genes and
cancer stem cell markers. Carcinogenesis. 31:1003–1009. 2010.
View Article : Google Scholar : PubMed/NCBI
|