Introduction
Although the incidence of gastric cancer (GC) is
declining overall, it remains the fifth most common type of cancer
and is the third leading cause of cancer-associated mortality
worldwide (1). In 2012, China
accounted for >40% of novel gastric cancer cases and associated
mortalities (1). Patients with GC were
often diagnosed at a late stage and had a low survival rate. The
5-year overall survival rate of gastric cancer is closely
associated with neoplasm staging. Stage I patients have a 5-year
survival rate of >90%, compared with <5% in stage IV patients
(2). Therefore, early diagnosis and
treatment are crucial to improving the prognosis of GC
patients.
Circulating microRNAs (miRNAs) are considered to be
ideal as disease biomarkers (3).
Recent studies demonstrated that circulating miRNAs have potential
as molecular tools for detecting, predicting and monitoring various
types of cancer (4–6). Circulating miRNAs may be encapsulated in
exosomes or bound to proteins and lipoproteins, increasing their
stability in circulating blood and protecting them from endogenous
RNAses (7). In addition, the levels of
serum or plasma miRNAs are similar in healthy individuals. The
levels of circulating miRNAs alter with the development and
progression of different types of cancer (8). Therefore, these characteristics give
circulating miRNAs great potential as molecular tumor markers.
Tsujiura et al (9) first reported that miRNAs were stable and
detectable in all GC plasma samples, and that detection of
circulating miRNAs may provide novel complementary tumor markers
for GC. Circulating miRNAs may also be used to predict metastasis,
early recurrence and poor prognosis in stomach cancer. Imaoka et
al (10) found that serum miR-203
had the potential to serve as a noninvasive molecular marker for
predicting metastases and prognosis. As the release of circulating
miRNA from donor cells and its uptake in recipient cells is not
fully understood there are few studies regarding the association
between circulating miRNAs and GC treatment. However, certain
studies have shown that circulating miRNAs could be used to treat
cancer. At present, treatment focuses on delivering antisense
miRNAs or tumor suppressive miRNAs into target tumor cells via
microvesicles (MVs) or exosomes (11).
Fonsato et al (12) revealed
that delivering anti-miRNAs (miR-451, miR-223, miR-24, miR-125b and
miR-31) by MVs derived from human adult liver stem cells inhibits
tumor growth induced in severe combined immunodeficiency mice with
primary hepatocellular carcinomas (12).
Materials and methods
Serum samples
The study was approved by the Ethics Committee of
the University of South China (Hengyang, China). Informed consent
was obtained from all participants included in the study. Serum
samples (1–2 ml; n=194) were collected from 127 individuals,
including 67 paired pre- and post-operative GC patients, and 12
cases of each of GC, nasopharyngeal cancer (NPC), colorectal
carcinoma (CRC), breast cancer (BC) and non-cancerous controls
(NC). All the malignant tumor patients were diagnosed by two
independent professors of pathology. The tumor serum samples were
collected from patients who had not undergone therapy, such as
radiotherapy or chemotherapy. The paired post-operative GC blood
specimens were obtained on the seventh day post-surgery. The
individuals with no evidence of carcinoma were selected as the NC
subjects. The serum samples were collected from the consenting
individuals from the First Hospital Affiliated of University of
South China and Second Hospital Affiliated of University of South
China from February 2012 to July 2013, according to our previously
published protocol (13). According to
AJCC cancer staging manual, each GC patient was staged (14).
Serum treatment and RNA
extraction
Serum samples (4 ml venous blood) were collected in
the morning before breakfast from all of the study subjects.
Samples were incubated at room temperature (15–25°C) for 1 h and
centrifuged at 1,200 × g at 4°C for 10 min. The upper phase of the
serum was collected and stored in a refrigerator at −80°C. For the
RNA isolation, a human serum sample (volume, 200 µl) was used for
extracting total RNA with an miRNeasy Serum/Plasma kit (Qiagen
GmbH, Hilden, Germany) according to the manufacturers protocol.
During the RNA extraction, cel-miR-39 was spiked at a fixed
concentration and served as an internal control. The total RNA was
eluted in 12 µl RNase-free water. Finally, the reverse
transcription (RT) reaction was prepared according to the
instructions of the miScript II RT kit (Qiagen GmbH). The template
cDNA was removed, according to the manufacturers protocol, and
diluted to 200 µl with RNase-free water for use in the quantitative
polymerase chain reaction (qPCR).
Serum miRNAs profiling assay
Six paired pre- and post-operative GC serum samples
were mixed at the same volume (50 µl) for microarray analysis using
miScript miRNA PCR Array (MIHS-106ZC; Qiagen GmbH), which contains
84 circulating miRNAs associated with human cancer. The experiment
was repeated once with six alternative paired pre- and
post-operative GC serum samples.
RT-qPCR and data analysis
Serum samples (n=170) from GC, NPC, CRC, BC and NC
were used for the validation of differentially expressed serum
miRNAs. Cel-miR-39 served as internal reference controls and
RT-qPCR was performed using a miScript SYBR-Green PCR kit (Qiagen
GmbH), according to the manufacturers protocol, to quantify
specific serum miRNAs. Relative expression of miRNAs was calculated
using ΔCq (ΔCq=CqmiRNA-CqCel-miR-39) and the
fold change was calculated using 2−ΔΔCq (ΔΔCq =
ΔCqsample 2 - ΔCqsample 1) (15).
Statistical analysis
SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used to
perform Kaplan-Meier survival analysis. In addition Cluster 3.0
(Stanford University, Stanford, CA, USA) and GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA) software were used.
Matched-pairs t-test was used to compare paired pre- and
post-operative GC serum samples. The Mann-Whitney U test was used
to analyze the difference between two groups, and the differences
among three groups were compared using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference and all P-values were two-tailed.
Results
Differentially expressed serum miRNA
profile between pre- and post-operative GC patients
The miRNA expression profiling of the serum between
pre- and post-operation GC patients was analyzed following
preliminary screening with microRNA Array analysis. The principles
for screening differentially expressed serum miRNAs are as follows:
i) The fold change of miRNAs in serum between the pre- and
post-operative GC samples is >2; ii) the miRNA Cq value should
be <35 in pre- or post-operative GC samples. Based on these
principles, the initial screening of the following 12
differentially expressed serum miRNAs between pre- and
post-operative GC: miR-15b-5p, miR-20a-5p, miR-29a-3p, miR-30a-5p,
miR-30c-5p, miR-30e-5p, miR-130a-3p, miR-130b-3p, miR-181b-5p,
miR-424-5p, let-7d-5p, let-7g-5p were subjected to the next step of
verification (Table I).
 | Table I.Differentially expressed serum miRNAs
screened by microarray in paired pre- and post-operative GC
samples. |
Table I.
Differentially expressed serum miRNAs
screened by microarray in paired pre- and post-operative GC
samples.
|
|
| Fold-change (pre- vs.
post-operative) |
|---|
|
|
|
|
|---|
| Gene name | Expression in
post-operative GC patients | Microarray 1 | Microarray 2 |
|---|
| hsa-miR-15b-5p | Downregulated | −2.60 | −3.34 |
| hsa-miR-20a-5p | Downregulated | −3.58 | −3.25 |
| hsa-miR-29a-3p | Downregulated | −45.89 | −3.34 |
| hsa-miR-30a-5p | Downregulated | −7.78 | −3.34 |
| hsa-miR-30c-5p | Downregulated | −2.79 | −2.04 |
| hsa-miR-30e-5p | Downregulated | −11.16 | −9.78 |
| hsa-miR-130a-3p | Downregulated | −2.60 | −3.01 |
| hsa-miR-130b-3p | Downregulated | −3.36 | −2.95 |
| hsa-miR-181b-5p | Downregulated | −3.16 | −3.20 |
| hsa-miR-424-5p | Downregulated | −2.11 | −4.63 |
| hsa-let-7d-5p | Downregulated | −4.56 | −3.63 |
| hsa-let-7g-5p | Downregulated | −5.39 | −79.89 |
Validation of the miRNAs
differentially expressed in paired pre- and post-operative GC serum
samples using RT-qPCR
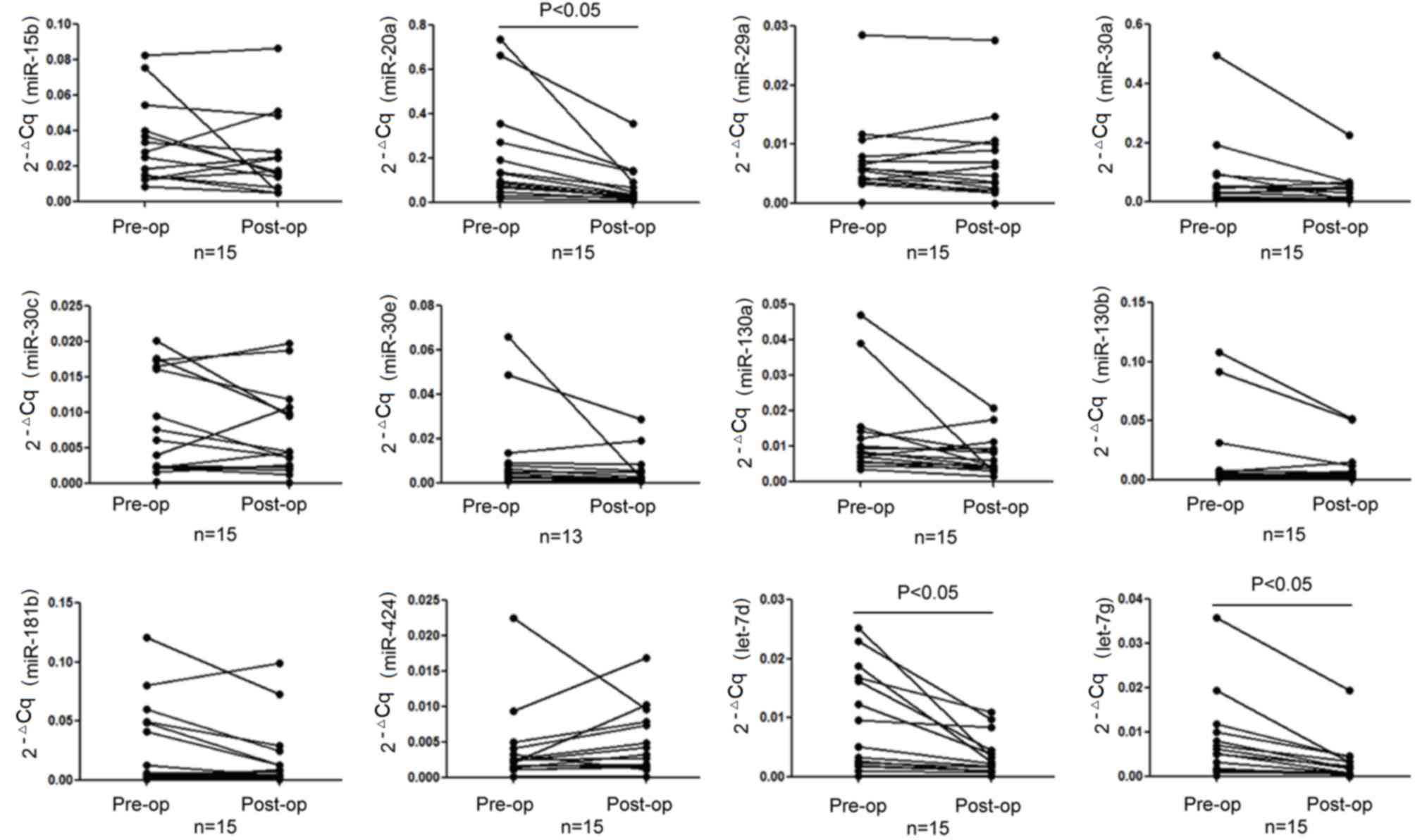
RT-qPCR was performed to verify the 12 miRNAs in the
15 paired pre- and post-operative GC serum samples. The data was
analyzed using the relative quantitative method. The expression
levels of miR-20a-5p, let-7d-5p and let-7g-5p were identified to be
significantly downregulated in the post-operative group (P<0.05;
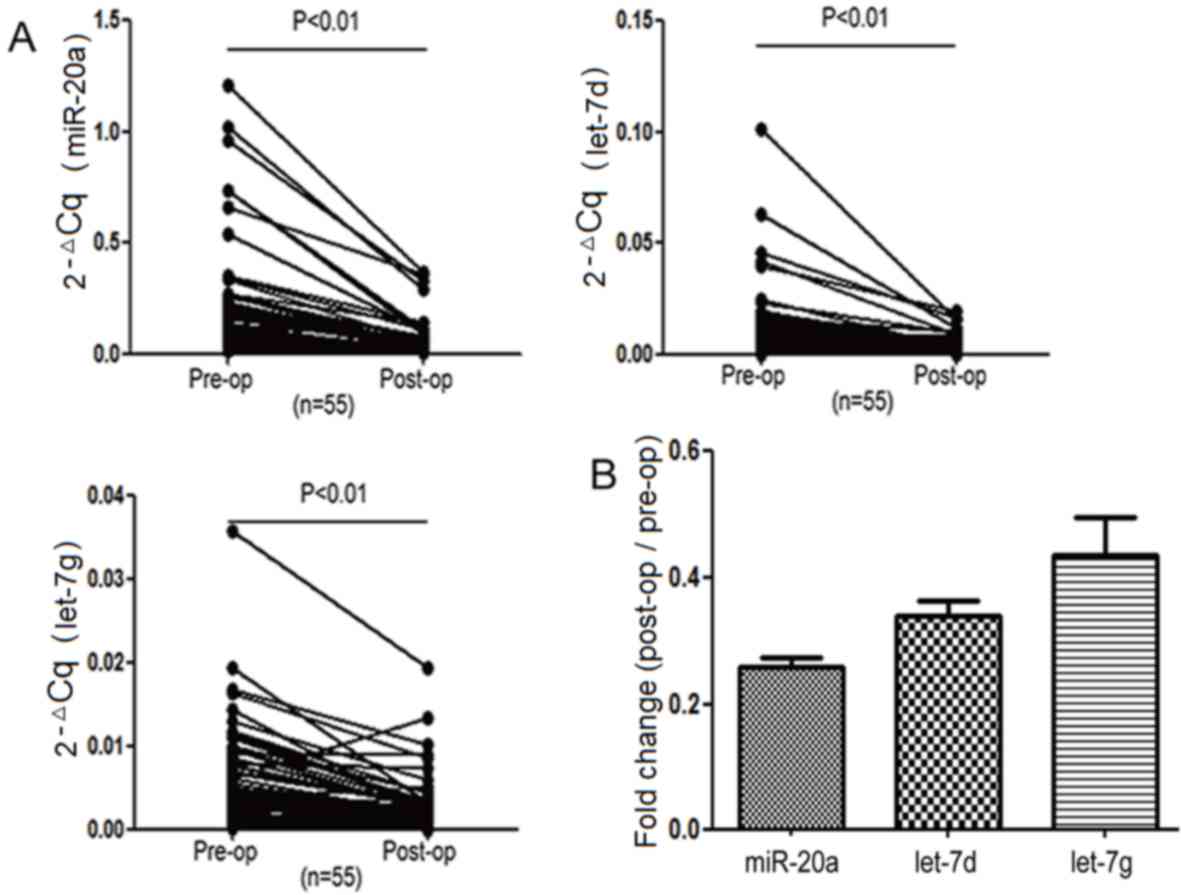
Fig. 1 and Table II). miR-20a-5p, let-7d-5p and
let-7g-5p were verified further in the remaining 40 paired GC serum
specimens. The results demonstrated that these three serum miRNAs
were significantly different between the pre- and post-operative
groups (P<0.05, Fig. 2A);
miR-20a-5p, let-7d-5p and let-7g-5p were downregulated in all 55
paired serum samples following surgery. Among these three miRNAs,
the difference between the GC patient pre- and post-operative serum
miR-20a-5p expression levels was the largest (Fig. 2B and Table
III).
 | Table II.Differences between the expression
levels of the 12 miRNAs from 15 cases of paired pre- and
post-operative gastric cancer patients. |
Table II.
Differences between the expression
levels of the 12 miRNAs from 15 cases of paired pre- and
post-operative gastric cancer patients.
| Gene name | ΔCq pre-operative -
ΔCq post-operative (mean ± SEM) | 95% CI | P-value |
|---|
| hsa-miR-15b-5p | −0.491±1.225 | (−1.170, 0.187) | 0.143 |
| hsa-miR-20a-5p | −1.422±0.614 | (−1.762, −1.082) | 0.000 |
| hsa-miR-29a-3p | −0.256±0.598 | (−0.587, 0.075) | 0.120 |
| hsa-miR-30a-5p | −0.157±1.368 | (−0.915, 0.600) | 0.663 |
| hsa-miR-30c-5p | −0.251±0.798 | (−0.694, 0.191) | 0.243 |
| hsa- miR-30e-5p | −0.123±0.903 | (−0.668, 0.422) | 0.632 |
| hsa-miR-130a-3p | −0.607±1.178 | (−1.259, 0.046) | 0.066 |
| hsa-miR-130b-3p | −0.423±0.976 | (−0.964,
0.117) | 0.115 |
|
hsa-miR-181b-5p | −0.726±1.530 | (−1.574,
0.121) | 0.087 |
| hsa-miR-424-5p | 0.361±0.967 | (−0.175,
0.896) | 0.171 |
| hsa-let-7d-5p | −1.198±0.842 | (−1.664,
−0.732) | 0.000 |
| hsa-let-7g-5p | −1.339±0.918 | (−1.847,
−0.830) | 0.000 |
 | Table III.Differences of miR-20a, let-7d and
let-7g between all 55 paired pre- and post-operative gastric cancer
serum samples. |
Table III.
Differences of miR-20a, let-7d and
let-7g between all 55 paired pre- and post-operative gastric cancer
serum samples.
| Gene name | ΔCq pre-operative -
ΔCq post-operative (mean ± SEM) | 95% CI |
|---|
| hsa-miR-20a-5p | −1.994±0.700 | (−2.183,
−1.805) |
| hsa-let-7d-5p | −1.581±0.645 | (−1.756,
−1.407) |
| hsa-let-7g-5p | −1.221±0.885 | (−1.459,
−0.982) |
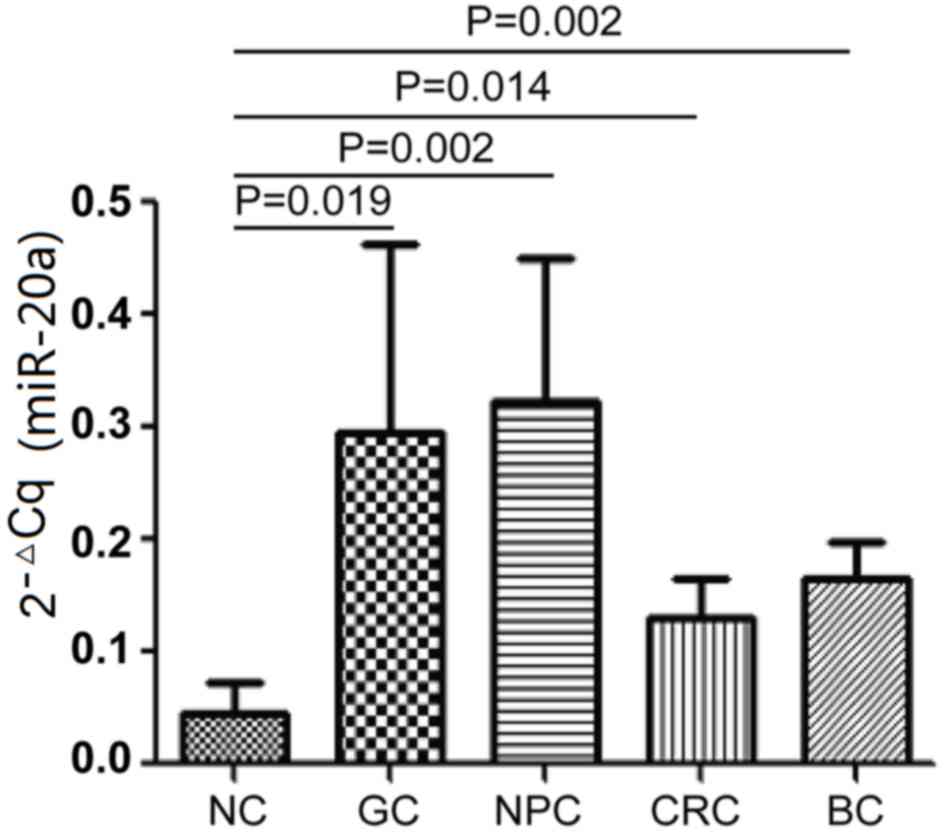
Detecting the serum levels of
miR-20a-5p in healthy volunteers and in subjects with other common
tumors
The expression levels of miR-20a-5p were measured in
the serum of 12 cases of each of GC, 12 NPC, 12 CRC, 12 BC and 12
NC. The results indicate that the serum levels of miR-20a-5p in GC,
NPC, CRC and BC are significantly higher than the NC (P<0.05;
Fig. 3). While no significant
difference was identified between the four tumor groups. The
present study proposes that serum miR-20a-5p may serve as a novel
biomarker to diagnose gastric carcinoma and may also be used to
detect NPC, CRC and BC.
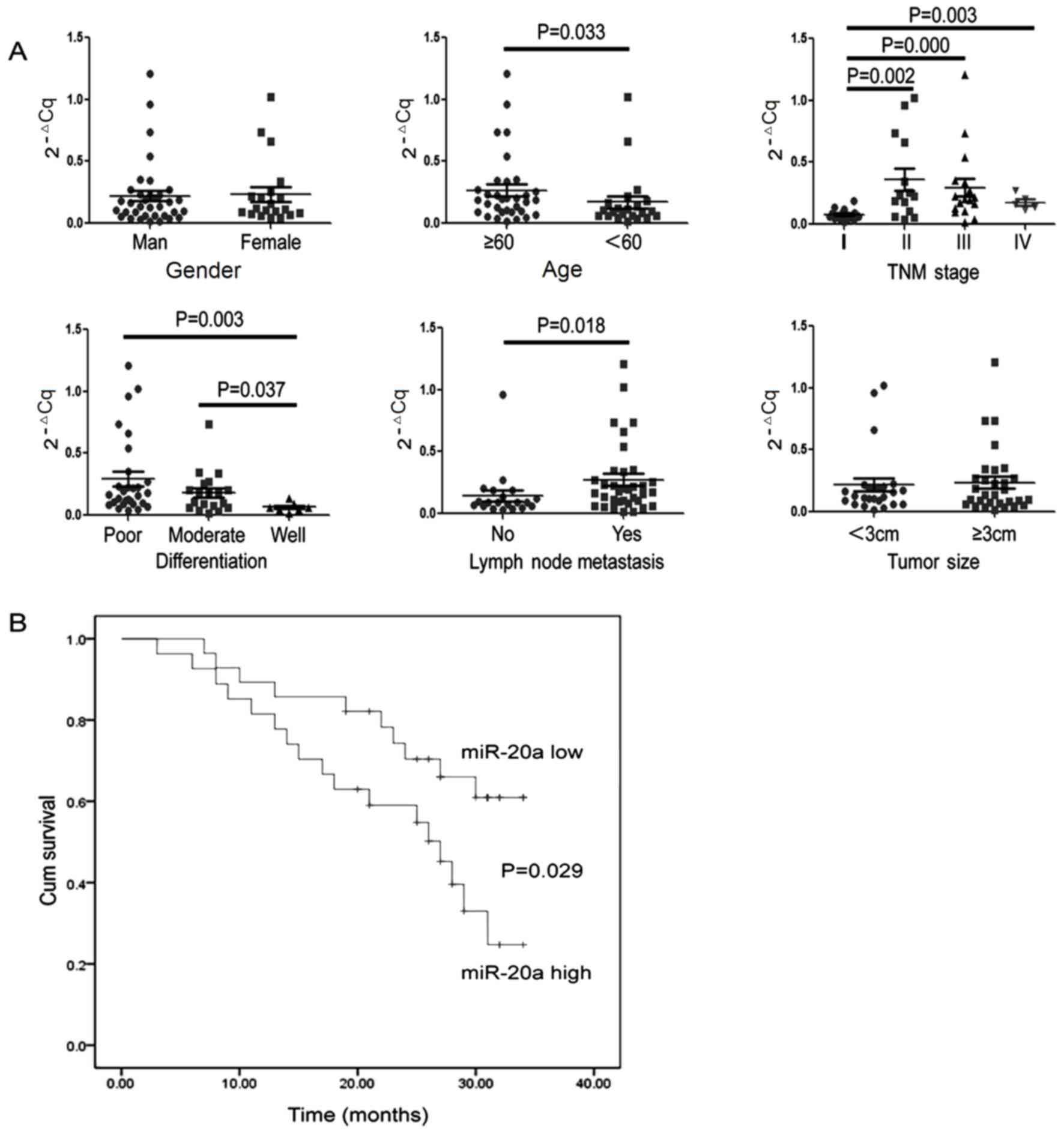
Clinical significance of serum
miR-20a-5p expression levels in the pre-operative GC patient
group
The serum miR-20a-5p expression levels of the 55 GC
patients were analyzed prior to surgery. The pre-operative serum
was divided into high miR-20a-5p and low miR-20a-5p expression
level groups according to the median value, and is presented in
combination with the clinicopathological parameters of GC patients
in Table IV. Next the concentrations
of serum miR-20a-5p was compared among different
clinicopathological characteristic groups and the clinical value
was evaluated. As shown in Table IV
and Fig. 4A, the expression levels of
serum miR-20a-5p were significantly associated with age, tumor
stage (14), degree of differentiation
and lymph node metastasis (Table IV).
The serum levels of miR-20a-5p in TNM stage II, III and IV were
significantly higher than TNM stage I, with no difference between
TNM stages II, III and IV. Concentrations of miR-20a-5p in the
well-differentiated group were lower than that of the moderately-
and poorly-differentiated groups, whereas no significant difference
was noted between the moderately- and poorly-differentiated groups.
GC patients with lymph node metastasis exhibited higher miR-20a-5p
expression levels than those patients who had no metastasis.
 | Table IV.Association between the expression
levels of serum miR-20a and clinicopathological characteristics of
patients. |
Table IV.
Association between the expression
levels of serum miR-20a and clinicopathological characteristics of
patients.
|
|
| Expression level of
serum miR-20a |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameter | Nos. | Low | High | P-value
(2-sided) |
|---|
| Gender |
|
|
| 0.396 |
|
Male | 35 | 17 | 18 |
|
|
Female | 20 | 11 | 9 |
|
| Age (years) |
|
|
| 0.048 |
|
≥60 | 33 | 15 | 18 |
|
|
<60 | 22 | 13 | 9 |
|
| TNM stage (14) |
|
|
|
0.000 |
| I | 19 | 13 | 6 |
|
| II | 14 | 6 | 8 |
|
|
III | 17 | 5 | 12 |
|
| IV | 5 | 4 | 1 |
|
|
Differentiation |
|
|
| 0.000 |
|
PDAC | 29 | 16 | 13 |
|
|
MDAC | 19 | 7 | 12 |
|
|
WDAC | 7 | 5 | 2 |
|
| Lymph node
metastasis |
|
|
| 0.002 |
|
Yes | 35 | 15 | 20 |
|
| No | 20 | 13 | 7 |
|
| Tumor size
(cm) |
|
|
| 0.777 |
| ≥3 | 30 | 15 | 15 |
|
|
<3 | 25 | 13 | 12 |
|
Predictive ability of serum miR-20a-5p
levels for GC prognosis
To evaluate the potential prognostic value of
miR-20a, The 55 GC patients who had undergone surgery were followed
up. At 34 months, 27 cases had succumbed during the process of
investigation and the overall survival rate was 50.9%. The results
of Kaplan-Meier survival analysis indicate that the overall
survival rate of those patients who exhibited low serum miR-20a-5p
expression levels was significantly higher than the GC patients who
had high serum expression levels of miR-20a-5p (P<0.05; Fig. 4B). Thus, the serum miR-20a-5p
expression levels may be used for predicting the prognosis of GC
patients.
Discussion
miR-20a is a member of the miR-17-92 cluster
(including miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and
miR-92a-1), which is commonly upregulated in malignant cancer and
involved in carcinogenesis as the classical oncogene (16). Previous studies have reported
upregulated expression levels of circulating miR-20a in malignant
neoplasms, such as non-small cell lung cancer (NSCLC), and in
cervical, hepatoma, colorectal and prostate cancers (17–22).
However, the association between the expression level of miR-20a
and GC is rarely reported. miR-20a is usually identified by
screening for circulating miRNA expression profiles in malignant
tumor patients. Liu et al (23)
reported that five serum miRNAs (miR-1, miR-20a, miR-27a, miR-34
and miR-423-5p) had potential in GC diagnosis. In addition, the
authors demonstrated that the areas under the receiver operating
characteristic curve of these five serum miRNAs (0.879) were
markedly higher than those of the classical biomarkers, such as
carcinoembryonic antigen (0.503) and cancer antigen 19–9 (0.600)
(23). Du et al (24) reported that expression levels of
miR-20a were significantly upregulated in GC tissue and plasma
samples. Their results indicated that miR-20a may promote
activation of the nuclear factor (NF)-κB signaling pathway and
downstream targets, livin and survivin by targeting NF-κB inhibitor
β (24).
In the present study, serum miR-20a expression
levels in 67 cases of paired pre- and post-operative GC samples
were evaluated by microarray and RT-qPCR technology. The use of
paired pre- and post-operative GC samples eliminated the individual
differences, such as age, gender and eating habits. C.
elegans spike-in non-human miR-39 served as an exogenous
reference gene in each experiment and RT-qPCR amplification was
repeated three times in each serum specimen of GC to ensure that
the results were highly accurate, repeatable and stable. miR-20a
was easily detected and identified to be downregulated following
surgery, indicating that the elevated expression levels of serum
miR-20a in pre-operative GC patients were released by GC cells.
Serum expression levels of miR-20a declined following surgery;
therefore, the expression levels may be useful for evaluating the
efficacy of GC therapy. In addition, serum miR-20a expression
levels were identified to be upregulated in GC, CRC, BC and NPC
patients, demonstrating that serum expression levels of miR-20a may
be used to diagnose GC and other types of cancer, and that the
miR-20a observed in the serum of tumor patients may be released by
various types of cancer cell.
In the present study, expression levels of serum
miR-20a were found to be significantly associated with age, TNM
stage, degree of differentiation and metastasis. Notably,
pre-operative GC patients with moderately- or poorly-differentiated
cancer cells, or with metastasis exhibited the highest expression
levels of serum miR-20a. GC patients that were ≥60 years exhibited
higher expression levels of serum miR-20a, which may be due to the
age distribution of GC, but this requires further investigation.
Patients in the pre-operative group with higher expression levels
of serum miR-20a demonstrated a poorer survival rate when compared
with GC patients with low expression levels of serum miR-20a. Thus,
concentrations of serum miR-20a may be used to predict the
prognosis and recurrence of GC.
In conclusion, the present results demonstrated that
serum miR-20a expression levels were upregulated in pre-operative
GC patients and significantly downregulated in post-operative GC
patients. Serum miR-20a could, therefore, serve as a biomarker for
diagnosing GC, as well as evaluating the curative effects,
predicting the prognosis and monitoring the recurrence of GC
patients. The exact efficiency of miR-20a as the tumor marker
remains to be confirmed by further validation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101643); the Hunan
Provincial Education Department document (document no. 2014-405);
the Foundation of the Construct Program of the Key Discipline in
Hunan Province of China (grant no. 2011-76); and the Hunan Province
Key Laboratory (grant no. 2016TP1015). The authors would like to
thank Dr Chun Wang of Washington University (St. Louis, MO, USA)
for assistance with language editing.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou X, Zhang M and Qiao H: Diagnostic
significance of miR-106a in gastric cancer. Int J Clin Exp Pathol.
8:13096–13101. 2015.PubMed/NCBI
|
|
3
|
Zeng X, Xiang J, Wu M, Xiong W, Tang H,
Deng M, Li X, Liao Q, Su B, Luo Z, et al: Circulating miR-17,
miR-20a, miR-29c, and miR-223 combined as non-invasive biomarkers
in nasopharyngeal carcinoma. PLoS One. 7:e463672012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarzenbach H, Nishida N, Calin GA and
Pantel K: Clinical relevance of circulating cell-free microRNAs in
cancer. Nat Rev Clin Oncol. 11:145–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bianchi F: Lung cancer early detection:
the role of circulating microRNAs. EBioMedicine. 2:1278–1279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mirzaei HR, Sahebkar A, Mohammadi M, Yari
R, Salehi H, Jafari MH, Namdar A, Khabazian E, Jaafari MR and
Mirzaei H: Circulating microRNAs in hepatocellular carcinoma:
potential diagnostic and prognostic biomarkers. Curr Pharm Des.
22:5257–5269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vickers KC and Remaley AT: Lipid-based
carriers of microRNAs and intercellular communication. Curr Opin
Lipidol. 23:91–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ganepola GA, Rutledge JR, Suman P,
Yiengpruksawan A and Chang DH: Novel blood-based microRNA biomarker
panel for early diagnosis of pancreatic cancer. World J
Gastrointest Oncol. 6:22–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsujiura M, Ichikawa D, Komatsu S,
Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi
K, Fujiwara H, et al: Circulating microRNAs in plasma of patients
with gastric cancers. Br J Cancer. 102:1174–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Imaoka H, Toiyama Y, Okigami M, Yasuda H,
Saigusa S, Ohi M, Tanaka K, Inoue Y, Mohri Y and Kusunoki M:
Circulating microRNA-203 predicts metastases, early recurrence, and
poor prognosis in human gastric cancer. Gastric Cancer. 19:744–753.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng G: Circulating miRNAs: Roles in
cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev.
81:75–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fonsato V, Collino F, Herrera MB,
Cavallari C, Deregibus MC, Cisterna B, Bruno S, Romagnoli R,
Salizzoni M, Tetta C, et al: Human liver stem cell-derived
microvesicles inhibit hepatoma growth in SCID mice by delivering
antitumor microRNAs. Stem Cells. 30:1985–1998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang M, Zeng Y, Yang R, Xu H, Chen Z,
Zhong J, Xie H, Xu Y and Zeng X: U6 is not a suitable endogenous
control for the quantification of circulating microRNAs. Biochem
Biophys Res Commun. 454:210–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer-Verlag; New York, NY: 2009
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Z, Yin J, Fu W, Mo Y, Pan Y, Dai L,
Huang H, Li S and Zhao J: miRNA 17 family regulates
cisplatin-resistant and metastasis by targeting TGFbetaR2 in NSCLC.
PLoS One. 9:e946392014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Babu KR and Muckenthaler MU: miR-20a
regulates expression of the iron exporter ferroportin in lung
cancer. J Mol Med (Berl). 94:347–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Safari A, Seifoleslami M, Yahaghi E,
Sedaghati F and Khameneie MK: RETRACTED ARTICLE: Upregulation of
miR-20a and miR-10a expression levels act as potential biomarkers
of aggressive progression and poor prognosis in cervical cancer.
Tumour Biol. Oct 1–2015.(Epub ahead of print).
|
|
19
|
Zhang Y, Zheng L, Ding Y, Li Q, Wang R,
Liu T, Sun Q, Yang H, Peng S, Wang W, et al: miR-20a induces cell
radioresistance by activating the PTEN/PI3K/Akt signaling pathway
in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
92:1132–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sokolova V, Fiorino A, Zoni E, Crippa E,
Reid JF, Gariboldi M and Pierotti MA: The effects of miR-20a on
p21: two mechanisms blocking growth arrest in TGF-β-responsive
colon carcinoma. J Cell Physiol. 230:3105–3114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azizian A, Kramer F, Jo P, Wolff HA,
Beißbarth T, Skarupke R, Bernhardt M, Grade M, Ghadimi BM and
Gaedcke J: Preoperative prediction of lymph node status by
circulating mir-18b and mir-20a during chemoradiotherapy in
patients with rectal cancer. World J Surg. 39:2329–2335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiang XF, Zhang ZW, Liu Q, Sun N, Pan LL,
Shen J, Li T, Yun C, Li H and Shi LH: miR-20a promotes prostate
cancer invasion and migration through targeting ABL2. J Cell
Biochem. 115:1269–1276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu R, Zhang C, Hu Z, Li G, Wang C, Yang
C, Huang D, Chen X, Zhang H, Zhuang R, et al: A five-microRNA
signature identified from genome-wide serum microRNA expression
profiling serves as a fingerprint for gastric cancer diagnosis. Eur
J Cancer. 47:784–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du Y, Zhu M, Zhou X, Huang Z, Zhu J, Xu J,
Cheng G, Shu Y, Liu P, Zhu W, et al: miR-20a enhances cisplatin
resistance of human gastric cancer cell line by targeting NFKBIB.
Tumour Biol. 37:1261–1269. 2016. View Article : Google Scholar : PubMed/NCBI
|