Introduction
Breast cancer is the most common type of cancer in
women in Western countries (1).
Furthermore, it is the second most frequent type of malignancy in
women worldwide after non-melanoma skin cancer. For the year 2012,
>1.6 million new cases and a breast cancer-associated mortality
rate of 522,000 have been estimated (2). In Brazil, breast cancer is the most
common type of cancer in women, its incidence shows yearly
increases and the National Institute of Cancer estimated that
57,960 new cases of breast cancer will occur in the year 2016.
However, due to the relatively low aggressiveness of the disease
and the efficacy of available treatments, the prognosis of breast
cancer patients is good with a mean 5-year survival rate of 61%
(3).
Breast cancer treatment is multidisciplinary and the
survival of affected women is associated with early diagnosis and
therapeutic strategies pursued. While systemic therapy is usually
applied for pre-menopausal women with early-stage breast cancer,
the optimal modalities remain to be determined (4). However, almost 60% of all breast cancers
in pre-menopausal women (5) and 70–80%
of post-menopausal women with breast cancer in Western countries
are estrogen receptor (ER)-positive and therefore eligible for
adjuvant endocrine therapy (6).
Tamoxifen, a first-generation selective ER
modulator, is currently the most widely used endocrine treatment
breast cancer worldwide. It has anti-estrogenic action and is used
for adjuvant treatment of hormone-sensitive breast cancer, as well
as for chemoprevention in women with a high risk of breast cancer
(7), although aromatase inhibitors are
generally preferred for post-menopausal patients (8).
Tamoxifen is a prodrug that is extensively
metabolized in the liver by the cytochrome P450 2D6 (CYP2D6) enzyme
into its most pharmacologically active metabolites, endoxifen and
4-hydroxitamoxifen (9). These
metabolites bind to ERs with an affinity 30–100-fold of that of
tamoxifen (10). The CYP2D6 gene that
encodes this enzyme is highly polymorphic and varies among
different genotypes and populations. It has been established that a
number of its genetic variants are deprived of any enzymatic
activity, resulting in low levels of endoxifen, its main
metabolite, resulting in a lack of response to tamoxifen with
unfavorable patient prognosis (11).
To date, >100 alleles have been described for the
CYP2D6 gene, which are stratified into alleles of null,
intermediate, normal and ultra-rapid enzymatic activity. Variant *4
is the most important null allelic variant responsible for
abolishing enzymatic activity and a poor metabolism (PM), while the
*10 and *17 variants are responsible for a severe decrease in
enzymatic activity and intermediate metabolism (IM). The most
important allelic variants are *4, *10 and *17, found in 25% of
Caucasians, 38–70% of Asians and 35% of Africans (12). The association of CYP2D6 gene
polymorphisms with the efficacy of tamoxifen in early-stage breast
cancer patients has been assessed by numerous studies, whose
results were conflicting. Certain studies have shown no alterations
in the efficacy of tamoxifen in breast cancer patients carrying the
CYP2D6 gene polymorphism in terms of recurrence and overall
survival (8,10,13), while
other studies have demonstrated a significant influence of CYP2D6
gene polymorphisms on the efficacy of tamoxifen (14,15). Only
one previous study has evaluated the effects of CYP2D6 gene
polymorphisms on the efficacy of tamoxifen in a Brazilian
population (13). Thus, due to the
controversies among studies on other populations and the scarcity
of investigations in Brazilian populations, the present study was
designed.
Patients and methods
Patients
The present study assessed 92 female patients aged
27–85 years with hormone-sensitive breast carcinoma with and
without disease recurrence taking adjuvant tamoxifen, who were seen
at the Department of Oncology of São Marcos Hospital (Teresina,
Brazil) and the Department of Mastology of Getulio Vargas Hospital
(the Federal University of Piauí, Teresina, Brazil) in March 2010
and followed up over five years until December 2015. Twelve
patients were excluded from the study due to incorrect use of
medication or technical problems, which precluded analysis. The
Internal Review Board of the Federal University of Piauí (Teresina,
Brazil) approved the protocol of the present study under the number
0311.0.045.000-11. All patients signed an informed consent form
prior to enrolment. The study included reproductive-age women aged
>18 years as well as post-menopausal women with no history of
thromboembolic disease, who had been diagnosed with estrogen-
and/or progesterone-positive and Her2-negative invasive carcinoma,
a clinical stage of I–III and who had received adjuvant endocrine
therapy with tamoxifen. Patients with a histological diagnosis of
non-epithelial malignant tumors, those who were pregnant and those
who had taken CYP2D6-inhibitory medication were not included.
Study design
The patients enrolled in the present study were
stratified into two groups of 40 patients each: Group 1 (without
recurrence) and group 2 (with distant recurrence). The patients
received 20 mg tamoxifen orally per day, which was prescribed for 5
years. The groups were considered homogeneous regarding age at
diagnosis, stage of the disease and hormonal status, and different
regarding axillary node status, with a significantly higher rate of
distant recurrence in the group with positive axilla (P=0.006;
Table I).
 | Table I.Characteristics, polymorphism
frequency and metabolizer phenotypes of patients without recurrence
(n=40) and with distant recurrence (n=40) treated with adjuvant
tamoxifen. |
Table I.
Characteristics, polymorphism
frequency and metabolizer phenotypes of patients without recurrence
(n=40) and with distant recurrence (n=40) treated with adjuvant
tamoxifen.
| Characteristics | Without recurrence, n
(%) | Distant recurrence, n
(%) | P-valuea |
|---|
| Age at diagnosis
(years) |
|
| 1.000 |
| Mean | 50.7 | 50.2 |
|
|
Range | 27–85 | 29–75 |
|
| Stage |
|
|
|
| I | 6 (15) | 2 (5) | 0.119 |
| II | 24 (60) | 20 (50) |
|
| III | 10 (25) | 18 (45) |
|
| Axillar lymph node
status |
|
| 0.006 |
|
Positive | 17 (42.5) | 30 (25.0) |
|
|
Negative | 23 (57.5) | 10 (75.0) |
|
| Hormonal status |
|
| 1.000 |
|
Pre-menopausal | 25 (62.5) | 26 (65.0) |
|
|
Post-menopausal | 15 (37.5) | 14 (40.0) |
|
| Alelle |
|
| 0.246 |
|
CYP2D6*1 | 26 (65.0) | 18 (45.0) |
|
|
CYP2D6*4 | 3 (7.5) | 8 (20.0) |
|
|
CYP2D6*10 | 7 (17.5) | 10 (25.0) |
|
|
CYP2D6*17 | 4 (10.0) | 4 (10.0) |
|
| Phenotype |
|
| 0.492 |
| Normal
(homozygous) | 31 (77.5) | 26 (65.0) |
|
| Normal
(heterozygous) | 6 (15.0) | 7 (17.5) |
|
|
Intermediate metabolisers | 2 (5.0) | 6 (15.0) |
|
| Poor
metabolisers | 1 (2.5) | 1 (2.5) |
|
Sample collection and DNA
extraction
From each patient, 3 ml peripheral blood was drawn
and stored in a tube containing anti-coagulant
ethylenediaminetetraacetic acid. The material was stored in a
sterile environment at 20°C. For DNA extraction of leukocytes, the
PureLinkGenomic® DNA Mini kit (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was used according to the manufacturer's
instructions.
Quantitative polymerase chain reaction
(qPCR)
qPCR technique was used for analysis of the
haplotypes (CYP2D6*4, *10 and *17). For allele detection,
TaqMan® probes (Applied Biosystems, Thermo Fisher
Scientific, Inc.) were used, whose sequences were in accordance
with the single nucleotide polymorphisms (SNPs) described in the
database of the National Center for Biotechnology Information
(Table II). These probes had a
fluorophore on one end and a quencher, which is a moiety that
accepts energy from the fluorophore in the form of light and
dissipates it in the form of light or heat, on the other end.
Reaction products were detected by fluorescence generated after
exonuclease activity 5′→3′ of Taq DNA polymerase (16). qPCR reactions were performed in
duplicate in MicroAmp® FastOptical 96-well plates, 0.1
ml (Thermo Fisher Scientific, Inc.). A negative control was
prepared by omission of target DNA. The PCR reaction mixture with a
final volume of 20 µl contained 10 µl 2X TaqMan®
Genotyping Master Mix (Thermo Fisher Scientific, Inc.), 0.5 µl 20X
TaqMan Drug Metabolism SNP Genotyping Assay reagent Mix (Thermo
Fisher Scientific, Inc.), 5.5 µl ultra-pure water free of nucleases
and 4.0 µl target DNA. Thermocycling conditions were as follows:
Initial denaturation for 10 min at 95°C, followed by 50 cycles of
denaturation for 15 sec at 92°C and annealing/extension for 90 sec
at 60°C and post-PCR for 1 min at 60°C. The device used for
analysis was the 7500 Real-Time PCR System with inbuilt SDS 2.2
software for SNP genotyping (Thermo Fisher Scientific, Inc.).
 | Table II.Identification of gene codes and SNPs
used in TaqMan® assays. |
Table II.
Identification of gene codes and SNPs
used in TaqMan® assays.
| Gene code | Product
IDa | SNP NCBI code |
|---|
| CYP2D6*4
g.1846G>A | C_27102431_D0 | rs3892097 |
| CYP2D6*10
g.100C>T | C_11484460_40 | rs1065852 |
| CYP2D6*17
g.1023C>T | C_2222771_40 | rs28371706 |
The results were preliminarily analyzed using the
complementary 7500 software version 2.5 (Applied Biosystems) of the
PCR equipment and subsequently with TaqMan Genotyper software
version 1.3 (Thermo Fisher Scientific, Inc.) for the calculation of
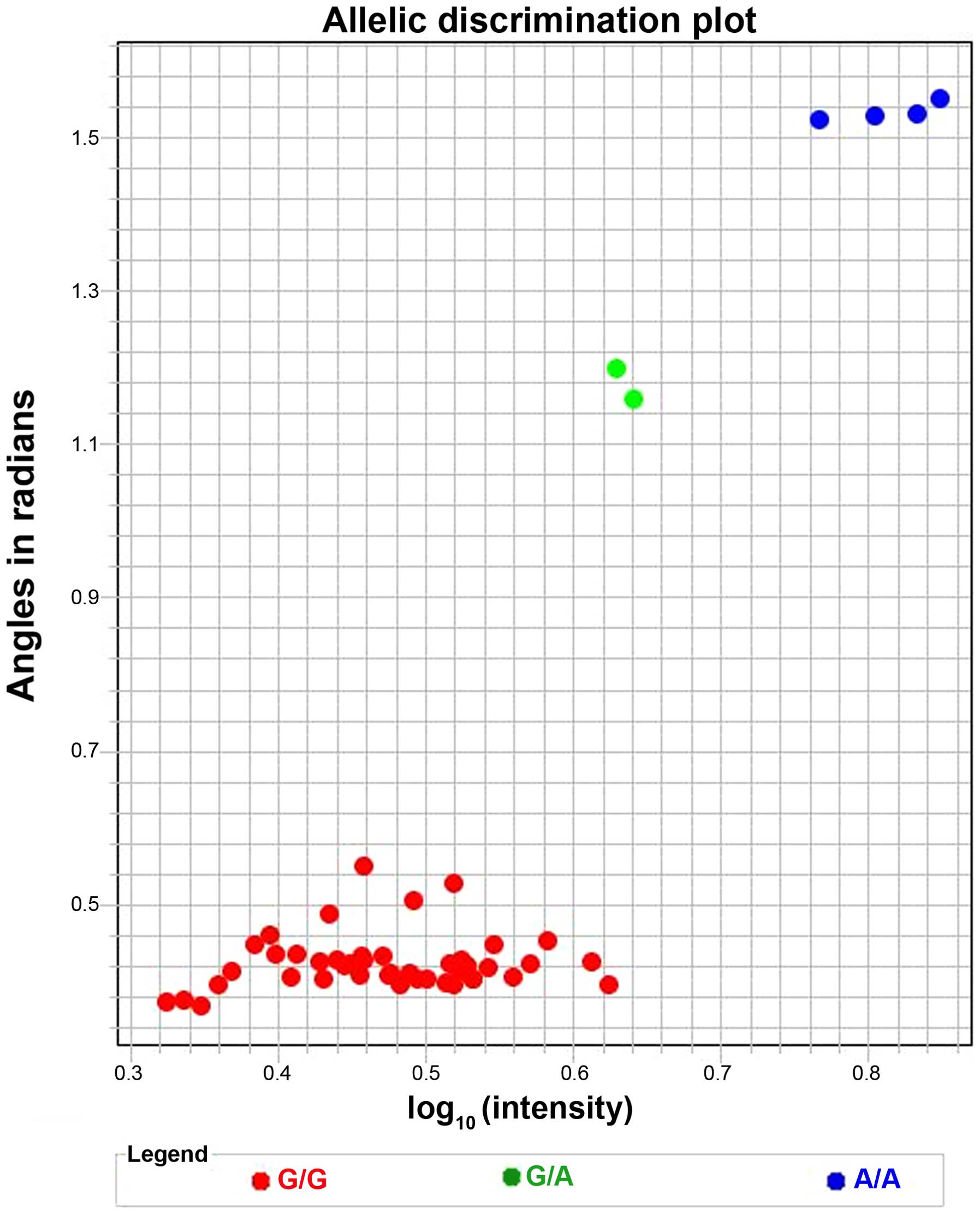
the allelic frequency, as illustrated in Fig. 1 for variant CYP2D6*17.
Statistical analysis
For statistical analysis of the PCR results,
Fisher's exact test was performed using Bioestat 5.3 software
(Mamirauá Institute, Tefé, Brazil) and P<0.05 was considered to
indicate a statistically significant difference between groups.
Results
Allele frequency within the
cohort
Genotyping of the 80 patients (Groups 1 and 2)
showed that the CYP2D6*4, CYP2D6*10 and CYP2D6*17 alleles occurred
in 11 (13.75%), 17 (21.25%) and eight patients (10%), respectively
(Table I).
Frequency of polymorphisms and
phenotype metabolizers
Variant *4 was present in 3 cases (7.5%) in Group 1
and 8 (20%) in Group 2. Furthermore, variant *10 was present in 7
cases (17.5%) from Group 1 and in 10 (25%) from Group 2.
Furthermore, variant *17 was found in 4 patients (10%) from each
group. There was no statistical difference between the groups
regarding the frequency of all polymorphisms, probably due to the
low patient number (P=0.246). Regarding the metabolizer phenotype,
normal metabolizers occurred in 37 (92.5%) and in 33 (82.5%) of
patients from Groups 1 and 2, respectively. IM occurred in 2 (5%)
and 6 (15%) patients from Groups 1 and 2, respectively, while null
or poor metabolizers occurred in 1 individual (2.5%) per group.
There was no significant difference between the two groups
regarding the frequency of all metabolizers, probably due to the
low number of patients (P=0.789; Table
I).
Discussion
Studies on genetic polymorphisms are of great
interest as they enhance the current understanding of the
metabolism of numerous drugs, in particular anticancer drugs
(17). This is particularly valid in
Brazil due to the pronounced ethnic diversity and regional
variability in miscegenation. The most commonly used endocrine
therapy used for chemoprevention and adjuvant breast cancer
treatment is the prodrug tamoxifen. Its efficacy depends on its
metabolisation by the CYP2D6 enzyme into active metabolites, the
major one being endoxifen. The gene encoding for CYP2D6 is highly
polymorphic and the variants show a great diversity among different
genotypes and populations (12).
In the present study, the most common allelic
variant in the 80 patients assessed was the normal variant *1,
followed by variant *10, which occurred in 7 patients from Group 1
and 10 from Group 2. Women in Group 1 (without recurrence) and 2
(with recurrence) showed the same frequency of poor metabolizers,
while patients in Group 2 using tamoxifen showed a higher frequency
of phenotypes of intermediate metabolizers compared to that in
Group 1. However, differences between groups were not statistically
significant, probably due to insufficient sample size.
The higher frequency of allelic variant *10, which
is pre-dominant in Asians but relatively rare in Caucasians
(18), was not expected in the
population from the northeast of Brazil assessed in the present
study, which has a high level of ethnic miscegenation with a
predominance of Afro-Brazilians. The CYP2D6 gene polymorphism
encodes for an unstable protein, which leads to reduced activity of
the CYP2D6 enzyme and is therefore classified as an intermediate
metabolizer phenotype (19–21).
In the present study, no significant association
between phenotypes of intermediate and null metabolizers with
breast cancer recurrence was identified in a population of women
from the Northeast of Brazil with hormone-sensitive breast cancer
treated with tamoxifen. The lack of significance may be due to the
small patient cohort. However, the results of the present study did
not show any influence of the CYP2D6 gene polymorphisms *4, *10 and
*17 on the recurrence of breast cancer within the cohort. Of note,
patients in Group 2 had a significantly higher frequency of
axillary metastases than those in Group 1 and the lymph node status
is a important prognostic factor and predictive of relapse.
Although certain studies have shown that patients with the CYP2D6
gene polymorphisms *4, *10 and *17 have more aggressive and
tamoxifen-resistant hormone-sensitive breast cancer of a recurring
nature, a lower overall survival and a lack of efficacy of
tamoxifen treatment (11,14,22), other
studies have not shown any significant effect of CYP2D6 genotype
variants on the risk of breast cancer recurrence in patients
receiving adjuvant tamoxifen (10,13), which
is in agreement with the results of the present study. However,
further studies with a larger sample size are required to identify
a possible association of CYP2D6 gene polymorphisms with relapse of
hormone-sensitive breast cancer in patients treated with adjuvant
tamoxifen.
In conclusion, the present study showed that in a
Northeast Brazilian population of hormone-sensitive breast cancer
patients treated with tamoxifen, the CYP2D6*4, *10 and *17 gene
polymorphisms were not significantly associated with disease
recurrence. However, further studies using a larger
population-based samples of patients with hormone-sensitive breast
cancer should be performed to elucidate of the role of CYP2D6 gene
polymorphism in the response to tamoxifen.
Acknowledgements
The authors would like to thank the Department of
Mastology (Getulio Vargas Hospital, the Federal University of
Piauí, Teresina, Brazil) and the Department of Oncology (Sao Marcos
Hospital Cancer Center, Teresina, Brazil) for their support in
performing the present study.
References
|
1
|
Smith RA, Cokkinides V, Brooks D, Saslow D
and Brawley OW: Cancer screening in the United States, 2010: A
review of current American Cancer Society guidelines and issues in
cancer screening. CA Cancer J Clin. 60:99–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International Agency for Cancer Research,
. Estimated Incidence, Mortality and Prevalence Worldwide in 2012.
http://globocan.iarc.fr/old/FactSheets/cancers/breast-new.aspAccessed.
February 16–2016
|
|
3
|
Parkin DM and Fernández LM: Use of
statistics to assess the global burden of breast cancer. Breast J.
12(Suppl 1): S70–S80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jankowitz RC, McGuire KP and Davidson NE:
Optimal systemic therapy for premenopausal women with hormone
receptor-positive breast cancer. Breast. 22(Suppl 2): S165–S170.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Montemurro F, Del Mastro L, De Laurentiis
M and Puglisi F: Endocrine therapy in premenopausal women with
breast cancer: A critical appraisal of current evidence. Expert Rev
Anticancer Ther. 16:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Howlader N, Chen VW, Ries LA, Loch MM, Lee
R, DeSantis C, Lin CC, Ruhl J and Cronin KA: Overview of breast
cancer collaborative stage data items - their definitions, quality,
usage, and clinical implications: A review of SEER data for
2004–2010. Cancer. 120(Suppl 23): 3771–3780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoskins JM, Carey LA and McLeod HL: CYP2D6
and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer.
9:576–586. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motamedi S, Majidzadeh K, Mazaheri M,
Anbiaie R, Mortazavizadeh SM and Esmaeili R: Tamoxifen resistance
and CYP2D6 copy numbers in breast cancer patients. Asian Pac J
Cancer Prev. 13:6101–6104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiyotani K, Mushiroda T, Sasa M, Bando Y,
Sumitomo I, Hosono N, Kubo M, Nakamura Y and Zembutsu H: Impact of
CYP2D6*10 on recurrence-free survival in breast cancer patients
receiving adjuvant tamoxifen therapy. Cancer Sci. 99:995–999. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morrow PK, Serna R, Broglio K, Pusztai L,
Nikoloff DM, Hillman GR, Fontecha M, Li R, Michaud L, Hortobagyi G,
et al: Effect of CYP2D6 polymorphisms on breast cancer recurrence.
Cancer. 118:1221–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goetz MP, Knox SK, Suman VJ, Rae JM,
Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL,
et al: The impact of cytochrome P450 2D6 metabolism in women
receiving adjuvant tamoxifen. Breast Cancer Res Treat. 101:113–121.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Schaik RH: Cancer treatment and
pharmacogenetics of cytochrome P450 enzymes. Invest New Drugs.
23:513–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martins DM, Vidal FC, Souza RD, Brusaca SA
and Brito LM: Determination of CYP2D6 *3, *4, and *10 frequency in
women with breast cancer in São Luís, Brazil, and its association
with prognostic factors and disease-free survival. Braz J Med Biol
Res. 47:1008–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schroth W, Antoniadou L, Fritz P, Schwab
M, Muerdter T, Zanger UM, Simon W, Eichelbaum M and Brauch H:
Breast cancer treatment outcome with adjuvant tamoxifen relative to
patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 25:5187–5193.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karle J, Bolbrinker J, Vogl S, Kreutz R,
Denkert C, Eucker J, Wischnewsky M, Possinger K and Regierer AC:
Influence of CYP2D6-genotype on tamoxifen efficacy in advanced
breast cancer. Breast Cancer Res Treat. 139:553–560. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De la Vega FM, Lazaruk KD, Rhodes MD and
Wenz MH: Assessment of two flexible and compatible SNP genotyping
platforms: TaqMan SNP Genotyping Assays and the SNPlex Genotyping
System. Mutat Res. 573:111–135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abraham JE, Maranian MJ, Driver KE, Platte
R, Kalmyrzaev B, Baynes C, Luccarini C, Shah M, Ingle S, Greenberg
D, et al: CYP2D6 gene variants: Association with breast cancer
specific survival in a cohort of breast cancer patients from the
United Kingdom treated with adjuvant tamoxifen. Breast Cancer Res.
12:R642010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ingelman-Sundberg M: Genetic polymorphisms
of cytochrome P450 2D6 (CYP2D6): Clinical consequences,
evolutionary aspects and functional diversity. Pharmacogenomics J.
5:6–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukuda T, Yamamoto I, Nishida Y, Zhou Q,
Ohno M, Takada K and Azuma J: Effect of the CYP2D6*10 genotype on
venlafaxine pharmacokinetics in healthy adult volunteers. Br J Clin
Pharmacol. 47:450–453. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goetz MP, Kamal A and Ames MM: Tamoxifen
pharmacogenomics: The role of CYP2D6 as a predictor of drug
response. Clin Pharmacol Ther. 83:160–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toyama T, Yamashita H, Sugiura H, Kondo N,
Iwase H and Fujii Y: No association between CYP2D6*10 genotype and
survival of node-negative Japanese breast cancer patients receiving
adjuvant tamoxifen treatment. Jpn J Clin Oncol. 39:651–656. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Irvin WJ Jr, Walko CM, Weck KE, Ibrahim
JG, Chiu WK, Dees EC, Moore SG, Olajide OA, Graham ML, Canale ST,
et al: Genotype-guided tamoxifen dosing increases active metabolite
exposure in women with reduced CYP2D6 metabolism: A multicenter
study. J Clin Oncol. 29:3232–3239. 2011. View Article : Google Scholar : PubMed/NCBI
|