Introduction
Nasopharyngeal carcinoma (NPC) has an incidence rate
of 80,000 new cases per year worldwide, making up 0.7% of all
cancers (1,2). However, NPC has a distinctive geographic
distribution, with a high prevalence in Southeast Asia, Northeast
India, and North Africa, and in the Inuits of Canada and Alaska.
The rates in Southeast China are particularly high (3), with the highest rate reported to be
35/100,000 in Guangdong province (4).
At the time of diagnosis, >70% of NPC patients are at stage III
and stage IV (5). Furthermore, ≤20% of
newly diagnosed NPC patients exhibit occult distant metastases
(6). Radiation therapy is the standard
treatment option for NPC. With the advent of intensity-modulated
radiotherapy (IMRT), local-regional control has been substantially
improved and distant metastasis is currently the main cause of
treatment failure (7). The 5-year
distant metastasis rate has been reported to be 19% for all disease
stages, and 25% for the stage III–IVB subgroup (8,9). Concurrent
or adjuvant chemotherapy is administered to reduce the distant
metastasis rate for locally advanced NPC patients. However, certain
patients, particularly the elderly and those with poor performance
status, cannot tolerate the toxicities. Additional therapeutic
strategies are required to control the metastasis, and improve
survival and the quality of life for patients with advanced NPC.
One such strategy is targeted therapy.
Proto-oncogene c-Kit, also termed tyrosine-protein
kinase it or CD117, is a protein encoded in humans by the KIT
proto-oncogene receptor tyrosine kinase (Kit) gene (10). The c-Kit mediated signal transduction
pathways appear to be involved in regulating cell differentiation
and proliferation, and antibodies to c-Kit have been widely applied
in immunohistochemistry to distinguish particular types of tumour,
for example, malignant gastrointestinal stroma tumours (GISTs).
c-Kit expression is of particular clinical interest, as it is one
of the targets of the tyrosine kinase inhibitor, imatinib mesylate
(termed Gleevec or Glivec). Imatinib has been shown to exert marked
clinical activity in malignant GISTs containing a Kit gene mutation
or c-Kit protein expression (11).
Studies concerning c-Kit expression in NPC are rare
according to our search of the scientific literature from the NCBI
PubMed database. To the best of our knowledge, whether c-Kit
expression levels change during the course of radiation therapy has
not yet been investigated. In the present study, the expression
levels of c-Kit were investigated immunohistochemically in 106 NPC
cases, and the changes of c-Kit expression during the course of
radiation therapy were evaluated in 41 of the 106 cases.
Materials and methods
Patients
The present study was approved by the Institutional
Review Board of Zhejiang Cancer Hospital (Hangzhou, China). Between
2007 and 2009, a total of 106 pathologically confirmed NPC patients
were treated in the same medical group of Zhejiang Cancer Hospital,
including 73 males and 33 females, ranging in age from 17 to 75
years (mean age, 48 years; median age, 49 years). Ninety-seven
patients received platinum-based concurrent chemoradiotherapy (38
cases with nedaplatin and 59 cases with cis-platinum) and nine
cases received radiotherapy alone. Nasopharyngeal endoscopy was
routinely performed at the time of diagnosis, and repeated weekly
during the 6–7 week course of radiation therapy. In 41 out of the
106 NPC patients, biopsies were obtained weekly, until no tumour
could be seen in the nasopharyngeal endoscopy examination (i.e., a
complete response; CR). In total, 2–5 biopsies were performed
during the course of radiotherapy for these 41 patients according
to the status of tumour regression.
The stage of disease was determined according to the
7th edition of the Union for International Cancer Control and the
American Joint Committee on Cancer staging system (12,13).
Clinical information was obtained from hospital
records and included gender, age, pathological classification,
disease stage, and concurrent chemoradiotherapy. Patient and tumour
characteristics of the analysed cases are presented in Table I.
 | Table I.Tumour and patient characteristics
(n=106). |
Table I.
Tumour and patient characteristics
(n=106).
| Characteristics | Patients | c-Kit
overexpression | c-Kit low
expression | P-value |
|---|
| Gender |
|
|
| 0.057 |
| Male |
73 | 15 | 58 |
|
|
Female |
33 | 13 | 20 |
|
| Histology | 0.822 |
|
|
|
|
Differentiated |
64 | 16 | 48 |
|
|
Non-differentiated |
42 | 12 | 30 |
|
| T-stage |
|
|
| 0.635 |
|
T1-T2 |
31 | 7 | 24 |
|
|
T3-T4 |
75 | 21 | 54 |
|
| N-stage |
|
|
| 0.378 |
|
N0-N1 |
55 | 17 | 38 |
|
|
N2-N3 |
51 | 11 | 40 |
|
| M-stage |
|
|
| 1.000 |
| M0 |
102 | 27 | 75 |
|
| M1 |
4 | 1 | 3 |
|
| Stage at
diagnosis |
|
|
| 1.000 |
|
II–III |
60 | 16 | 44 |
|
| IV |
46 | 12 | 34 |
|
| Concurrent
chemoradiotherapy |
|
|
| 0.542 |
| No
CCRT |
9 | 1 | 8 |
|
|
Nedaplatin |
38 | 11 | 27 |
|
|
Cis-platinum |
59 | 16 | 43 |
|
| Total |
106 | 28 | 78 |
|
c-Kit staining
The tissue samples were fixed in 4% buffered
formalin, dehydrated in graded alcohol, and fully embedded in
paraffin. Subsequently, 4-µm thick sections were sliced and
deparaffinized in xylene at 60°C for 20 min, and hydrated through
graded alcohol to distilled water. Enzymatic antigen retrieval was
conducted in citric acid buffer solution (pH 6) for 20 min at 98°C.
Endogenous peroxidase was blocked in 3% H2O2
in phosphate-buffered saline for 10 min. The slides were blocked in
bovine serum (Sijiqing, Hangzhou, China), followed by incubation in
rabbit anti-human c-Kit (cat. no. ab32363; Abcam, Cambridge, USA)
diluted in 1:100 at 4°C overnight. After the sections were
incubated in secondary antibody conjugated with AlexaFluor 488
(cat. no. A-11008; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 30 min at room temperature, the slides were
finally counterstained with hematoxylin and mounted.
c-Kit scores
The c-Kit expression was scored using the scoring
criterion as reported by Bar-Sela et al (14) with modifications as follows. All the
slides were evaluated by investigators who were blinded to the
patients' clinical data using an Eclipse E200 microscope (Nikon
Corporation, Tokyo, Japan). The staining intensities were graded
according to the percentage of stained tumour cells, using a scale
of 0–3+. The positive staining appeared as cytoplasmic
immunoreactivity with accentuation along the cell membranes. The
score was based on a scale as follows: Positive staining of ≥50% of
the tumour cells was considered as 3+; positive staining of≥10% and
<50% of the tumour cells was considered as 2+;
2+ and 3+ were considered to be
overexpression. Positive staining of <10% of the tumour cells
was considered as 1+; No cellular staining was
considered as 0; and 1+ and 0 were considered as low
expression.
Follow-up
The patients were followed up every 3 months for the
first 2 years, then every 6 months for a further 3 years and
annually thereafter. Physical examination, including palpation of
the neck, direct flexible fiberoptic nasopharyngoscopy, blood
examination, magnetic resonance imaging scans of the nasopharynx
and neck, chest radiography, and abdominal ultrasound were
performed at each follow-up visit. Whole-body bone scans were
obtained at least once per year.
Statistical analysis
The patients were divided into two groups; the
overexpression group and low expression group, with respect to
c-Kit expression. The Pearson χ2 test was used to
compare groups regarding categorical variables, such as gender,
age, tumour stage, histology classification, and concurrent
chemoradiotherapy (Table I).
Progression-free survival was defined as the time from treatment to
the time of disease progression. Overall survival was defined as
the time from treatment to date of death from any cause.
Progression-free survival, overall survival and the nasopharyngeal
recurrence, regional recurrence, distant metastasis were analysed
using the Kaplan-Meier curves. Data were analysed using SPSS 19.0
software (IBM SPSS, Armonk, NY, USA).
Results
Expression levels of c-Kit in NPC
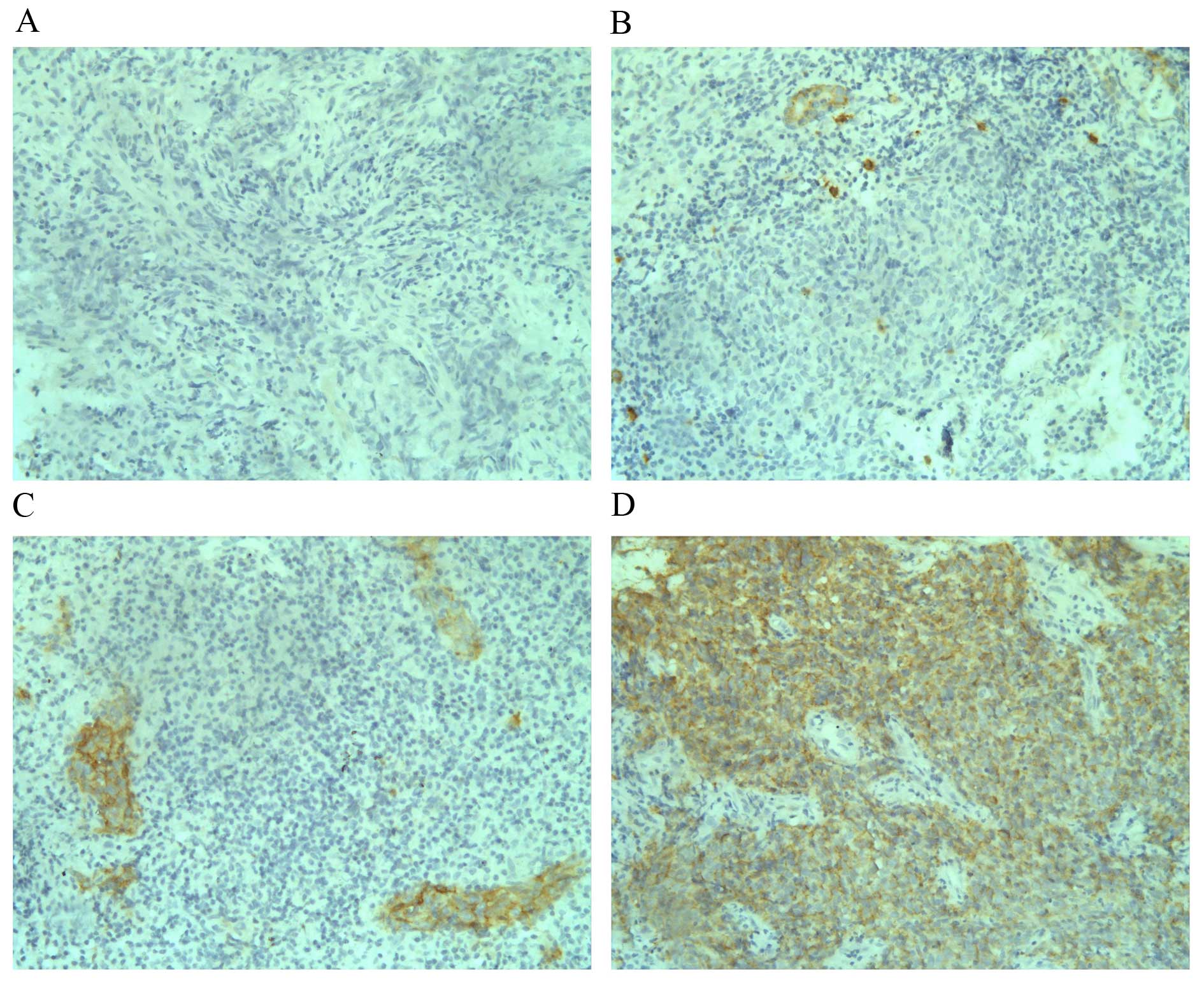
In the current study, among the 106 analysed NPC
cases, 12 (11.3%) had c-Kit expression scores of 3+, 16
(15.1%) had c-Kit expression scores of 2+, 35 (33.0%)
had c-Kit expression scores of 1+. Negative c-Kit
staining was observed in 43 cases (40.6%) of the analysed tumours.
In total, c-Kit overexpression (2+ or 3+) was
observed in 26.4% (28 of 106) of NPC cases, and low expression
(1+ or 0) was found in 73.6% (78 of 106) of cases. As
shown in Table I, no difference of
c-Kit expression was identified among patients with different
clinical staging. Examples of staining patterns for the different
c-Kit expression levels are presented in Fig. 1.
Alteration of c-Kit expression during
the course of radiotherapy in NPC
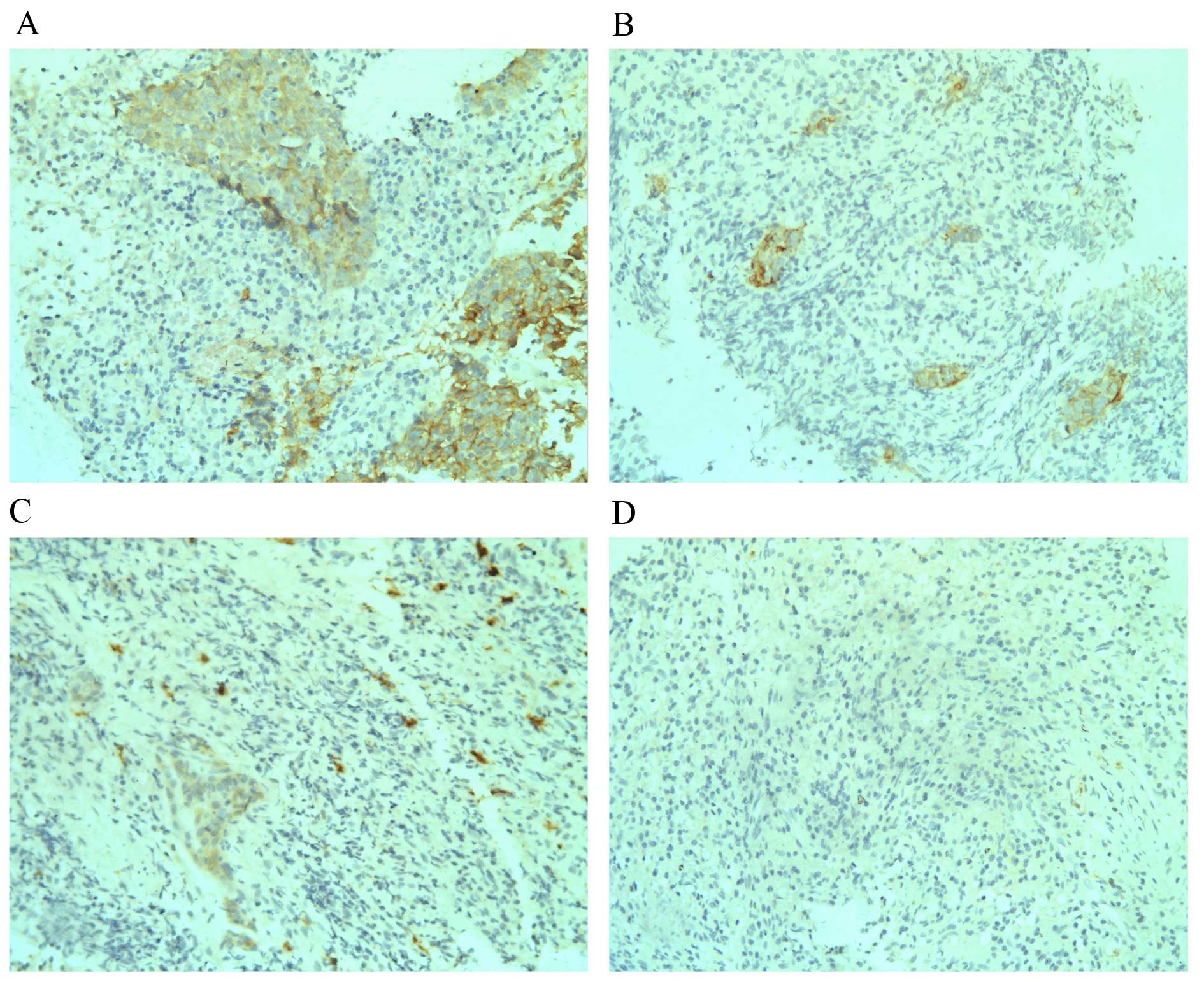
In the current study, weekly nasopharyngeal
endoscopies were performed during the 6–7 week course of radiation
therapy. In 41 out of the 106 NPC patients tumour biopsies were
also performed weekly, until no tumour could be seen (complete
response; CR) during nasopharyngeal endoscopy examination. Overall,
2–5 biopsies were made during the course of radiotherapy for these
41 patients according to the status of tumour regression. When
c-Kit expression levels at different time points for the same
patients were compared, a trend of decreased c-Kit expression was
observed following the start of radiotherapy. For the five cases
with 3+ c-Kit expression at diagnosis prior to
radiotherapy, three exhibited reduced c-Kit expression
(1+ and 0) in the second week of radiotherapy and,
thereafter, one showed reduced c-Kit expression (2+)
from the fourth week, and the other case showed stable
3+ c-Kit expression for the first 2 weeks of radiation
and the tumour had disappeared under endoscopy in the third week.
For the 8 cases with 2+ c-Kit expression at diagnosis
prior to radiotherapy, all showed reduced c-Kit expression
following radiotherapy (five cases exhibited reduced c-Kit
expression from the second week of the course of radiotherapy, and
the other three cases exhibited reduced c-Kit expression from the
third week). Seven of the 10 cases (70.0%) with 1+ c-Kit
expression prior to radiotherapy showed negative c-Kit expression
(scored as 0). The other three cases retained low c-Kit expression
(1+) during the course of radiation therapy. None of the
cases with c-Kit expression scored as 1+ or
2+ demonstrated increased c-Kit expression subsequent to
radiation. For the 18 cases with negative c-Kit expression, one
case showed increased c-Kit expression (scored as 1+)
after radiation, the other 15 cases maintained negative expression
throughout. Examples of c-Kit expression alteration during
radiation therapeutic course are presented in Fig. 2.
Treatment outcome
The median follow-up time was 50 months (range, 2–75
months). Local recurrence occurred in three patients, regional
recurrence occurred in three patients and distant metastases in 15
patients: Four cases to the bone, five to the liver, four to the
lung, one to the bone and lung, and the other case to the bone and
liver. Fifteen patients had succumbed at the time of this analysis;
three from progression of residual disease, three from locoregional
recurrence and 9 as a result of distant metastasis.
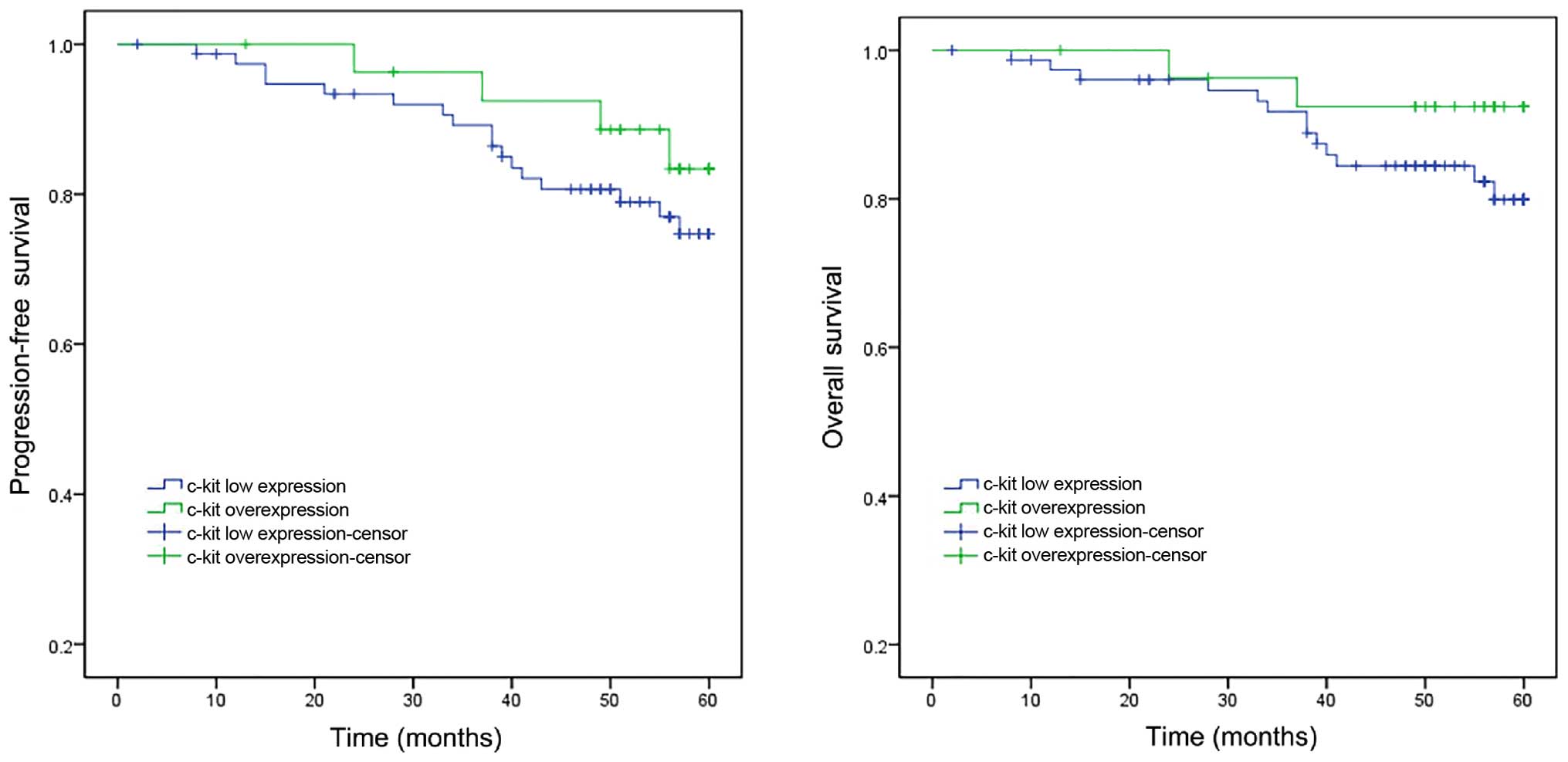
The results of Kaplan-Meier analysis are presented
in Fig. 3. The 5-year progression-free
survival rates were 83.4% for the cases with c-Kit overexpression
compared with 74.7% for those with low c-Kit expression (P=0.769).
Statistical analysis did not identify any significant differences.
The overall survival rates at 5 years were 92.4 and 79.9% for the
cases with c-Kit overexpression and low c-Kit expression,
respectively; the difference was not statistically significant
(P=0.199).
Discussion
In the present study, c-Kit expression levels of
patients with NPC were examined, the dynamic changes of c-Kit
expression during the course of radiation were evaluated, and the
correlation between c-Kit expression and clinical characteristics
was analysed.
The data regarding c-Kit expression in NPC are
limited. In the study by Bar-Sela et al (14), 49 NPC tissue samples were analysed for
c-Kit (CD117) expression. Overexpression of c-Kit was identified in
33% (16/49) of the patients, with 18.4% (9/49) strongly positive
for the c-Kit protein (staining of >50% of the tumour cells). In
another report by Bar-Sela et al (15), c-Kit overexpression was observed in 28%
(9/32) of the adult NPC cases. In the present study, a relatively
large population, of 106 NPC cases were analysed in total, and
similar c-Kit scoring criteria were used as in the study Bar-Sela
et al (14). Twelve (11.3%)
cases exhibited c-Kit expression that was scored as 3+ and 16
(15.1%) that was scored as 2+. Thus, c-Kit
overexpression (2+ or 3+) was observed in
26.4% (28/106) of NPC cases, which is comparable to the former
reports (28 and 33%, respectively) by Bar-Sela et al
(14,15). However, higher expression rates of
c-Kit have also been reported. In the study by Sheu et al
(16), positive staining of >1% of
the tumour cells was considered to be positive for c-Kit
expression, and the proportion of NPC expressing c-Kit was 86% in
that series.
Children with NPC demonstrate increased c-Kit
expression when compared with adults with NPC. In a study by Charfi
et al (17), expression of
c-kit was detected in 79% of the cases for patients aged
<30-years-old. Bar-Sela et al (15) reported on 16 NPC patients aged
<20-years-old. Overexpression of c-Kit was found in 88% of the
cases, as compared to 28% in adults (15). In the present study, only one patient
was <20 years old. Furthermore, attempts were made to analyse
the expression rates in different age ranges; however, no
difference was identified between the younger adults and older
patients.
To the best of our knowledge, the present study is
the first to report whether c-Kit expression levels change during
the course of radiation therapy. A trend of decreased c-Kit
expression was observed subsequent to commencing radiotherapy when
the c-Kit expression levels were compared at different time points
for each individual patient. For the 13 cases exhibiting c-Kit
overexpression (2+ and 3+) at diagnosis prior
to radiotherapy, 12 cases demonstrated reduced c-Kit expression
following radiotherapy, and the majority of patients with
1+ c-Kit expression showed negative staining subsequent
to radiotherapy. Notably, none of the cases with c-Kit expression
scored as 1+ or 2+ demonstrated increased
c-Kit expression following radiation. For those cases exhibiting
negative c-Kit staining, the majority maintained negative
expression during radiation. Although the functional involvement of
c-Kit in tumorigenesis and the therapeutic response of NPC remains
to be elucidated in detail, the current study provides valuable
data regarding dynamic changes of c-Kit expression during the
course of radiation therapy.
C-kit expression has been reported as an independent
prognostic factor in patients with other types of cancer, such as
small cell lung cancer (18). However,
in the current study, no association was identified between c-Kit
expression and survival of NPC patients. The present results are
consistent with previous reports by Bar-Sela et al (14,15). In the
present study, the majority of the cases were adults, with only one
patient aged 17-years-old. For paediatric patients, tumours with
strongly positive c-Kit expression were reported to have a lower
recurrence rate (15). However, larger
studies are required to evaluate c-Kit as a potential prognostic
factor.
c-Kit overexpression had been reported in various
NPC cell lines (19). In preclinical
studies, imatinib and sunitinib induced a dose-dependent inhibitory
effect on the proliferation of NPC cells (19,20). As yet,
to the best of our knowledge, targeted therapy against c-Kit has
not been investigated in NPC patients. As c-Kit overexpression has
been reported in ~30% of adult NPC patients (14,15), it may
be of interest to evaluate c-Kit as a therapeutic target for
metastatic NPC patients with c-Kit overexpression, particularly for
those whose first line treatment failed.
c-Kit overexpression was identified to be common in
NPC, therefore, it may be of interest to evaluate c-Kit as a
therapeutic target for metastatic NPC with c-Kit overexpression as
a second line treatment. A trend of decreased c-Kit expression was
observed during the course of radiotherapy. However, the prognostic
value of c-Kit in patients with NPC remains to be elucidated.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81572952).
References
|
1
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang JB, Jiang Y, Liang H, Li P, Xiao HJ,
Ji J, Xiang W, Shi JF, Fan YG, Li L, et al: Attributable causes of
cancer in China. Ann Oncol. 23:2983–2989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia WH, Huang QH, Liao J, Ye W, Shugart
YY, Liu Q, Chen LZ, Li YH, Lin X, Wen FL, et al: Trends in
incidence and mortality of nasopharyngeal carcinoma over a 20-25
year period (1978/1983-2002) in Sihui and Cangwu counties in
southern China. BMC Cancer. 6:1782006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi JL, Gao L, Huang XD, Li SY, Luo JW, Cai
WM, Xiao JP and Xu GZ: Nasopharyngeal carcinoma treated by radical
radiotherapy alone: Ten-year experience of a single institution.
Int J Radiat Oncol Biol Phys. 65:161–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ong YK, Heng DM, Chung B, Leong SS, Wee J,
Fong KW, Tan T and Tan EH: Design of a prognostic index score for
metastatic nasopharyngeal carcinoma. Eur J Cancer. 39:1535–1541.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu
LZ, Sun Y, Lin AH, Liu MZ and Ma J: How does intensity-modulated
radiotherapy versus conventional two-dimensional radiotherapy
influence the treatment results in nasopharyngeal carcinoma
patients? Int J Radiat Oncol Biol Phys. 80:661–668. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee AW, Sze WM, Au JS, Leung SF, Leung TW,
Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al: Treatment results
for nasopharyngeal carcinoma in the modern era: The Hong Kong
experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hui EP, Leung SF, Au JS, Zee B, Tung S,
Chua D, Sze WM, Law CK, Leung TW and Chan AT: Lung metastasis alone
in nasopharyngeal carcinoma: A relatively favorable prognostic
group. A study by the Hong Kong Nasopharyngeal Carcinoma Study
Group. Cancer. 101:300–306. 2004.
|
|
10
|
Yarden Y, Kuang WJ, Yang-Feng T, Coussens
L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U and
Ullrich A: Human proto-oncogene c-kit: A new cell surface receptor
tyrosine kinase for an unidentified ligand. EMBO J. 6:3341–3351.
1987.PubMed/NCBI
|
|
11
|
Dagher R, Cohen M, Williams G, Rothmann M,
Gobburu J, Robbie G, Rahman A, Chen G, Staten A, Griebel D, et al:
Approval summary: Imatinib mesylate in the treatment of metastatic
and/or unresectable malignant gastrointestinal stromal tumors. Clin
Cancer Res. 8:3034–3038. 2002.PubMed/NCBI
|
|
12
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 7th. Wiley Blackwell;
Chichester: 2010
|
|
13
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A III: American Joint Committee on Cancer
(AJCC)Cancer staging manual. 7th. Springer; New York: 2010
|
|
14
|
Bar-Sela G, Kuten A, Ben-Eliezer S,
Gov-Ari E and Ben-Izhak O: Expression of HER2 and C-KIT in
nasopharyngeal carcinoma: Implications for a new therapeutic
approach. Mod Pathol. 16:1035–1040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bar-Sela G, Ben Arush MW, Sabo E, Kuten A,
Minkov I and Ben-Izhak O: Pediatric nasopharyngeal carcinoma:
Better prognosis and increased c-Kit expression as compared to
adults. Pediatr Blood Cancer. 45:291–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheu LF, Lee WC, Lee HS, Kao WY and Chen
A: Co-expression of c-kit and stem cell factor in primary and
metastatic nasopharyngeal carcinomas and nasopharyngeal epithelium.
J Pathol. 207:216–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charfi S, Khabir A, Ayadi L, Mseddi M,
Makni H, Gorbel A, Daoud J, Frikha M, Jlidi R, Busson P, et al:
Expression of c-kit in North African nasopharyngeal carcinomas:
Correlation with age and LMP1. Cancer Radiother. 11:247–251.
2007.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rohr UP, Rehfeld N, Pflugfelder L, Geddert
H, Müller W, Steidl U, Fenk R, Gräf T, Schott M, Thiele KP, et al:
Expression of the tyrosine kinase c-kit is an independent
prognostic factor in patients with small cell lung cancer. Int J
Cancer. 111:259–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang PY, Hong MH, Zhang X, Mai HQ, Luo DH
and Zhang L: C-KIT overexpression and mutation in nasopharyngeal
carcinoma cell lines and reactivity of Imatinib on these cell
lines. Chin J Cancer. 29:131–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hui EP, Lui VW, Wong CS, Ma BB, Lau CP,
Cheung CS, Ho K, Cheng SH, Ng MH and Chan AT: Preclinical
evaluation of sunitinib as single agent or in combination with
chemotherapy in nasopharyngeal carcinoma. Invest New Drugs.
29:1123–1131. 2011. View Article : Google Scholar : PubMed/NCBI
|