Introduction
Aortic valve stenosis (AoV), a global cardiovascular
health concern, is the most frequent valve disease, the third most
frequent cardiovascular disease after arterial hypertension and
coronary heart disease, and the most widespread indication for
surgical valve replacement (1–3). Data of
systemic studies showed that calcific AoV sclerosis is present in
approximately 9% of patients aged 54 and above, to 42% at age 81.
In 1.8–1.9% of patients, AoV sclerosis progresses to clinical
calcific AoV stenosis. These patients have increased risk of
coronary heart disease (68%), myocardial infarction (27%), cardiac
death (69%), and risk of other etiology death, which makes calcific
AoV stenosis a significant risk factor for morbidity and mortality
in the age groups mentioned (4). If
the AoV is not replaced, 75% of patients succumb in 3 years and 50%
above the age of 65 years succumb within 2 years after the
occurrence of symptoms such as chest pain, syncope, and heart
failure (5). Historically, AoV
stenosis was considered a degenerative disease related to aging,
and wear and tear of the valve. Nevertheless, there is no distinct
answer as to whether it is the cause of AoV stenosis onset and
progression, as many elderly people do not suffer from this disease
(6).
Regarding pathogenetic aspects of AoV stenosis,
valves are made of specialized cells with distinct functions:
Valvular endothelial cells, valvular interstitial cells, and the
extracellular matrix, which consists of collagen, elastin, and
glycosaminoglycans. Valvular interstitial cells are of five
different phenotypes with distinct biological functions; embryonic
endothelial and mesenchymal progenitor cells, quiescent, activated,
progenitor, and osteoblast-like valvular interstitial cells, with
the latter contributing to the calcification of AoV (1).
Calcification at the tissue level is the most common
finding in AoV stenosis (7). There
are findings to prove the active inflammation process, such as the
increased expression of C-reactive protein (CRP), increased
temperature in stenotic valve leaflets, and a special non-invasive
method combining positron emission tomography and computed
tomography using a ligand 18 fluordesoxyglucose, therefore proving
the activity of macrophages in stenotic valve leaflets (8,9).
Chemerin is an adipokine known as a ligand for G
protein-coupled chemokine-like receptor 1, also known as chemerin
receptor 23, which was first identified in macrophages and
dendritic cells (10–12). The main source of chemerin is white
adipose tissue, but a high concentration was also found in the
liver (13,14), lower lungs, brown adipose tissue,
heart, ovaries, kidneys, skeletal muscle, and pancreas (15). The circulating chemerin level strongly
correlates with the level of inflammatory markers such as tumor
necrosis factor (TNF)-α, interleukin (IL)-6, high-sensitivity CRP,
resistin, and leptin (15,16) in humans and animals (17–19).
Chemerin regulates the inflammatory process by promoting the
migration of macrophages to certain tissues such as adipose tissue
(20).
It is clear that chemerin has a role in inflammatory
processes, but there is still no consensus whether it is a pro- or
anti-inflammatory activity (21–27).
Eisinger et al showed that chemerin has no positive
correlation with the severity of inflammation (17). A link between the severity grade of
liver cirrhosis and the severity of chronic hepatitis C and plasma
chemerin levels has been investigated, and a negative correlation
was found in both cases, which leads to the conclusion that
chemerin may have a protective role in the inflammation process
(17,28). Studies revealed that various chemerin
fragments with pro- and anti-inflammatory activity are produced
depending on predominating proteases in the inflammatory process
(29,30).
Fibroblast growth factor-21 (FGF-21) has an
important role in cardiovascular diseases. It is a protein secreted
in the liver, skeletal muscle, adipose tissue, and pancreas
(31). It has been found that the
FGF-21 serum level is increased in patients with abnormal lipid
profiles, obesity, metabolic syndrome, reduced glucose tolerance,
diabetes type 2, hypertension, non-alcoholic fatty liver disease,
and coronary artery disease (31–33).
Similarly, the FGF-21 level is increased in patients with coronary
heart disease and atherosclerotic plaques of the carotid arteries;
thus, FGF-21 can be used as a biomarker in atherosclerotic diseases
(34–36).
FGF-21 has been shown to reduce IL-6, IL-β, and
TNF-α production, which assume the main role in the pathogenesis of
inflammation (37).
Findings support the protective role of FGF-21's in
different cardiovascular diseases, such as atrial fibrillation,
myocardial infarction, coronary artery disease, and hypertension.
Han et al stated that the FGF-21 level was markedly higher
in patients with atrial fibrillation compared to a control group
(38).
It is also stated that cardiomyocytes are capable of
secreting FGF-21 as a response to various stress factors, such as
cardiac hypertrophy or myocardial infarction (39–42).
FGF-21 has an autocrine effect on preventing cardiac inflammation,
development of hypertrophy, apoptosis of cardiomyocytes, and the
emergence of oxygen-free radicals, as well as promoting the
oxidation of fatty acids (42).
The aim of the present study was to evaluate serum
chemerin and serum FGF-21 as possible biomarkers of AoV stenosis
and to study inflammation in the pathogenesis of AoV stenosis.
Patients and methods
Study population
This prospective study was conducted from 2013 to
2016 in different hospitals in Latvia. The study protocol had prior
approval from the Riga Stradins University (Riga, Latvia) Ethics
Committee on research on humans and the study protocol conforms to
the ethical guidelines of the 1975 Declaration of Helsinki.
A total of 102 patients were selected on the basis
of inclusion and exclusion criteria and divided in two core groups,
a control and an AoV stenosis group. The control group included
patients without AoV stenosis aged from 55 to 80 years, which
complies with the average age of AoV stenosis patients according to
the Guidelines on the Management of Valvular Heart Disease 2012 of
the European Society of Cardiology (43). Written informed consent was obtained
from each patient included in the study. Patients were included in
the control group according to echocardiographically confirmed
results of a healthy AoV. Exclusion criteria in the two groups were
obesity, systemic connective tissue, infectious and oncological
diseases, diabetes, thyroid dysfunction and arterial hypertension,
atherosclerotic coronary artery disease, heart failure, myocardial
infarction, cerebral infarction and transient ischemic attack.
Exclusion criteria in AoV stenosis group were patients with
congenital AoV stenosis and rheumatic AoV stenosis.
AoV stenosis assessment
Patients were examined echocardiographically and the
data were archived using GE VIVID 7 Dimension and Philips IE 33,
both from KPI Healthcare (Yorba Linda, CA, USA). Each
echocardiography examination was evaluated by two
professionals.
Patients with AoV stenosis were subdivided into
three groups (Table I) depending on
the severity grade according to the echocardiography criteria:
aortic jet velocity (Vmax) (m/sec); mean pressure gradient, PG
(mmHg); aortic valve area, AVA (cm2) and indexed AVA
(cm2/m2). Data were graded as: Severe: Vmax
>4 m/sec, PG >40 mmHg, AVA <1.0 cm2, indexed
AVA <0.6; moderate: Vmax 3.0–4.0 m/sec, PG 20–40 mmHg, AVA
1.0–1.5 cm2, indexed AVA 0.60–0.85; mild: Vmax 2.5–2.9
m/sec, PG <20 mmHg, AVA >1.5 cm2, indexed AVA
>0.85.
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
|
Characteristics | Control n=50 | AoV mild stenosis
n=18 | AoV moderate
stenosis n=19 | AoV severe stenosis
n=15 |
|---|
| Sex (%) |
|
|
|
|
|
Male | 11 (22.0) | 2 (11.1) | 8 (42.1) | 7 (46.7) |
|
Female | 39 (78.0) | 16 (88.9) | 11 (57.9) | 8 (53.3) |
| Age, mean ± SD | 65.18 (9.74) | 70.53 (6.08) | 72.16 (8.20) | 65.27 (8.13) |
| P-value vs.
control |
| P=0.127 | P=0.012 | P>0.999 |
| BMIa, mean ± SD | 26.04 (4.31) | 27.39 (3.10) | 25.81 (4.58) | 27.40 (3.18) |
| P-value vs.
control |
| P=0.399 | P=0.682 | P=0.869 |
| LDLb, mmol/l mean ± SD | 3.28 (1.18) | 3.05 (0.97) | 2.59 (0.92) | 3.10 (1.12) |
| P-value vs.
control |
| P>0.999 | P=0.057 | P>0.999 |
| Triglycerides
mmol/l, mean ± SD | 1.47 (0.71) | 1.64 (0.84) | 1.11 (0.56) | 1.27 (0.57) |
| P-value vs.
control |
| P=0.406 | P=0.178 | P=0.406 |
| Total cholesterol
mmol/l, mean ± SD | 5.49 (1.28) | 5.01 (1.34) | 4.21 (1.18) | 4.68 (1.08) |
| P-value vs.
control |
| P=0.056 | P=0.001 | P=0.016 |
| hs-CRPc mg/l, | 5.03 | 4.69 | 5.27 | 3.7 |
| Median (IQR) | (2.12–14.23) | (3.42–7.35) | (1.71–11.14) | (1.56–11.9) |
| P-value vs.
control |
| P>0.999 | P>0.999 | P>0.999 |
| dSV ml, mean ± SD | 81.86 (20.11) | 73.63 (18.92) | 77.72 (18.65) | 75.93 (15.49) |
| P-value vs.
control |
| P=0,299 | P=0.501 | P=0.501 |
| eEF %, mean ± SD | 62.22 (6.35) | 57.16 (9.23) | 61.78 (8.23) | 56.13 (7.84) |
| P-value vs.
control |
| P=0.014 | P=0.291 | P=0.007 |
Biochemical analysis
Venous blood samples were taken and serum was
obtained in accordance with the serum preparation instructions.
Serum samples were stored at −80°C and were available in 102
patients. We obtained laboratory analyses for all the patients at
the Biochemistry Laboratory of the Riga Stradins University,
including chemerin and FGF-21 with the ELISA method, using human
Chemerin ELISA (cat. no. EZHCMRN-57K) and human FGF-21 ELISA (cat.
no. EZHFGF21-19K) kits, both produced by EMD Millipore (Billerica,
MA, USA). Results were detected using a TECAN Infinite 200 PRO
multimode reader (Tecan Group, Ltd., Mannedorf, Switzerland). The
procedure was carried out in accordance with the manufacturer's
instructions.
Statistical analysis
The data were analyzed using IBM SPSS version 23.0
(IBM Corp., Armonk, NY, USA). Descriptive statistics were performed
according to the data type. The results for age, body mass index,
low-density lipoprotein cholesterol, total cholesterol,
triglycerides and ejection fraction, as well as stroke volume are
expressed as mean (M) and standard deviation (± SD). Results for
high-sensitivity CRP are expressed as median and interquartile
range.
Laboratory parameters between the groups were
compared using analysis of covariance (ANCOVA) while controlling
age as a covariate. Tukey's test was used as a post-hoc test to
determine any significant differences between the groups. The
performance of the chemerin and FGF-21 was assessed using
receiver-operating characteristic (ROC) curves, sensitivity,
specificity, and negative and positive predictive values. The
P-value was reported for the area under the curve (AUC) for the
best cut-off level. Diagnostic tests were assessed by this
classification: 0.90–1=excellent; 0.80–0.90=good; 0.70–0.80=fair;
0.60–0.70=poor; and 0.50–0.60=fail. A two-tailed P-value less than
0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Patient characteristics are provided in Table I and the results of laboratory
measurements in Tables II and
III. A total of 102 patients were
included and divided into three AoV stenosis severity groups and a
control group: A total of 18 patients had mild, 19 moderate, and 15
severe AoV stenosis, and there were 50 patients in the control
group. The average age of the patients in the aortic stenosis
groups and the control group were similar, and the mean level of
body mass index did not differ between the groups.
 | Table II.Sensitivity and specificity of the
chemerin and FGF-21 in patients with aortic valve stenosis
(including all severity degrees of stenosis). |
Table II.
Sensitivity and specificity of the
chemerin and FGF-21 in patients with aortic valve stenosis
(including all severity degrees of stenosis).
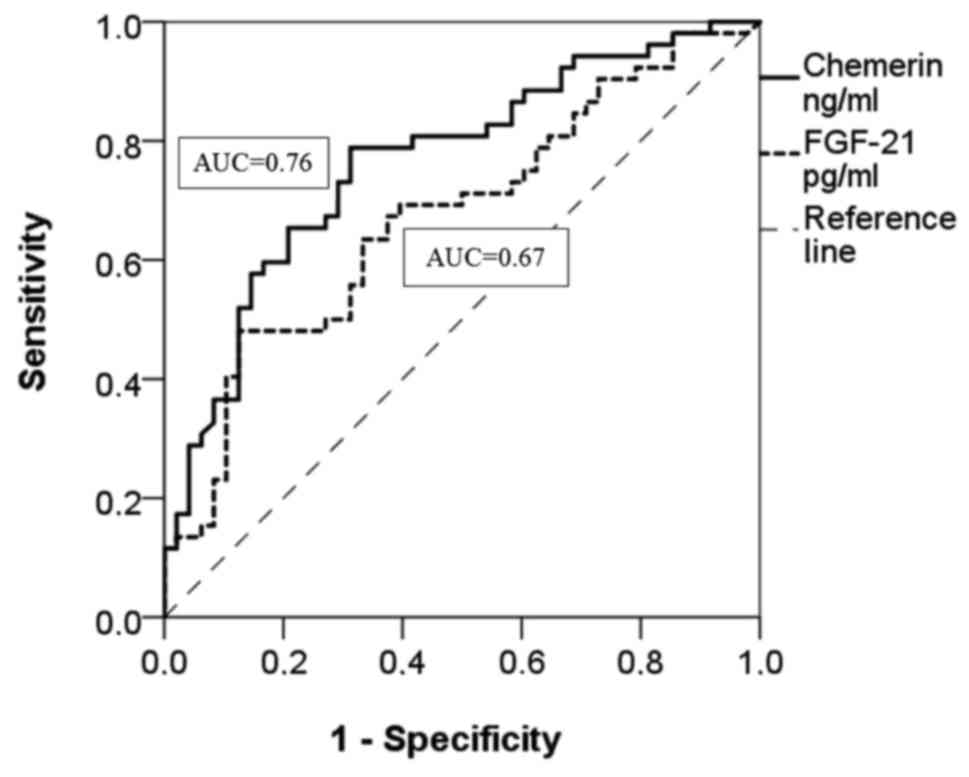
| Biomarker | AUC (95% CI) | P-value | Cut-off value | Sp % | Se % | NPV % | PPV % | Accuracy % |
|---|
| Chemerin
(ng/ml) | 0.76
(0.67–0.85) | <0.001 | 38.60 | 55 | 80 | 72.2 | 63.6 | 67.5 |
| FGF (pg/ml) | 0.67
(0.56–0.77) | 0.003 | 309.83 | 67 | 61.5 | 61.5 | 66.6 | 64.2 |
 | Table III.Sensitivity and specificity of
chemerin and FGF-21 in the mild AoV stenosis group. |
Table III.
Sensitivity and specificity of
chemerin and FGF-21 in the mild AoV stenosis group.
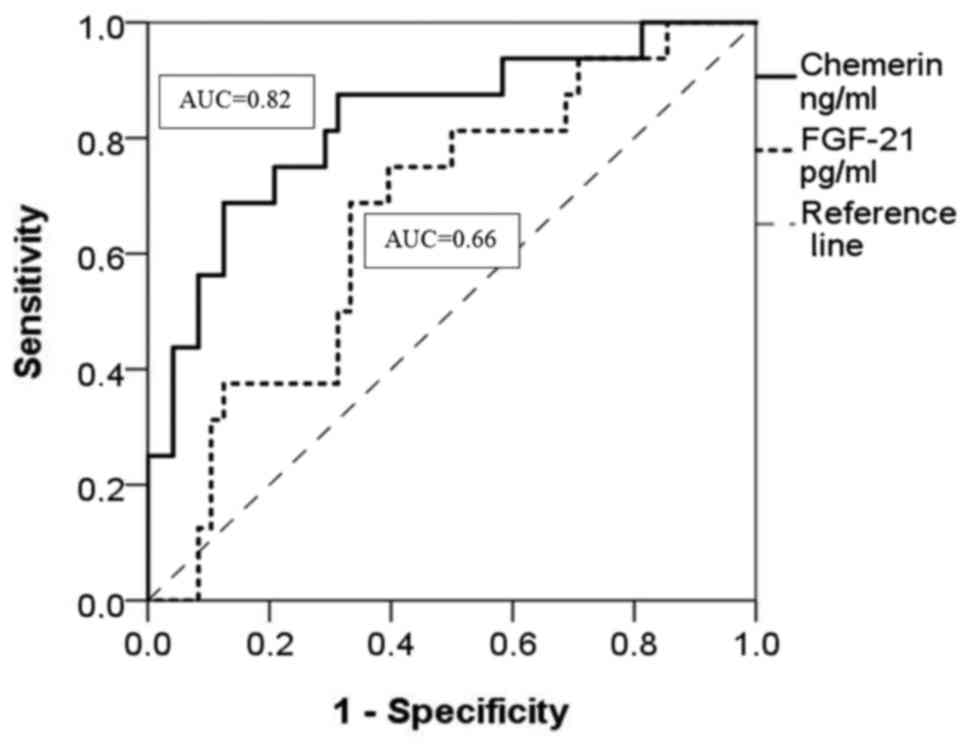
| Biomarker | AUC (95% CI) | P-value | Cut-off value | Sp % | Se % | NPV % | PPV % | Accuracy % |
|---|
| Chemerin
(ng/ml) | 0.82
(0.70–0.95) | <0.001 | 43.12 | 69 | 87 | 75.5 | 71.9 | 78 |
| FGF (pg/ml) | 0.66
(0.51–0.81) | 0.04 | 283.78 | 61 | 75 | 64.4 | 65.4 | 68 |
Chemerin and FGF-21 level differences
between the patient groups
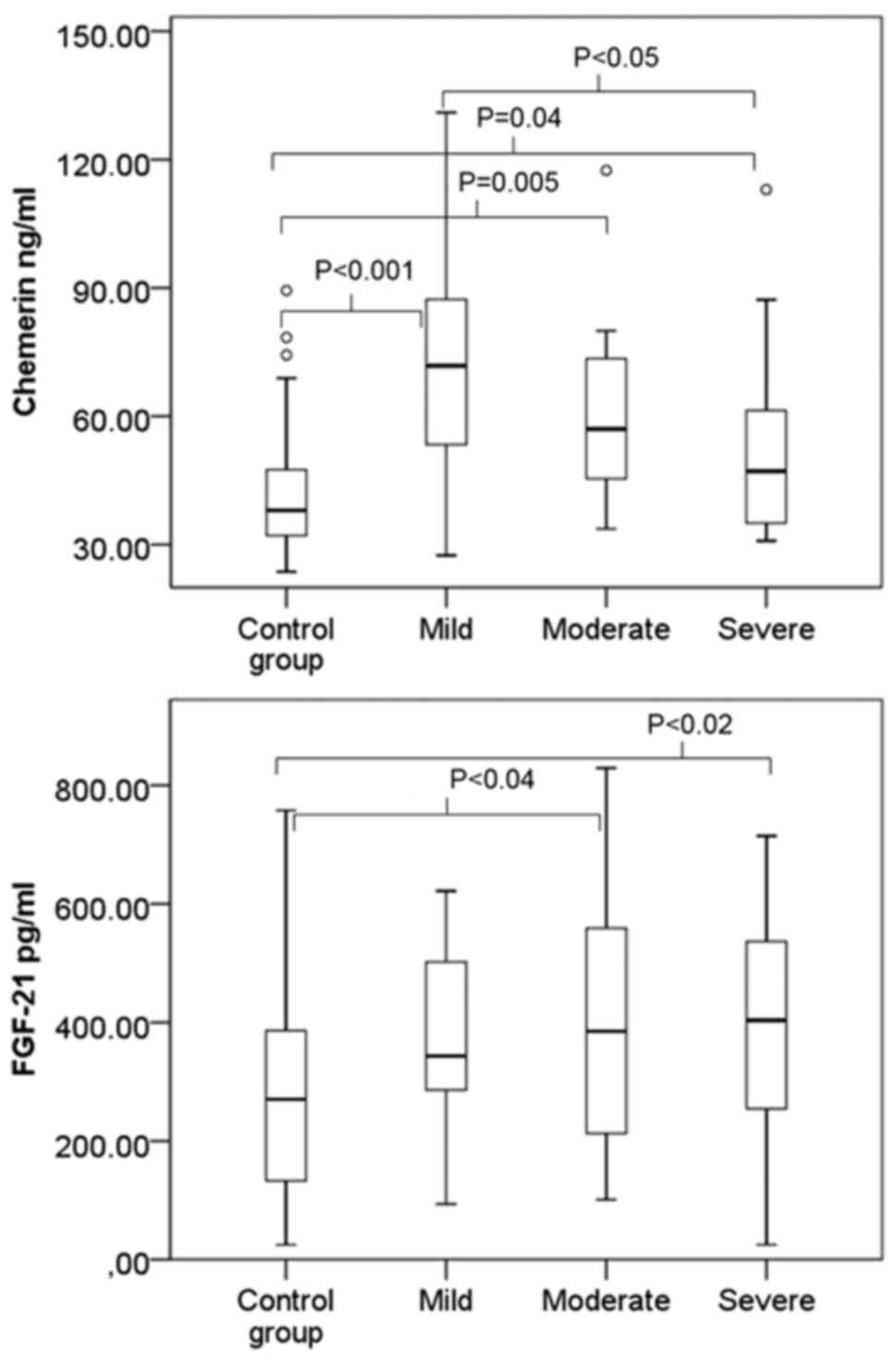
A box and whisker plot of chemerin and FGF-21 for
the control and AoV stenosis groups is shown in Fig. 1. The mean chemerin and FGF-21 levels
had a statistically significant difference in the study groups
(ANCOVA, P<0.001, and P=0.03; age as covariate). To evaluate a
difference between the groups, a post-hoc analysis with Tukey's
correction for chemerin was performed. Results showed that the
control group had a statistically significant difference with the
mild AoV stenosis group (P<0.001), with the moderate AoV
stenosis group (P=0.005), and with the severe AoV stenosis group
(P=0.04). The mild AoV stenosis group had a statistically
significant difference with the severe AoV stenosis group
(P<0.05), while the moderate and severe AoV stenosis groups had
no statistically significant difference (P>0.05). Post-hoc
analysis for FGF-21 revealed that the control group with moderate
and severe stenosis groups had a statistically significant
difference (P<0.05), but for the rest of the groups, there was
no statistically significant difference (P>0.05) (Fig. 1).
The chemerin serum level increased in all three
stenosis groups. The highest chemerin levels were found in mild and
moderate AoV stenosis and decreased along with the grade of
severity, compared to the control group (Fig. 1). The FGF-21 level increased in all of
the stenosis groups, but the FGF-21 level in serum increased along
with the AoV stenosis severity grade, reaching the maximum for
severe stenosis (Fig. 1).
Predictive values of chemerin as
biomarker
The ROC analysis showed that chemerin is a moderate
diagnostic marker in all AoV stenosis groups without grading the
severity (AUC =0.76; 0.70–0.80=fair; P<0.001) (Fig. 2 and Table
II). At the same time, the ROC curve shows that chemerin is a
specific and sensitive biomarker for mild AoV stenosis (AUC= 0.82;
0.80–0.90=good; P<0.001) (Fig. 3
and Table III). The optimal cut-off
value for chemerin in the ROC curve was 43.12 ng/ml at Se=87%,
Sp=69%. FGF-21 is a poor diagnostic marker among all AoV stenosis
groups without grading the severity (AUC=0.67; 0.56–0.77=poor;
P=0.003) (Fig. 2 and Table II) and in the mild AoV stenosis group
(AUC=0.66; 0.51–0.81=poor; P=0.04) (Fig.
3 and Table III).
Discussion
The results of the present study indicate that
chemerin is a novel biomarker for the detection of AoV stenosis in
the early stages, with a high sensitivity and specificity in the
mild AoV stenosis group. Chemerin is found in different
inflammatory and systemic processes all over the body (13,30,44–51):
Therefore, patients with systemic and inflammatory diseases were
excluded from this study. Thus, in the early stages of AoV
stenosis, an active inflammation process is dominant, as chemerin
is mainly considered an inflammatory biomarker (16,20,32) and in
our study its level was the highest in mild AoV stenosis. There is
a possibility that chemerin is secreted due to the activation of
valvular myofibrils, although there is a lack of evidence to
support this theory and further research should be conducted. In
more severe stages, the serum chemerin level decreased, leading to
the conclusion that passive extracellular matrix changes and
calcification predominate in moderate and severe calcific AoV
stenosis. The ROC analysis proves that chemerin sensitivity and
specificity as a diagnostic biomarker in mild AoV stenosis is high;
thus, this test may be engrained in cardiovascular risk panels for
laboratory analysis. Early detection of AoV stenosis may improve
prognosis and quality of life of patients, improve therapy
efficiency, and help to avoid late compensatory complications of
AoV stenosis, such as cardiac hypertrophy. It would be useful to
study other biomarkers that represent the inflammation process in
AoV stenosis and find appropriate medications for treatment. Along
with other studies mentioned above (21–27), our
study does not state whether chemerin has a pro- or
anti-inflammatory role and further research should be conducted
with other biomarkers that support varying theories.
Of note is that the FGF-21 level increases along
with the AoV stenosis severity grade. These results have led to the
conclusion that FGF-21 plays a protective role in calcific AoV
stenosis pathogenesis, because in the initial stages only a slight
increase in plasma FGF-21 was evident, but in the more severe grade
of AoV stenosis, it significantly increased. It has been suggested
that FGF-21 may have a cardioprotective role in different
cardiovascular diseases such as atrial fibrillation, myocardial
infarction, coronary artery disease, hypertension, and cardiac
hypertrophy, which supports our findings (38,42).
In conclusion, our study indicates that chemerin is
a good diagnostic biomarker in mild AoV stenosis and FGF-21 is a
poor diagnostic biomarker for AoV stenosis. The present data show
that the inflammatory process is dominant at the early stages of
stenosis, which leads us to the conclusion that it is an active
process not caused by atherosclerosis. Chemerin as a biomarker may
play a clinical role if we can prove that it is one of the causes
of inflammation; therefore, a possible treatment may be found.
FGF-21 undergoes a marked increase in severe AoV stenosis compared
to healthy controls, and it may have a cardioprotective role.
Further research is necessary to confirm these findings and to
specify what pathogenetic role these markers play in AoV
stenosis.
Glossary
Abbreviations
Abbreviations:
|
CMKLR1
|
chemokine-like receptor 1
|
|
ChemR23
|
chemerin receptor 23
|
|
FGF-21
|
fibroblast growth factor-21
|
|
AoV
|
aortic valve
|
|
CRP
|
C-reactive protein
|
References
|
1
|
Akerström F, Barderas MG and
Rodríguez-Padial L: Aortic stenosis: A general overview of
clinical, pathophysiological and therapeutic aspects. Expert Rev
Cardiovasc Ther. 11:239–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bossé Y, Mathieu P and Pibarot P:
Genomics: The next step to elucidate the etiology of calcific
aortic valve stenosis. J Am Coll Cardiol. 51:1327–1336. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wypasek E, Potaczek DP and Undas A:
Association of the C-reactive protein gene (CRP) rs1205 C>T
polymorphism with aortic valve calcification in patients with
aortic stenosis. Int J Mol Sci. 16:23745–23759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coffey S, Cox B and Williams MJA: The
prevalence, incidence, progression, and risks of aortic valve
sclerosis: A systematic review and meta-analysis. J Am Coll
Cardiol. 63:2852–2861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carabello BA: Aortic stenosis: A fatal
disease with but a single cure. JACC Cardiovasc Interv. 1:127–128.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Novaro GM and Griffin BP: Calcific aortic
stenosis: Another face of atherosclerosis? Cleve Clin J Med.
70:471–477. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fondard O, Detaint D, Iung B, Choqueux C,
Adle-Biassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel
JB, et al: Extracellular matrix remodelling in human aortic valve
disease: The role of matrix metalloproteinases and their tissue
inhibitors. Eur Heart J. 26:1333–1341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galante A, Pietroiusti A, Vellini M,
Piccolo P, Possati G, De Bonis M, Grillo RL, Fontana C and Favalli
C: C-reactive protein is increased in patients with degenerative
aortic valvular stenosis. J Am Coll Cardiol. 38:1078–1082. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fayad ZA, Mani V, Woodward M, Kallend D,
Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, et
al: dal-PLAQUE Investigators: Safety and efficacy of dalcetrapib on
atherosclerotic disease using novel non-invasive multimodality
imaging (dal-PLAQUE): A randomised clinical trial. Lancet.
378:1547–1559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duvic M, Nagpal S, Asano AT and
Chandraratna RAS: Molecular mechanisms of tazarotene action in
psoriasis. J Am Acad Dermatol. 37:S18–S24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roh SG, Song S-H, Choi K-C, Katoh K,
Wittamer V, Parmentier M and Sasaki S: Chemerin-a new adipokine
that modulates adipogenesis via its own receptor. Biochem Biophys
Res Commun. 362:1013–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wittamer V, Franssen J-D, Vulcano M,
Mirjolet J-F, Le Poul E, Migeotte I, Brézillon S, Tyldesley R,
Blanpain C, Detheux M, et al: Specific recruitment of
antigen-presenting cells by chemerin, a novel processed ligand from
human inflammatory fluids. J Exp Med. 198:977–985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Döcke S, Lock JF, Birkenfeld AL, Hoppe S,
Lieske S, Rieger A, Raschzok N, Sauer IM, Florian S, Osterhoff MA,
et al: Elevated hepatic chemerin mRNA expression in human
non-alcoholic fatty liver disease. Eur J Endocrinol. 169:547–557.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weigert J, Neumeier M, Wanninger J,
Filarsky M, Bauer S, Wiest R, Farkas S, Scherer MN, Schäffler A,
Aslanidis C, et al: Systemic chemerin is related to inflammation
rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf).
72:342–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rourke JL, Dranse HJ and Sinal CJ: Towards
an integrative approach to understanding the role of chemerin in
human health and disease. Obes Rev. 14:245–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lehrke M, Becker A, Greif M, Stark R,
Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker
C, et al: Chemerin is associated with markers of inflammation and
components of the metabolic syndrome but does not predict coronary
atherosclerosis. Eur J Endocrinol. 161:339–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisinger K, Krautbauer S, Wiest R, Weiss
TS and Buechler C: Reduced serum chemerin in patients with more
severe liver cirrhosis. Exp Mol Pathol. 98:208–213. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu P, Jiang W, Lu B and Shi Z: Chemerin is
associated with inflammatory markers and metabolic syndrome
phenotypes in hypertension patients. Clin Exp Hypertens.
36:326–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kostopoulos CG, Spiroglou SG, Varakis JN,
Apostolakis E and Papadaki HH: Chemerin and CMKLR1 expression in
human arteries and periadventitial fat: A possible role for local
chemerin in atherosclerosis? BMC Cardiovasc Disord. 14:562014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goralski KB, McCarthy TC, Hanniman EA,
Zabel BA, Butcher EC, Parlee SD, Muruganandan S and Sinal CJ:
Chemerin, a novel adipokine that regulates adipogenesis and
adipocyte metabolism. J Biol Chem. 282:28175–28188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bondue B, De Henau O, Luangsay S, Devosse
T, de Nadaï P, Springael JY, Parmentier M and Vosters O: The
chemerin/ChemR23 system does not affect the pro-inflammatory
response of mouse and human macrophages ex vivo. PLoS One.
7:e400432012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bondue B, Wittamer V and Parmentier M:
Chemerin and its receptors in leukocyte trafficking, inflammation
and metabolism. Cytokine Growth Factor Rev. 22:331–338. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ernst MC and Sinal CJ: Chemerin: At the
crossroads of inflammation and obesity. Trends Endocrinol Metab.
21:660–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hart R and Greaves DR: Chemerin
contributes to inflammation by promoting macrophage adhesion to
VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J
Immunol. 185:3728–3739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaur J, Adya R, Tan BK, Chen J and Randeva
HS: Identification of chemerin receptor (ChemR23) in human
endothelial cells: Chemerin-induced endothelial angiogenesis.
Biochem Biophys Res Commun. 391:1762–1768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monnier J, Lewén S, O'Hara E, Huang K, Tu
H, Butcher EC and Zabel BA: Expression, regulation, and function of
atypical chemerin receptor CCRL2 on endothelial cells. J Immunol.
189:956–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao RJ and Wang H: Chemerin/ChemR23
signaling axis is involved in the endothelial protection by K(ATP)
channel opener iptakalim. Acta Pharmacol Sin. 32:573–580. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kukla M, Zwirska-Korczala K, Gabriel A,
Waluga M, Warakomska I, Szczygiel B, Berdowska A, Mazur W,
Wozniak-Grygiel E and Kryczka W: Chemerin, vaspin and insulin
resistance in chronic hepatitis C. J Viral Hepat. 17:661–667.
2010.PubMed/NCBI
|
|
29
|
Cash JL, Hart R, Russ A, Dixon JPC,
Colledge WH, Doran J, Hendrick AG, Carlton MB and Greaves DR:
Synthetic chemerin-derived peptides suppress inflammation through
ChemR23. J Exp Med. 205:767–775. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mariani F and Roncucci L: Chemerin/chemR23
axis in inflammation onset and resolution. Inflamm Res. 64:85–95.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Semba RD, Crasto C, Strait J, Sun K,
Schaumberg DA and Ferrucci L: Elevated serum fibroblast growth
factor 21 is associated with hypertension in community-dwelling
adults. J Hum Hypertens. 27:397–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van de Voorde J, Pauwels B, Boydens C and
Decaluwé K: Adipocytokines in relation to cardiovascular disease.
Metabolism. 62:1513–1521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Yeung DCY, Karpisek M, Stejskal
D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, et al: Serum
FGF21 levels are increased in obesity and are independently
associated with the metabolic syndrome in humans. Diabetes.
57:1246–1253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
An SY, Lee MS, Yi SA, Ha ES, Han SJ, Kim
HJ, Kim DJ and Lee KW: Serum fibroblast growth factor 21 was
elevated in subjects with type 2 diabetes mellitus and was
associated with the presence of carotid artery plaques. Diabetes
Res Clin Pract. 96:196–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chow WS, Xu A, Woo YC, Tso AWK, Cheung
SCW, Fong CHY, Tse HF, Chau MT, Cheung BM and Lam KS: Serum
fibroblast growth factor-21 levels are associated with carotid
atherosclerosis independent of established cardiovascular risk
factors. Arterioscler Thromb Vasc Biol. 33:2454–2459. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S,
Xiao J, Wang X, Feng W and Li X: Serum levels of FGF-21 are
increased in coronary heart disease patients and are independently
associated with adverse lipid profile. PLoS One. 5:e155342010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu P, Zhang Y, Liu Y, Yuan Q, Song L, Liu
M, Liu Z, Yang Y, Li J, Li D, et al: Fibroblast growth factor 21
attenuates hepatic fibrogenesis through TGF-β/smad2/3 and NF-κB
signaling pathways. Toxicol Appl Pharmacol. 290:43–53. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han X, Chen C, Cheng G, Xie C, Yang M,
Shou X and Sun C: Serum fibroblast growth factor 21 levels are
increased in atrial fibrillation patients. Cytokine. 73:176–180.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Itoh N and Ornitz DM: Fibroblast growth
factors: From molecular evolution to roles in development,
metabolism and disease. J Biochem. 149:121–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Patel V, Adya R, Chen J, Ramanjaneya M,
Bari MF, Bhudia SK, Hillhouse EW, Tan BK and Randeva HS: Novel
insights into the cardio-protective effects of FGF21 in lean and
obese rat hearts. PLoS One. 9:e871022014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Planavila A, Redondo I, Hondares E,
Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt
M, van Bilsen M and Villarroya F: Fibroblast growth factor 21
protects against cardiac hypertrophy in mice. Nat Commun. 4:2019
06;2013. doi: 10.1038/ncomms3019.
|
|
42
|
Planavila A, Redondo-Angulo I and
Villarroya F: FGF21 and cardiac physiopathology. Front Endocrinol.
6:1332015. View Article : Google Scholar
|
|
43
|
Vahanian A and Iung B: The new ESC/EACTS
guidelines on the management of valvular heart disease. Arch
Cardiovasc Dis. 105:465–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gisondi P, Lora V, Bonauguri C, Russo A,
Lippi G and Girolomoni G: Serum chemerin is increased in patients
with chronic plaque psoriasis and normalizes following treatment
with infliximab. Br J Dermatol. 168:749–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Herenius MMJ, Oliveira ASF, Wijbrandts CA,
Gerlag DM, Tak PP and Lebre MC: Anti-TNF therapy reduces serum
levels of chemerin in rheumatoid arthritis: A new mechanism by
which anti-TNF might reduce inflammation. PLoS One. 8:e578022013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Makrilakis K, Fragiadaki K, Smith J,
Sfikakis PP and Kitas GD: Interrelated reduction of chemerin and
plasminogen activator inhibitor-1 serum levels in rheumatoid
arthritis after interleukin-6 receptor blockade. Clin Rheumatol.
34:419–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rutkowski P, Sledzinski T, Zielinska H,
Lizakowski S, Goyke E, Szrok-Wojtkiewicz S, Swierczynski J and
Rutkowski B: Decrease of serum chemerin concentration in patients
with end stage renal disease after successful kidney
transplantation. Regul Pept. 173:55–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stepan H, Philipp A, Roth I, Kralisch S,
Jank A, Schaarschmidt W, Lössner U, Kratzsch J, Blüher M, Stumvoll
M, et al: Serum levels of the adipokine chemerin are increased in
preeclampsia during and 6 months after pregnancy. Regul Pept.
168:69–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weigert J, Obermeier F, Neumeier M,
Wanninger J, Filarsky M, Bauer S, Aslanidis C, Rogler G, Ott C,
Schäffler A, et al: Circulating levels of chemerin and adiponectin
are higher in ulcerative colitis and chemerin is elevated in
Crohn's disease. Inflamm Bowel Dis. 16:630–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu QL, Zhu M, Jin Y, Wang N, Xu HX, Quan
LM, Wang SS and Li SS: The predictive value of the first-trimester
maternal serum chemerin level for pre-eclampsia. Peptides.
62:150–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang J, Jin HC, Zhu AK, Ying RC, Wei W
and Zhang FJ: Prognostic significance of plasma chemerin levels in
patients with gastric cancer. Peptides. 61:7–11. 2014. View Article : Google Scholar : PubMed/NCBI
|