Introduction
Industrially synthesized polyphosphate (sodium
polyphosphate) is a complex, glassy material of approximate
composition (NaPO3)x, where x≥20, but unknown
constitution. It is produced by the fusion and rapid quenching of
monosodium orthophosphate in accordance with the following
reaction: n(NaH2PO4) →
(NaPO3)n + nH2O (1,2).
Polyphosphates are widely distributed in nature and are present in
archaebacteria, eubacteria, fungi, algae and protozoa, as well as
higher plants and animals (3,4). However, as it has no known function,
polyphosphate has previously been dismissed as a ‘molecular fossil’
(1). Recently, however, studies have
indicated that long chain polyphosphate (>45 phosphate units)
may induce thrombosis and infection, linked with the activation of
coagulation factor XII (FXII), via a bradykinin-dependent mechanism
(5–7).
However, Faxälv et al found that polyphosphate released from
platelets did not activate FXII (5),
and the relationship between synthetic polyphosphate, platelets and
coagulation remains unclear. Therefore, the present study explored
the effect of industrially synthesized polyphosphate on hemostasis
and coagulation in a clinical laboratory setting and investigated
the potential underlying mechanisms.
Materials and methods
Primary reagents and instruments
Synthetic polyphosphate (marketed as ‘phosphate
glass, water’ or polyP), 4′,6-diamidino-2-phenylindole
dihydrochloride (DAPI), Fluo-3 and adenosine diphosphate (ADP) were
purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Other reagents were purchased from Aladdin (Shanghai, China) unless
otherwise stated. To reduce the levels of very short polymers, the
synthetic polyphosphate (1 g) was dissolved twice in 10 ml purified
water in a 10-ml tube and then resuspended in 10 ml 250 mM LiCl
(6). The supernatant was considered as
synthetic polyphosphate solution, and the residue was removed via
centrifugation (5,000 × g for 10 min) (6). The synthetic polyphosphate solution was
stored at 4°C.
Venous blood samples were collected by health worker
volunteers. For each sample, 3 ml blood was collected into a
citrate anticoagulation tube, and platelet-poor plasma (PPP) was
extracted within 30 min. The blood was centrifuged at 1,000 × g for
10 min, and the upper 1 ml, comprising the platelet-rich plasma
(PRP), was collected. The PRP was centrifuged at 2,000 × g for 10
min, and the upper 300 µl was retained as PPP. All study
participants gave informed, signed consent, and the study was
approved by the Institutional Ethics Committee of Xiangya Hospital,
Central South University (Changsha, China). The study was carried
out in accordance with the Code of Ethics of the World Medical
Association (Declaration of Helsinki) for experiments involving
humans. The blood samples were collected in 1:9 sodium citrate
human venous blood specimen collection containers for clinical
testing (Shandong Weigao Group Medical Polymer Co., Ltd., Weihai,
China). All of the products and instruments as well as the methods
of platelet aggregation, coagulation routines, thromboelastograms,
and clotting factor activity tests were appropriate for clinical
assessment.
Evaluation of the effects of synthetic
polyphosphate using routine coagulation tests
Different quantities (0, 5, 10, 20 and 40 µl) of
synthetic polyphosphate in LiCl (250 mM) were added to 3 ml whole
blood, and a routine coagulation test was carried out. This test
measured the prothrombin time (PT), the international normalized
ratio (INR), fibrinogen level (FIB), the activated partial
thromboplastin time (APTT), D-dimer (DD) level and the thrombin
time (TT) using a high-throughput hemostasis analyzer (Destiny Max;
Trinity Biotech Plc., Bray, Ireland). The assay kits were purchased
from Beijing Biochem Medical Technology Co., Ltd. (Beijing, China).
All analyses were based on disseminated intravascular coagulation
(DIC) diagnostic criteria (8). All of
the experiments were performed in triplicate.
Effect of synthetic polyphosphate on
clotting factor activity
Different quantities (0, 10, 20 and 40 µl) of
synthetic polyphosphate in LiCl (250 mM) were added to 3 ml whole
blood, and the activities of factor VIII (FVIII), factor IX (FIX),
factor XI (FXI) and FXII were detected using Siemens Healthcare
Diagnostics products (item ids: OTXW17, OTXX17, OSDF13 and OSDG13;
Siemens Healthcare AG, Munich, Germany) in a high-throughput
hemostasis analyzer. Coagulation factor-deficient plasma, provided
with the diagnostic assay kits, was used to confirm factor activity
and to identify and quantify the activity of coagulation factors in
the treated plasma. A mixture of the respective factor-deficient
plasma and the treated plasma was tested by APTT assay. All
experiments were performed in triplicate.
Evaluation of the effects of synthetic
polyphosphate using thromboelastography (TEG)
Different quantities (20, 40 and 80 µl) of
polyphosphate were added to 3 ml whole blood in a citrated tube,
and all TEG analyses were performed with a kaolin and
CaCl2 active assay kit and TEG instrument (CFMS™; Lepu
Medical Technology Co., Ltd., Beijing, China) (9). All experiments were performed in
triplicate, and 250 mM LiCl was used as a control treatment. The
following variables were determined: Reaction time (R); time taken
to reach 20 mm amplitude (K); angle degree; maximum amplitude (MA);
shear elastic simulation strength (G); time to maximum amplitude
(TMA); coagulation index (CI); lysis time estimate (LTE); estimated
percent lysis (EPL); and percentage of clot lysed after 30 min
(LY30).
Effect of synthetic polyphosphate on
platelet aggregation
Aggregation tests were performed via the turbidity
method using PRP with a TYXN-96 multi-functional intelligent blood
condensate meter (Shanghai General Electromechanical Technology
Research Institute, Shanghai, China). A total of 200 µl PRP was
tested for aggregation. A 10 µl aliquot of 250 mM LiCl with one of
several platelet agonists was used for control group treatments.
Platelet aggregation was induced using 10 µmol/l ADP, 10 µmol/l
epinephrine, 1.0 mmol/l arachidonic acid (AA), 1.5 mg/ml
ristocetin, 0.769 U/ml thrombin (Hunan Yige Pharmaceutical Co.,
Ltd., Dalian China), 1.304 U/ml oxytocin (Shanghai Harvest
Pharmaceutical Co., Ltd., Shanghai, China) or 0.291 U/ml pituitrin
(Anhui Hongye Pharmaceuticals Co., Ltd., Bengbu, China). Each
agonist was applied alone, or in combination with synthetic
polyphosphate (6 or 10 µl), and the aggregation rate was
determined.
Platelet Ca2+ evaluation
via flow cytometry
A total of 20 µl PRP, treated using 10 µmol/l ADP as
described in the platelet aggregation assay, was diluted 1:10 in
modified Tyrode's solution. Platelet Ca2+ concentrations
were then determined as previously described (10), with the modification that the platelets
were labeled with CD61-PerCP (BD Biosciences, Franklin Lakes, NJ,
USA), not CD41. The CD61-PerCP-labeled platelets and Fluo3-chelated
calcium concentrations in the platelets were detected using a BD
FACSAria III cell sorter (BD Biosciences). All experiments were
performed in triplicate.
Platelet Ca2+ evaluation
via imaging
A total of 10 µl PRP, treated with 10 µmol/l ADP as
described above, was placed in 96-well plates (PerkinElmer, Inc.,
Waltham, MA, USA) and dyed with CD61-PerCP and Fluo-3 for 20 min
prior to 1:10 dilution with modified Tyrode's buffer. The plate was
centrifuged at 13,000 × g for 5 min (Centrifuge 5804R; Eppendorf,
Hamburg, Germany) and immediately analyzed using the 40x long
working distance objective of a high-throughput screening system
(Operetta; PerkinElmer, Inc.). The system was equipped with a
460–490 nm excitation filter, a 500–530 nm long-pass (LP) emission
filter for Fluo-3, and a 650–690 nm LP emission filter for
CD61-PerCP. All experiments were performed in triplicate.
Thromboxane B2 (TXB2) measurement
To assess thromboxane A2 (TXA2) levels, the stable
metabolite TXB2 was analyzed using a commercial ELISA kit (R&D
Systems, Inc., Minneapolis, MN, USA) (11). Briefly, PRP, treated with 10 µmol/l ADP
as described above, was quenched for 5 min with 5 mM
ethylenediamine tetra-acetic acid and 200 µM indomethacin to
inhibit further TXA2 formation. The samples were centrifuged for 4
min at 12,000 × g. The supernatant was removed and stored at −80°C
for subsequent TXB2 analysis using an Infinite M200pro NanoQuant
plate reader (Tecan, Männedorf, Switzerland).
Statistical analysis
The data were analyzed with SAS 8.1 (SAS Institute,
Cary, NC, USA), and P<0.05 was considered to indicate a
statistically significant difference. The data were analyzed using
nonparametric analysis of variance (ANOVA) based on the
heterogeneity of variance, paired t-test or one-way ANOVA.
Results
Synthetic polyphosphate inhibits
coagulation
The addition of 250 mM LiCl did not impact routine
coagulation and was used as the control group treatment. Since FIB,
DD and PT showed no significant changes from the control values
when polyphosphate was added, this indicated that synthetic
polyphosphate did not significantly affect the exogenous
coagulation pathway and fibrinolysis. However, APTT, representing
endogenous coagulation, increased significantly following the
addition of synthetic polyphosphate (F=51.00, P<0.0001; Table I). As the half-life of synthetic
polyphosphate is ~2 h (6), the whole
blood samples were allowed to stand for 4 h following the addition
of 5, 10 or 20 µl polyphosphate in LiCl, or LiCl alone, prior to
APTT testing. The APTT changed little over 4 h in the LiCl group,
whereas the APTT was significantly increased in the synthetic
polyphosphate groups (F=15.25, P=0.0298; Table II).
 | Table I.Routine coagulation test results
following the addition of synthetic polyphosphate (polyP). |
Table I.
Routine coagulation test results
following the addition of synthetic polyphosphate (polyP).
| Parameters | Control | 5 µl polyP | 10 µl polyP | 20 µl polyP | 40 µl polyP | Reference range |
|---|
| PT, sec |
11.72 |
12.40 |
12.10 |
13.20 |
13.90 | 10.0–16.0 |
| PT% | 122.73 | 114.77 | 119.32 | 105.68 |
97.73 |
70–140 |
| INR |
0.91 |
0.97 |
0.93 |
1.03 |
1.08 |
0.8–1.2 |
| APTT, sec |
37.30 |
50.90 |
60.50 |
83.63 | >180.0 |
25.0–43.0 |
| TT, sec |
19.50 |
19.60 | 18.9 | 24.5 | 26.9 |
14.0–21.0 |
| FIB, g/l |
2.17 |
3.19 |
3.41 |
2.01 |
1.98 |
2.0–4.0 |
| DD, mg/l |
0.48 |
0.46 |
0.16 |
0.15 |
0.07 | 0–0.5 |
 | Table II.APTT test results at 0 and 4 h after
the addition of synthetic polyphosphate (polyP). |
Table II.
APTT test results at 0 and 4 h after
the addition of synthetic polyphosphate (polyP).
|
| APTT (sec) |
|---|
|
|
|
|---|
| Group | 0 h | 4 h | Reference range |
|---|
| Control | 37.10 | 35.10 | 25.0–43.0 |
| 5 µl polyP | 50.90 | 37.30 |
|
| 10 µl polyP | 66.90 | 54.10 |
|
| 20 µl polyP | 80.10 | 63.00 |
|
Synthetic polyphosphate inhibits
intrinsic coagulation factor activity
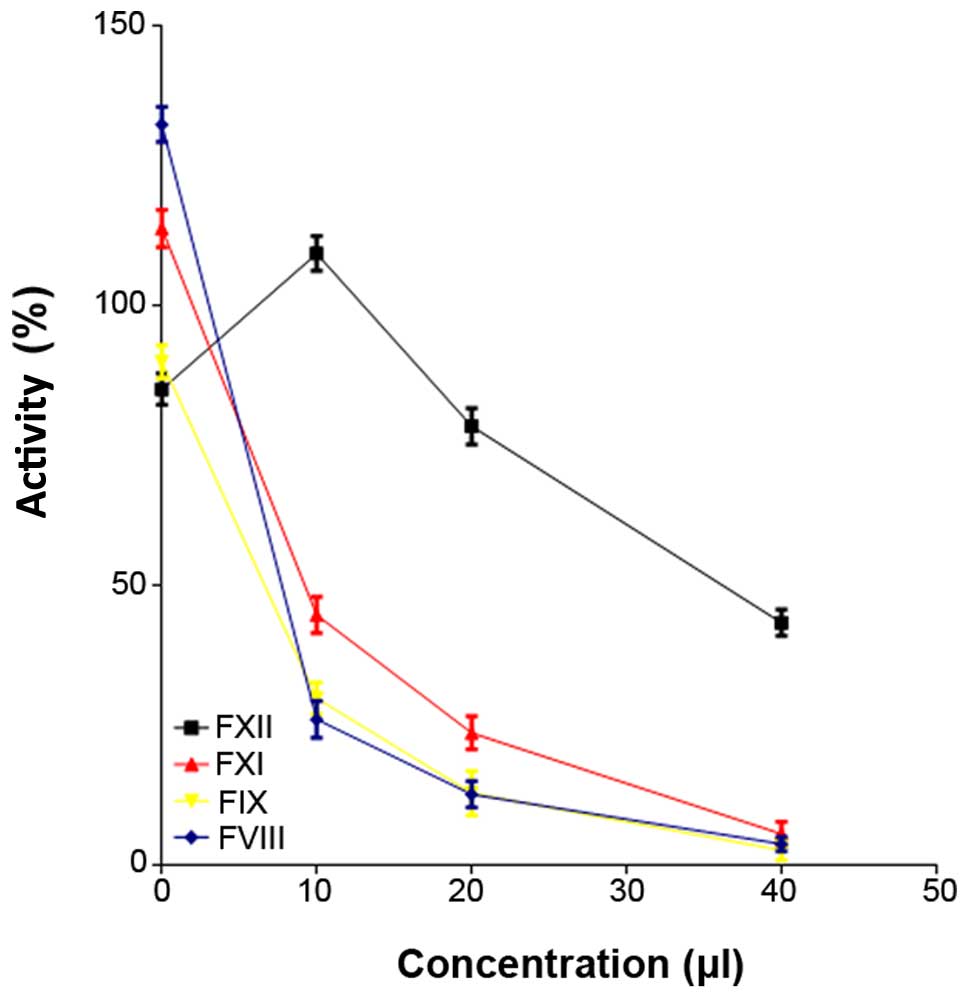
The FVIII (F=3908.37, P=0.0001), FIX (F=1590.24,
P<0.0001) and FXI (F=542.24, P<0.0001) activities were
decreased following the addition of polyphosphate (Fig. 1 and Table
III), whereas LiCl (control) did not affect coagulation factor
activity (F=50.02, P=0.9971). A 10-µl volume of synthetic
polyphosphate increased FXII activity, but the FXII activity
remained within the normal range. Furthermore, FXII activity
declined as the concentration of polyphosphate increased (F=489.10,
P<0.0001). Overall, the synthetic polyphosphate inhibited
clotting factor activity and blood coagulation, as assessed using
conventional coagulation tests.
 | Table III.Activity of coagulation factors FXII,
FXI, FVIII and FIV declined with increasing concentrations of
synthetic polyphosphate (polyP). |
Table III.
Activity of coagulation factors FXII,
FXI, FVIII and FIV declined with increasing concentrations of
synthetic polyphosphate (polyP).
| Group | FVIII (%) | FIX (%) | FXI (%) | FXII (%) |
|---|
| Control | 132.5 | 90 | 113.9 | 85.1 |
| 10 µl polyP | 26 | 29.8 | 44.7 | 109.4 |
| 20 µl polyP | 12.6 | 12.8 | 23.6 | 78.5 |
| 40 µl polyP |
3.7 |
2.6 |
5.5 |
42.2 |
| Reference
range | 60–150 | 60–120 | 70–120 | 70–150 |
TEG confirms that synthetic
polyphosphate inhibits blood clotting
LiCl did not affect TEG and was therefore used as a
control treatment. As the concentration of synthetic polyphosphate
increased, R (F=3030.29, P<0.0001) and TMA (F=3180.50,
P<0.0001) increased but the MA (F=115.78, P<0.0001), G value
(F=159.55, P<0.0001) and CI (F=3581.71, P<0.0001) decreased.
These results indicate that polyphosphate inhibited coagulation.
Thromboelastograms indicated that polyphosphate decreased
significantly clotting factor activity and platelet function, since
the angle degree decreased (F=455.28, P<0.0001) and the K value
increased (F=285.50, P<0.0001). However, when 20 µl
polyphosphate was added to 3 ml whole blood, the APTT for routine
coagulation was approximately twice the normal value, and was
significantly abnormal (P=0.0026). Furthermore, at this time, the
R, TMA, MA, G value and CI obtained by TEG, representing the
clotting factor activity or platelet function, and EPL and LY30,
representing the fibrinolytic system, were in the normal range
(Table IV). The LTE is an estimate of
the clot lysis time. However, the results indicate that LTE more
accurately predicts decreased clotting factor activity and platelet
function. When 20 µl polyphosphate was added to 3 ml whole blood,
the R and MA remained in the normal range, and at this time the LTE
increased from 32.8 to 127.2 min, which far exceeded the normal
value. When 80 µl polyphosphate was added to 3 ml whole blood, TEG
was not able to provide results for LTE, EPL and LY30; this may be
due to synthetic polyphosphate severely inhibiting blood
coagulation by depressing the activity of platelets and coagulation
factors, so that neither blood coagulation nor fibrinolysis
occurred. The fibrinolytic system might not necessarily be
abnormal. Thus, the routine coagulation test is more accurate for
the detection of coagulation and fibrinolysis than
thromboelastograms.
 | Table IV.Thromboelastography results. |
Table IV.
Thromboelastography results.
| Group | R (min) | K (min) | Angle degree | MA (mm) | G (K) | TMA (min) | CI | LTE (min) | EPL (%) | LY30 (%) |
|---|
| Control |
5.8 |
1.7 |
63.4 |
64.7 |
9.2 |
27.1 |
0.5 |
32.8 | 0 | 0 |
| 20 µl polyP |
6.8 |
2.1 |
61.5 |
57.9 |
6.9 |
28.2 | −1.2 | 127.2 |
0.8 |
0.8 |
| 40 µl polyP |
9.8 |
2.6 |
54.5 |
58.6 |
7.1 |
32.1 |
−3.4 | >3 h | 0 | 0 |
| 80 µl polyP |
10.9 |
3.3 |
47.1 |
56.7 |
6.5 |
39.2 |
−5.6 | >3 h | No result | No result |
| Reference
range | 2–8 | 1–3 | 56–69 | 51–69 |
4.6–10.9 | – | −3 to +3 | – | 0–15 | 0–8 |
Synthetic polyphosphate inhibits
platelet aggregation
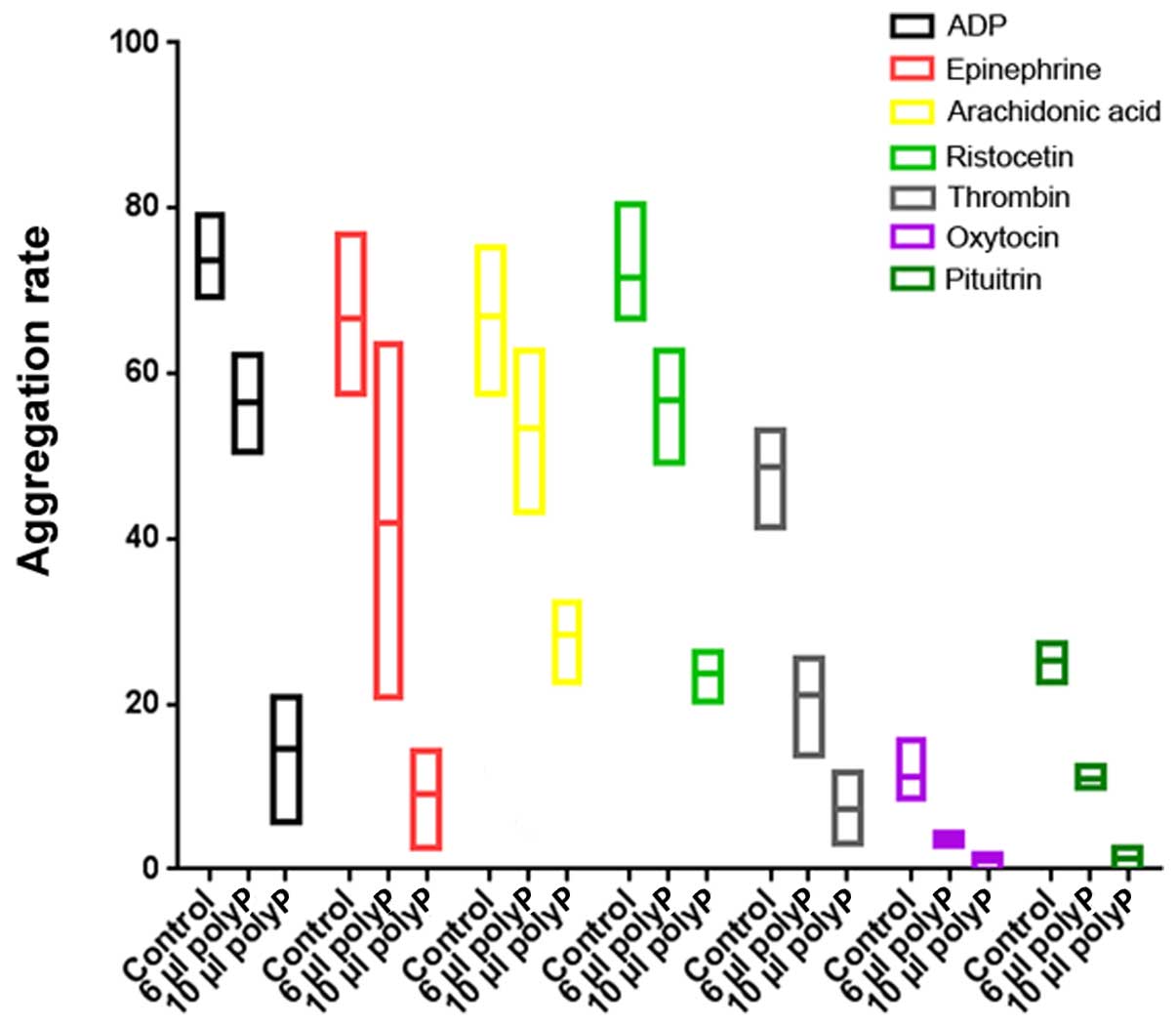
Synthetic polyphosphate inhibited ADP (F=72.51,
P<0.0001)-, platelet epinephrine (F=13.74, P=0.0058)-, AA
(F=19.19, P=0.0025)-, ristocetin (F=66.16, P<0.0001)-, thrombin
(F=41.59, P=0.0003)-, oxytocin (F=30.61, P=0.0007)- and pituitrin
(F=202.14, P<0.0001)-induced platelet aggregation. As the
concentration of polyphosphate increased, suppression was increased
(Fig. 2).
Synthetic polyphosphate reduces
calcium concentrations with platelet aggregation
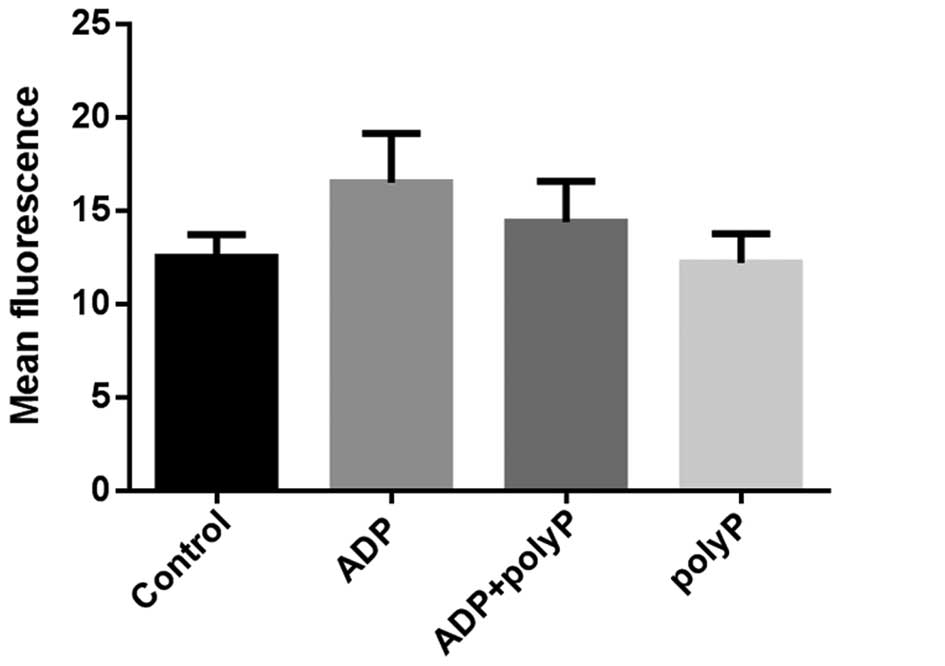
The mean fluorescence intensity of platelet calcium
in the ADP group was higher than that in the control group
(16.49±2.65 vs. 12.48±1.23; P=0.0135). When 10 µl synthetic
polyphosphate and ADP were both applied, the mean calcium
fluorescence intensity decreased to 14.39±2.18 (P=0.0031); however,
there was no significant difference between the control group and
the polyphosphate group (12.48±1.23 vs. 12.19±1.57; P=0.4462;
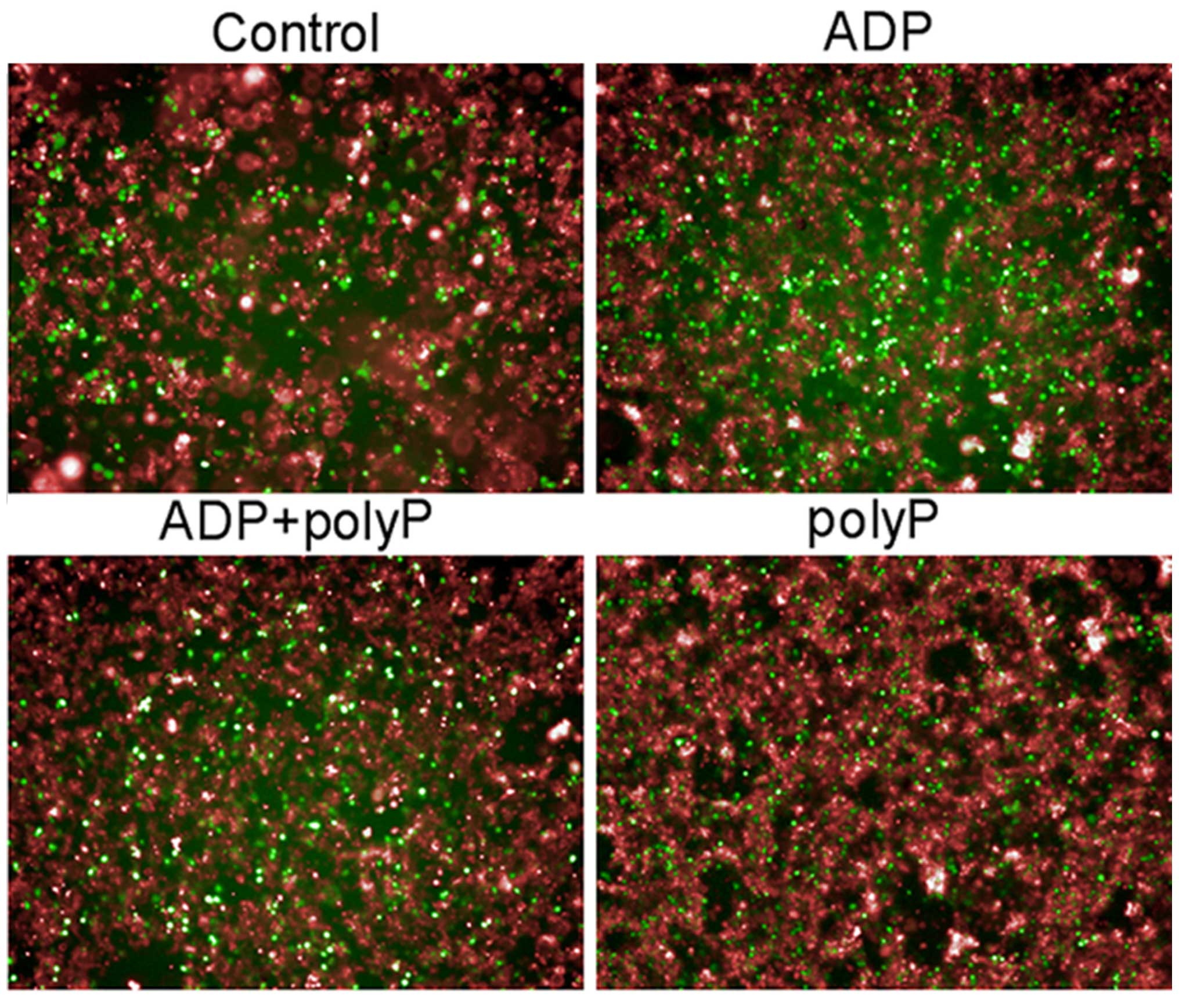
Fig. 3). The same pattern of results
was obtained via high-throughput imaging (Fig. 4). Thus, it appears that synthetic
polyphosphate inhibits platelet aggregation and activation by
reducing calcium levels.
Synthetic polyphosphate inhibits
TXA2
Treatment of platelets with polyphosphate
significantly decreased TXB2 levels compared with those in
untreated platelets (1.416±0.248 vs. 0.409±0.464; P<0.0001). By
contrast, ADP increased TXB2 levels, and the ADP-induced increases
were attenuated by polyphosphate (1.701±0.367 vs. 1.146±0.235;
P<0.0001).
Discussion
Previous studies have revealed that platelet-derived
polyphosphate promotes blood coagulation (5,6). However,
the effects obtained when adding synthetic polyphosphate have been
conflicting. In the present study, endogenous clotting factor
(i.e., FXII, FVIII, FIV and FXI) activity was inhibited following
the addition of synthetic polyphosphate. APTT, which represents the
endogenous coagulation time and is used to guide clinical
treatment, was prolonged and higher than the diagnostic criteria of
DIC (12). The effects on fibrinogen
and DD were small, with the results varying within the normal
range. The changes in routine coagulation caused by synthetic
polyphosphate gradually weakened as the polyphosphate underwent
degradation for 4 h, as assessed in the routine coagulation test of
the present study (F=15.25, P=0.0298). Synthetic polyphosphate
turnover is dynamic; after the polyphosphate enters the plasma,
changes in its integrity, size and concentration occur (13). In addition, its half-life in plasma is
1.5–2 h (14). Therefore, it is
difficult to accurately measure the concentration of synthetic
polyphosphate with linked phosphate groups (13).
LTE, an estimate of clot lysis time, may predict
platelet function more accurately than fibrinolysis, as determined
by comparing thromboelastograms, conventional coagulation times and
reports from the literature. Thromboelastograms may not be accurate
for predicting fibrinolysis. Routine coagulation tests exhibit
difficulty in detecting the effects of higher concentrations of
synthetic polyphosphate, and thromboelastograms exclusively detect
abnormalities. However, at lower concentrations, routine
coagulation tests are more accurate than thromboelastograms.
Therefore, TEG may accurately predict severe, life-threatening
coagulopathy, while routine coagulation tests perform better than
TEG when predicting small changes in coagulopathy (15–17).
Synthetic polyphosphate and platelet-derived
polyphosphate have different effects on coagulation. One possible
reason for this is that these two phosphates are have different
chemical structures. The polyphosphate anion is able to bind
calcium ions (2). The mechanism of the
reaction between calcium ions and sodium polyphosphate is not
thoroughly understood but is considered to be as follows:
(NaPO3)n → 2Na+ +
Nan-2 (PO3)2−
xCa2+ + Nan-2
(PO3)n2− → 2xNa+ +
Nan-(2+2x)Cax
(PO3)n2−
The synthetic polyphosphate used in the present
study, and polyphosphate derived from cells have different
structures, which may affect their functions. The polyphosphate in
platelets or cells is calcium-saturated polyphosphate (calcium
polyphosphate). The synthetic polyphosphate used in the present
study was sodium polyphosphate. Sodium polyphosphate can bind to
calcium in plasma and inhibit coagulation and the activity of
coagulation factors. Calcium polyphosphate cannot bind calcium ions
in plasma, and accelerates coagulation by activating factor V or
factor XI (5).
Platelet aggregation tests in plasma are
calcium-free; therefore synthetic polyphosphate could not bind
calcium in the plasma. Therefore, the mechanisms by which synthetic
sodium polyphosphate inhibited platelet aggregation were
investigated in the present study, and it was found that the
synthetic polyphosphate inhibited both calcium and TXA2.
TXA2 is produced by activated platelets and has
prothrombotic properties. It also stimulates the activation of new
platelets and increases platelet aggregation. TXA2 is unstable in
aqueous solution and is hydrolyzed within ~30 sec to the
biologically inactive TXB2 (18). In
human studies, TXB2 levels are used to indirectly measure TXA2
production (19). When clotting begins
in the body, calcium mobilization occurs in the dense tubular
system. Increased intracellular calcium is associated with the
activation of several kinases that are necessary for morphological
changes, presentation of the procoagulant surface, secretion of
platelet granules, activation of glycoproteins, and activation of
phospholipase A2 (PLA2). PLA2 activation releases AA, a TBXA2
precursor, with prostaglandin G/H synthase 1 catalyzing the first
step in the formation of TBXA2 from AA. These processes result in
the local accumulation of molecules such as thrombin, TXA2 and ADP,
which are important for further platelet aggregation (20). The results of the TXA2 assay conducted
in the present study indicate that this reaction was blocked by
synthetic polyphosphate, thereby reducing platelet aggregation.
In conclusion, synthetic polyphosphate inhibits
endogenous coagulation and platelet aggregation in vitro.
The effects of synthetic polyphosphate on coagulation are different
from those of platelet-derived polyphosphate. Synthetic
polyphosphate may be a potential drug for the prevention or
treatment of thrombosis. Obviously, whether the complex compound
binds with calcium or not, or changes in concentration, may induce
different effects or cause damage to the body. Further
investigation of the role of synthetic polyphosphate in the body is
necessary in future studies.
Acknowledgements
The authors would like to express their warmest
gratitude to all of the nurses of Xiangya Hospital and Xiangya
Third Hospital of Central South University as well as all their
colleagues in the clinical testing laboratory of Xiangya Hospital
and Xiangya Third Hospital of Central South University.
References
|
1
|
Brown MR and Kornberg A: Inorganic
polyphosphate in the origin and survival of species. Proc Natl Acad
Sci USA. 101:16085–16087. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartz C, Jones KK, Mack TW and Vance
RW: The use of sodium metaphosphate for the preparation of
soft-curd milk. J Dairy Sci. 23:19–35. 1940. View Article : Google Scholar
|
|
3
|
Wiame JM: Yeast metaphosphate. Fed Proc.
6:3021947.PubMed/NCBI
|
|
4
|
Jimenez-Nuñez MD, Moreno-Sanchez D,
Hernandez-Ruiz L, Benítez-Rondán A, Ramos-Amaya A, Rodríguez-Bayona
B, Medina F, Brieva JA and Ruiz FA: Myeloma cells contain high
levels of inorganic polyphosphate which is associated with
nucleolar transcription. Haematologica. 97:1264–1271. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Faxälv L, Boknäs N, Ström JO, Tengvall P,
Theodorsson E, Ramström S and Lindahl TL: Putting polyphosphates to
the test: evidence against platelet-induced activation of factor
XII. Blood. 122:3818–3824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith SA, Choi SH, Davis-Harrison R, Huyck
J, Boettcher J, Rienstra CM and Morrissey JH: Polyphosphate exerts
differential effects on blood clotting, depending on polymer size.
Blood. 116:4353–4359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Wan M, Yang K and Chen F: Long
chain polyphosphates identified in infectious fever patients in the
department of hematology. Acta Med Mediterr. 32:377–383. 2016.
|
|
8
|
Levi M, Toh CH, Thachil J and Watson HG:
British Committee for Standards in Haematology: Guidelines for the
diagnosis and management of disseminated intravascular coagulation.
Br J Haematol. 145:24–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kol A and Borjesson DL: Application of
thrombelastography/thromboelastometry to veterinary medicine. Vet
Clin Pathol. 39:405–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
do Céu Monteiro M, Sansonetty F, Gonçalves
MJ and O'Connor JE: Flow cytometric kinetic assay of calcium
mobilization in whole blood platelets using Fluo-3 and CD41.
Cytometry. 35:302–310. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walsh TG, Harper MT and Poole AW: SDF-1α
is a novel autocrine activator of platelets operating through its
receptor CXCR4. Cell Signal. 27:37–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wada H, Matsumoto T and Yamashita Y:
Diagnosis and treatment of disseminated intravascular coagulation
(DIC) according to four DIC guidelines. J Intensive Care. 2:152014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lorenz B and Schröder HC: Methods for
investigation of inorganic polyphosphates and
polyphosphate-metabolizing enzymes. Prog Mol Subcell Biol.
23:217–239. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith SA, Mutch NJ, Baskar D, Rohloff P,
Docampo R and Morrissey JH: Polyphosphate modulates blood
coagulation and fibrinolysis. Proc Natl Acad Sci USA. 103:903–908.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katz-Summercorn AC, Cuffolo G, Hossain MA
and Wilde M: The use of rapid thromboelastogram for trauma
mortality prediction. Am J Surg. 208:3162014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanriverdi S, Koroglu OA, Uygur O, Balkan
C, Yalaz M and Kultursay N: The effect of inhaled nitric oxide
therapy on thromboelastogram in newborns with persistent pulmonary
hypertension. Eur J Pediatr. 173:1381–1385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valeri CR and Ragno G: In vitro testing of
platelets using the thromboelastogram, platelet function analyzer,
and the clot signature analyzer to predict the bleeding time.
Transfus Apheresis Sci. 35:33–41. 2006. View Article : Google Scholar
|
|
18
|
Fontana P, Zufferey A, Daali Y and Reny
JL: Antiplatelet therapy: targeting the TxA2 pathway. J Cardiovasc
Transl Res. 7:29–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lordkipanidzé M, Pharand C, Schampaert E,
Turgeon J, Palisaitis DA and Diodati JG: A comparison of six major
platelet function tests to determine the prevalence of aspirin
resistance in patients with stable coronary artery disease. Eur
Heart J. 28:1702–1708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sangkuhl K, Shuldiner AR, Klein TE and
Altman RB: Platelet aggregation pathway. Pharmacogenet Genomics.
21:516–521. 2011. View Article : Google Scholar : PubMed/NCBI
|