Introduction
Approximately 80% of the aging population suffers
from lower back pain (LBP) in their lifetime (1–3). It markedly
affects the quality of life and work productivity, and
significantly impacts health care spending (4). Intervertebral disc degeneration (IDD) is
a common degenerative physiological process with normal aging,
which has been established as a causal factor with LBP (5,6).
Degenerative disorders of intervertebral discs (IVDs) are generally
characterized by disequilibrium between extracellular matrix repair
and degradative processes, including morphological and cellular
changes (7–11).
A solution to managing disc degeneration would be to
repair the IVDs, producing a matrix with similar or improved
biological and biomechanical properties compared with the original
(12). With this aim, cell
transplantation has been widely investigated as an effective
approach (12–14), including bone marrow mesenchymal stem
cell (BMSC) transplantation. Using this method, BMSCs are
transplanted into IVDs to replenish the matrix-producing cells,
compensating for the decrease in matrix components. Thus far, the
majority of studies focus on allogeneic BMSC transplantation in
IVDs to observe the regeneration effect (15–18).
However, there have been few reports regarding the survival status
of human BMSCs in the IVDs post-transplantation in vivo. The
current study was designed to observe the survival status of human
BMSCs following transplantation into rabbit IVDs, providing an
experimental basis for further investigation of disc degeneration
treatment.
Materials and methods
Animals and BMSCs
Fifteen New Zealand white rabbits [Animal Husbandry
and Veterinary Bureau, Shandong Academy of Agricultural sciences;
permission no. SCXK (Lu) 20040013], aged 6-months and of either
gender (weight, 2.0–3.0 kg) were used in the study. The rabbits
were maintained apart at room temperature with 16:8 h light and
dark cycles, and were fed with rabbit feed (Qingdao Kangda
Foodstuffs Co., Ltd., Qingdao, China). All animals were raised, and
procedures were performed, in strict accordance with Animal Ethical
Standards, and the study was approved by the Ethics Committee of
the Affiliated Hospital of Qingdao University (Qingdao, China). The
IVDs of these rabbits were divided into three groups: Punctured
blank control group (L1-2), punctured physiological saline (PS)
control group (L2-3) and punctured human BMSCs transfected with
green fluorescent protein (GFP) group (L3-4, L4-5 and L5-6).
GFP-labeled human BMSCs and the complete medium
[Dulbecco's modified Eagle's medium with 15% fetal bovine serum]
were purchased from Cyagen Bioscience Co., Ltd. (Santa Clara, CA,
USA). The BMSCs were frozen prior to use.
Preparation of GFP-labeled human BMSC
suspension
Frozen GFP-labeled human BMSCs were thawed and
cultured in a 25 cm2 culture flask with complete medium.
After overspreading the bottom of the flask, cells were
trypsinized, centrifuged at 520 × g for 5 min at room temperature
and suspended in normal saline at a final concentration of
1×106 cells/ml and prepared for transplantation.
Transplantation of GFP-labeled human
BMSCs
After tranquilizing the rabbits by intramuscular
injection of ketamine hydrochloride (40 mg/kg; Selleck Chemicals,
Houston, TX, USA) and xylazine (2.5 mg/kg; Selleck Chemicals), 25
µl GFP-labeled human BMSC suspension was injected into the L3-L4,
L4-L5, L5-L6 discs of the rabbits using a posterolateral approach
with a 29-gauge needle on a 100 µl microinjector. The same method
was used to inject the equivalent PS into the L2-3 discs. Following
the surgical procedure, 20 units benzylpenicillin sodium and 0.25
mg streptomycin were administered by injection to prevent
infection.
Evaluation of the survival of
BMSCs
The rabbits were sacrificed by ear vein air
injection at 1, 2, 4, 6 and 8 weeks post-treatment (n=3 per
time-point). Their spines were harvested and the discs were
isolated. The specimens were frozen in liquid nitrogen immediately
after harvest and cryosectioned axially at 6 µm. A fluorescence
microscope was used to observe the cell density in the different
groups. To compare the ralative cell density, cells in five
sections crossing the center of the discs per group were counted
using ×200 magnification images.
Statistical analysis
Statistical analysis was performed with SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). A signed-rank test was used to
analyze the data and P<0.05 was considered to indicate a
statistically significant difference.
Results
General status of laboratory
animals
Prior to transplantation, two rabbits succumbed due
to environment inadaptation and were substituted with two different
rabbits.
There were no complications or mortalities during
and after transplantation. After the specimens were harvested, no
significant changes were observed macroscopically and,
microscopically, no vascularization or inflammation was apparent in
the nucleus pulposus (NP).
Survival status of human BMSCs at
different periods post-transplantation
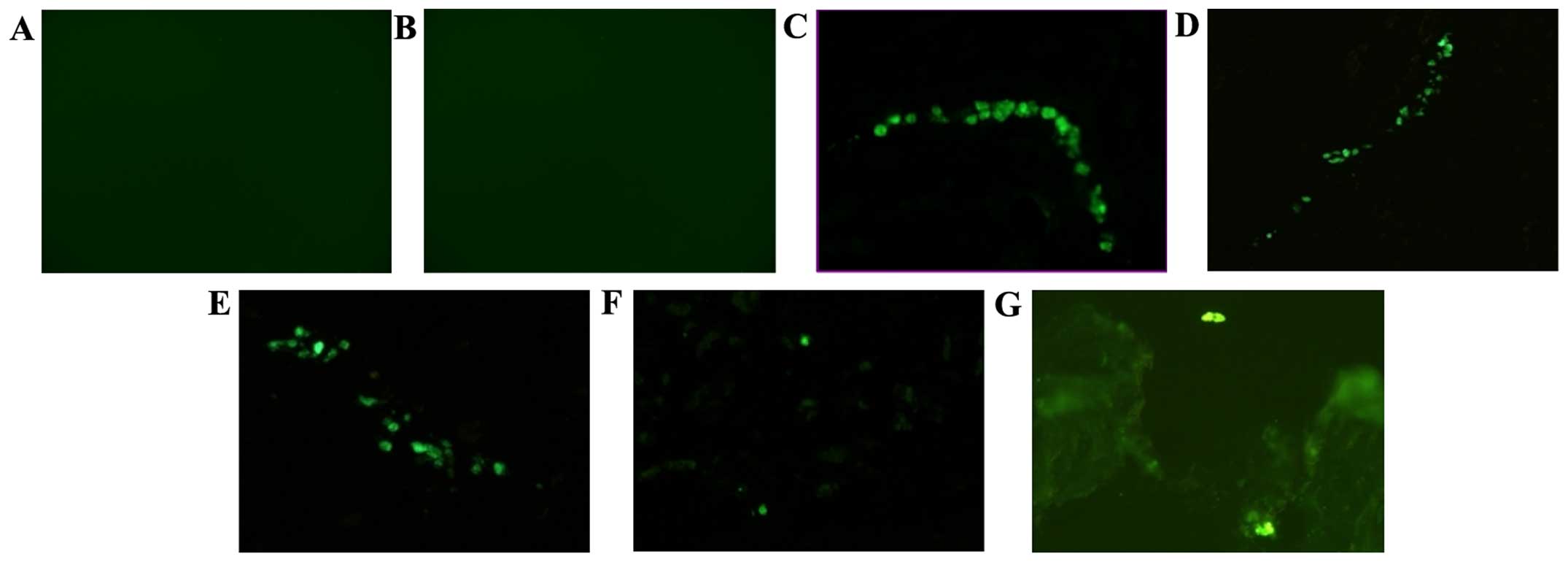
No GFP-positive cells were detected in the blank and
PS control groups, whereas GFP-positive cells were observed in the
NP at all periods post-transplantation in the BMSCs group,
demonstrating the survival of the transplanted BMSCs (Fig. 1). In the BMSCs group, a mean of 11, 10,
11, 2 and 1 GFP-positive cells was detected in one section at 1, 2,
4, 6 and 8 weeks post-transplantation. Significant differences were
identified between the cell densities at different periods
(H=28.27; P<0.001). The cell density at 6 and 8 weeks was
significantly less than that at 1, 2 and 4 weeks
post-transplantation (H=16.50–24.44; P<0.001) and no statistical
differences were observed between the cell density counted at 1, 2,
4, 6 and 8 weeks post-transplantation (H=2.06–5.44; P>0.05).
Discussion
More than 20% of all LBP originates from
degenerative changes in IVDs, leading to a gradual process of
cellular loss and degradation of the extracellular matrix within
the IVDs (19). While the pathology of
IDD is not fully understood, insufficient nutritional supply to the
disc has been proposed as a possible initiator (20). As the largest avascular organs in
humans, IVDs obtain nutrition from adjacent vertebral bodies by
diffusion through the annulus fibrosus (AF) and endplate. Among
these nutritional routes, the endplate pathway has been identified
to be more significant than the other AF signaling pathways
(21). In cases with loss of endplate
permeability and/or disruptions therein, as well as changes in
geometry and fall in diffusivity associated with fluid expression,
the nutrient concentrations could fall to levels inadequate to
maintain cellular activity or viability, thus initiating or
accelerating disc degeneration (22).
Current treatments for degenerative disc disease
including conservative treatment (medications and physiotherapy)
and surgical treatment (spinal fusion or total disc replacement)
may alleviate pain, but fail to address the underlying issue and
often result in reoccurrence in the same or adjacent discs
(23). Particularly in treatment with
spinal fusion, it accelerates the degeneration of adjacent IVDs and
the severity of adjacent segment degeneration increases with the
fused segment numbers (24).
Cell-based tissue engineering, which aims to restore IVD structure
and function, represents a promising approach for IVD repair or
regeneration.
Since NP cells possess a chondrocyte-like phenotype
(25), MSCs, which were capable of
differentiating into a chondrocyte-like phenotype following
appropriate stimulation (12,26), have shown promise as suitable seed
cells to be widely applied for IVD regeneration (27–29). In
addition, MSCs have the advantage of easy availability and
proliferative ability, better potential in producing matrix (mainly
comprising type II collagen and proteoglycan) and low
immunogenicity (29–32). BMSCs, as the most focused seed cells,
have been demonstrated as effective in decelerating disc
degeneration in experimental models by autologous transplantation
(29) and allogeneic BMSC
transplantation has also become a focus of investigation worldwide
(15–18).
Among research investigating allogeneic BMSC
transplantation, there are few studies regarding human BMSCs. In
the current study, a GFP gene was used as a marker to observe the
survival status of human BMSCs in rabbit IVDs following
transplantation. The results indicate that human BMSCs may survive
in rabbits for 8 weeks, but the cell density decreased
significantly from 6 to 8 weeks. Rather than applying scaffolds,
direct injection avoids the underlying influence on the
microenvironment within IVDs. Furthermore, no vascularization and
inflammation was identified in the NP, which implies little
interference of surgical procedures and low immunogenicity of human
BMSCs. In the GFP-labeled human BMSCs group, the density of
GFP-positive cells marginally decreased at 2 weeks and
significantly decreased after 6 weeks post-transplantation, which
may be due to various reasons, including inadaptation to the IVD
microenvironment, insufficient nutrients for cell survival and cell
migration to the outer NP.
In conclusion, the present study observed the
survival status of human BMSCs in rabbit IVDs and demonstrated that
the cells were able to survive during 8 weeks of transplantation.
The finding provides fundamental information for tissue engineering
repair used to decelerate the degeneration of discs. The status of
the cells 8 weeks post-transplantation, such as their ability to
survive, proliferate and differentiate, requires further
investigation.
Acknowledgements
The authors would like to thank the members for
their enthusiastic participation in the present study. The study
was supported by a research grant award from the National Natural
Science Foundation of China (grant no. 81371998) and the National
Key Clinical Specialty construction projects of China (grant no.
201030402).
References
|
1
|
Hoy D, Brooks P, Blyth F and Buchbinder R:
The Epidemiology of low back pain. Best Pract Res Clin Rheumatol.
24:769–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K, Aoki Y and Ohtori S:
Resolving discogenic pain. Eur Spine J. 17(Suppl 4): 428–431. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hillman M, Wright A, Rajaratnam G, Tennant
A and Chamberlain MA: Prevalence of low back pain in the community:
Implications for service provision in Bradford, UK. J Epidemiol
Community Health. 50:347–352. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McMeeken J, Tully E, Stillman B, Nattrass
C, Bygott IL and Story I: The experience of back pain in young
Australians. Man Ther. 6:213–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pye SR, Reid DM, Smith R, Adams JE, Nelson
K, Silman AJ and O'Neill TW: Radiographic features of lumbar disc
degeneration and self-reported back pain. J Rheumatol. 31:753–758.
2004.PubMed/NCBI
|
|
6
|
Hoyland JA, Le Maitre C and Freemont AJ:
Investigation of the role of IL-1 and TNF in matrix degradation in
the intervertebral disc. Rheumatology (Oxford). 47:809–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
LeMaitre CL, Freemont AJ and Hoyland JA:
Localization of degradative enzymes and their inhibitors in the
degenerate human intervertebral disc. J Pathol. 204:47–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
LeMaitre CL, Freemont AJ and Hoyland JA:
Accelerated cellular senescence in degenerate intervertebral discs:
A possible role in the pathogenesis of intervertebral disc
degeneration. Arthritis Res Ther. 9:R452007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richardson SM, Knowles R, Tyler J,
Mobasheri A and Hoyland JA: Expression of glucose transporters
GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate
human intervertebral disc. Histochem Cell Biol. 129:503–511. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao CQ, Wang LM, Jiang LS and Dai LY: The
cell biology of intervertebral disc aging and degeneration. Ageing
Res Rev. 6:247–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim KW, Chung HN, Ha KY, Lee JS and Kim
YY: Senescence mechanisms of nucleus pulposus chondrocytes in human
intervertebral discs. Spine J. 9:658–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richardson SM, Walker RV, Parker S, Rhodes
NP, Hunt JA, Freemont AJ and Hoyland JA: Intervertebral disc
cell-mediated mesenchymal stem cell differentiation. Stem Cells.
24:707–716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobajima S, Vadala G, Shimer A, Kim JS,
Gilbertson LG and Kang JD: Feasibility of a stem cell therapy for
intervertebral disc degeneration. Spine J. 8:888–896. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakai D, Mochida J, Iwashina T, Watanabe
T, Nakai T, Ando K and Hotta T: Differentiation of mesenchymal stem
cells transplanted to a rabbit degenerative disc model: Potential
and limitations for stem cell therapy in disc regeneration. Spine.
30:2379–2387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang YH, Yang B, Li WL and Li JM: Effect
of the mixture of bone marrow mesenchymal stromal cells and annulus
fibrosus cells in repairing the degenerative discs of rabbits.
Genet Mol Res. 14:2365–2373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi Z, Guanjun T, Lin C and Zifeng P:
Effects of transplantation of htimp1-expressing bone marrow
mesenchymal stem cells on the extracellular matrix of degenerative
intervertebral discs in an in vivo rabbit model. Spine.
39:E669–E675. 2014. View Article : Google Scholar
|
|
17
|
Wang Y, Wu X, Wang F, Hong X, Bao J and
Zhu L: An in vitro study on biological characteristics of bone
marrow mesenchymal stem cells in microenvironment of premature
senescence of nucleus pulposus cells. Zhongguo Xiu Fu Chong Jian
Wai Ke Za Zhi. 28:758–762. 2014.(In Chinese). PubMed/NCBI
|
|
18
|
Subhan RA, Puvanan K, Murali MR,
Raghavendran HR, Shani S, Abdullah BJ, Abbas AA, Mohamed JA and
Kamarul T: Fluoroscopy assisted minimally invasive transplantation
of allogenic mesenchymal stromal cells embedded in HyStem reduces
the progression of nucleus pulposus degeneration in the damaged
ntervertebral disc: A preliminary study in rabbits. Scientific
World Journal. 2014:8185022014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dagenais S, Caro J and Haldeman S: A
systematic review of low back pain cost of illness studies in the
United States and internationally. Spine J. 8:8–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grunhagen T, ShiraziAdl A, Fairbank JC and
Urban JP: Intervertebral disk nutrition: A review of factors
influencing concentrations of nutrients and metabolites. Orthop
Clin North Am. 42465–477. (vii)2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogata K and Whiteside LA: 1980 Volvo award
winner in basic science. Nutritional pathways of the intervertebral
disc. An experimental study using hydrogen washout technique.
Spine. 6:211–216. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Soukane DM, ShiraziAdl A and Urban JP:
Computation of coupled diffusion of oxygen, glucose and lactic acid
in an intervertebral disc. J Biomech. 40:2645–2654. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Richardson SM, Mobasheri A, Freemont AJ
and Hoyland JA: Intervertebral disc biology, degeneration and novel
tissue engineering and regenerative medicine therapies. Histol
Histopathol. 22:1033–1041. 2007.PubMed/NCBI
|
|
24
|
Levin DA, Hale JJ and Bendo JA: Adjacent
segment degeneration following spinal fusion for degenerative disc
disease. Bull NYU Hosp Jt Dis. 65:29–36. 2007.PubMed/NCBI
|
|
25
|
Sive JI, Baird P, Jeziorsk M, Watkins A,
Hoyland JA and Freemont AJ: Expression of chondrocyte markers by
cells of normal and degenerate intervertebral discs. Mol Pathol.
55:91–97. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steck E, Bertram H, Abel R, Chen B, Winter
A and Richter W: Induction of intervertebral disc-like cells from
adult mesenchymal stem cells. Stem Cells. 23:403–411. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hiyama A, Mochida J, Iwashina T, Omi H,
Watanabe T, Serigano K, Tamura F and Sakai D: Transplantation of
mesenchymal stem cells in a canine disc degeneration model. J
Orthop Res. 26:589–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakai D, Mochida J, Iwashina T, Hiyama A,
Omi H, Imai M, Nakai T, Ando K and Hotta T: Regenerative effects of
transplanting mesenchymal stem cells embedded in atelocollagen to
the degenerated intervertebral disc. Biomaterials. 27:335–345.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakai D, Mochida J, Yamamoto Y, Nomura T,
Okuma M, Nishimura K, Nakai T, Ando K and Hotta T: Transplantation
of mesenchymal stem cells embedded in Atelocollagen gel to the
intervertebral disc: A potential therapeutic model for disc
degeneration. Biomaterials. 24:3531–3541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartholomew A, Sturgeon C, Siatskas M,
Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R,
et al: Mesenchymal stem cells suppress lymphocyte proliferation in
vitro and prolong skin graft survival in vivo. Exp Hematol.
30:42–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horwitz EM, Prockop DJ, Fitzpatrick LA,
Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz
RE, et al: Transplantability and therapeutic effects of bone
marrow-derived mesenchymal cells in children with osteogenesis
imperfecta. Nat Med. 5:309–313. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
LeBlanc K, Tammik C, Rosendahl K,
Zetterberg E and Ringdén O: HLA expression and immunologic
properties of differentiated and undifferentiated mesenchymal stem
cells. Exp Hematol. 31:890–896. 2003. View Article : Google Scholar : PubMed/NCBI
|