Introduction
Rejection is a major threat to tissue and organ
transplantation, and immunosuppression is required to preserve
graft function and survival (1).
Although >90% of graft survival in the majority of organ
transplants survive by immunosuppression, the immunosuppressive
drugs have adverse side effects on various cells and tissues.
Long-term administration of the immunosuppressive compounds cause
nephrotoxicity, susceptibility to infection and onset of diabetes
(2,3).
Therefore, additional treatment options are required.
CD4+ T cells have long been known to be
central in mediating transplant rejection (4). Acute allograft rejection is a T
cell-dependent phenomenon and may be triggered by different types
of helper T (Th) cell. Th1 cell responses initiate allograft
rejection by promoting proliferation of alloreactive
CD8+ T cells or by inducing a delayed type
hypersensitivity reaction mediated by macrophages. In previous
years, it has been reported that Th1 cells and Th17 cells mediate
acute allograft rejection by recruiting neutrophils and monocytes
into the graft, which subsequently contributes to transplant
inflammation (5–7). Furthermore, it has been demonstrated
that regulatory T cells (Tregs) induce and maintain tolerance to
the allograft in experimental and clinical transplantation
(8).
Proteoglycan (PG) consists of a core protein and one
or more covalently attached glycosaminoglycan chain(s). It is a
compound of extracellular matrix materials that exist in connective
tissue, such as skin, bone, cartilage and vascular walls by forming
a complex with collagen, fibronectin, laminin, hyaluronic acid and
other glycoproteins. In corporation with collagen, fibronectin and
laminin, PG has been demonstrated to be involved in cellular
proliferation and adhesion (9). It
has previously been demonstrated that PG extracted from salmon
nasal cartilage exerts a potent effect on suppression of
inflammatory responses induced by heat-killed Escherichia
coli in mouse macrophages (10).
In addition, daily oral administration of PG attenuates the
severity of experimental inflammatory colitis (11), autoimmune encephalomyelitis (EAE)
(12) and collagen-induced arthritis
(13). Attenuation of the systemic
inflammation in colitis and EAE models by daily oral administration
of PG depends on suppression of Th17 lineage differentiation and an
induction of Foxp3+ Treg cells (11,12).
Additionally, daily oral administration of PG reduced the
accumulation of M1 macrophages, which induce inflammation via the
production of proinflammatory cytokines, in the adipose tissue of
high-fat diet-induced obesity mice (14).
In the present study, the effect of salmon nasal
cartilage PG on skin graft transplantation was examined to
determine whether oral administration of PG could prolong graft
survival.
Materials and methods
Mice
C57BL/6 mice and BALB/c mice (age, 6–8 weeks), were
purchased from CLEA Japan, Inc. (Tokyo, Japan). The mice were
provided with food and water ad libitum. The C57BL/6 mice
and BALB/c mice were kept separately in a temperature-controlled
room (22°C) under a 12 h light/dark cycle and specific
pathogen-free conditions at the Institute for Animal
Experimentation, Hirosaki University Graduate School of Medicine
(Hirosaki, Japan). All animal experiments in the present study were
conducted in accordance with the Animal Research Ethics Committee
of the Hirosaki University Graduate School of Medicine, and
followed the Hirosaki University Guidelines for Animal
Experimentation.
Preparation and administration of
PG
Salmon nasal cartilage PG was purchased from
Kakuhiro Co., Ltd. (Hirosaki, Japan). Lyophilized PG powder was
dissolved in phosphate-buffered saline (PBS) to a concentration of
10 mg/ml. C57BL/6 mice were administrated with 2 mg PG per os
daily. PBS served as a control.
Skin graft model
C57BL/6 recipient mice were orally administrated
with PG for 10 days, then skin grafting was performed. For the
experimental models of skin grafts, the procedure described by
Billingham and Medawar (15) was
adopted. Briefly, BALB/c donor mice were sacrificed by cervical
dislocation, and 0.5×0.5 cm sections of tail skin were removed and
immersed in RPMI-1640 medium (Nissui Pharmaceutical Co., Ltd.,
Tokyo, Japan) supplemented with 10% fetal calf serum (JRH
Biosciences, Lenexa, KS, USA). The C57BL/6 recipient mice were
anesthetized with nembutal (Dainippon Sumitomo Pharma Co., Ltd.,
Osaka, Japan), and the fur was shaved off the dorsal trunk. At the
shaved area, 0.5×0.5 cm of skin in each recipient mouse was
removed. One piece of donor tail skin was sutured to the exposed
tissue of each recipient. Animals were maintained in individual
cages and observed daily. PG was continuously administered daily
until graft rejection was observed. In each experiment, 3–4 mice
were used and three independent experiments were performed.
Skin graft survival
To detect graft rejection, the sizes of the grafts
were recorded. The initial graft size is referred to as 100%. The
criterion for graft rejection was based on the graft size being
<20%.
Histology
The skin tissue was collected from the graft site of
C57BL/6 recipient mice. After the tissue was fixed with 10%
neutral-buffered formalin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), it was embedded in paraffin and sliced into 4 µm thick
sections. The sections were stained at room temperature with
hematoxylin for 10 min and eosin for 15 min, and observed under a
BZ-X700 microscope (Keyence Corporation, Osaka, Japan). Three
histologists evaluated the sections of the skin tissue.
Statistical analysis
Data are expressed as means ± standard deviation
(mean ± SD). For graft survival, statistical analysis was performed
using Kaplan-Meier method and the statistical significance was
evaluated using the log rank test. For graft size, statistical
analysis was calculated using the unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
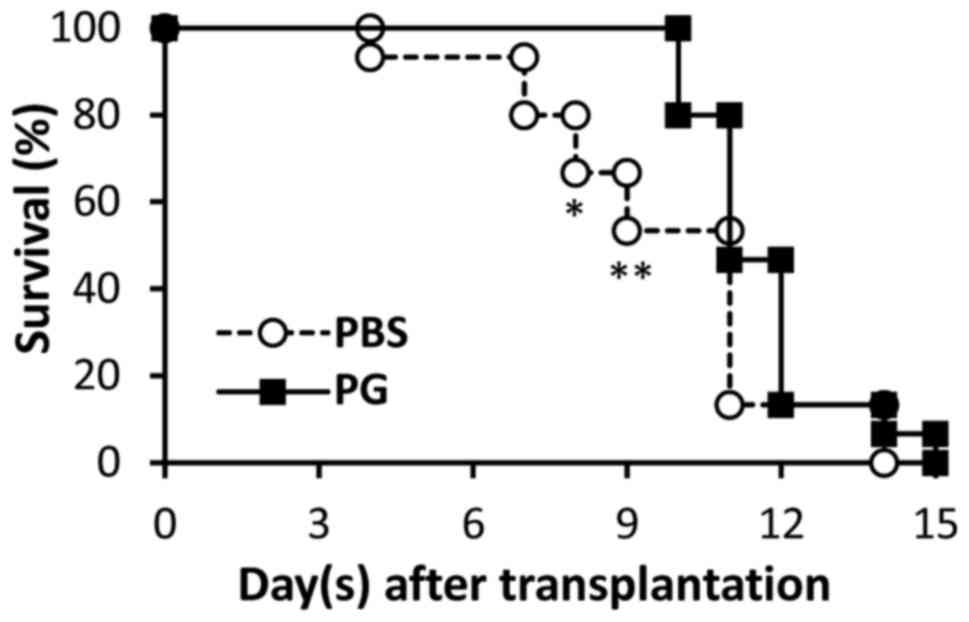
Skin graft survival
Following transplantation, survival of skin grafts
was observed daily. The results are presented in Fig. 1. Graft rejection was observed from day
4 in the control mice, whereas the grafts were rejected from day 10
in the PG-administered mice. Significant differences in graft
survival between the control and PG-administered mice were observed
on day 8 and 9. On day 15, the grafts from the PG-administered mice
were totally rejected, which was identified in the control
mice.
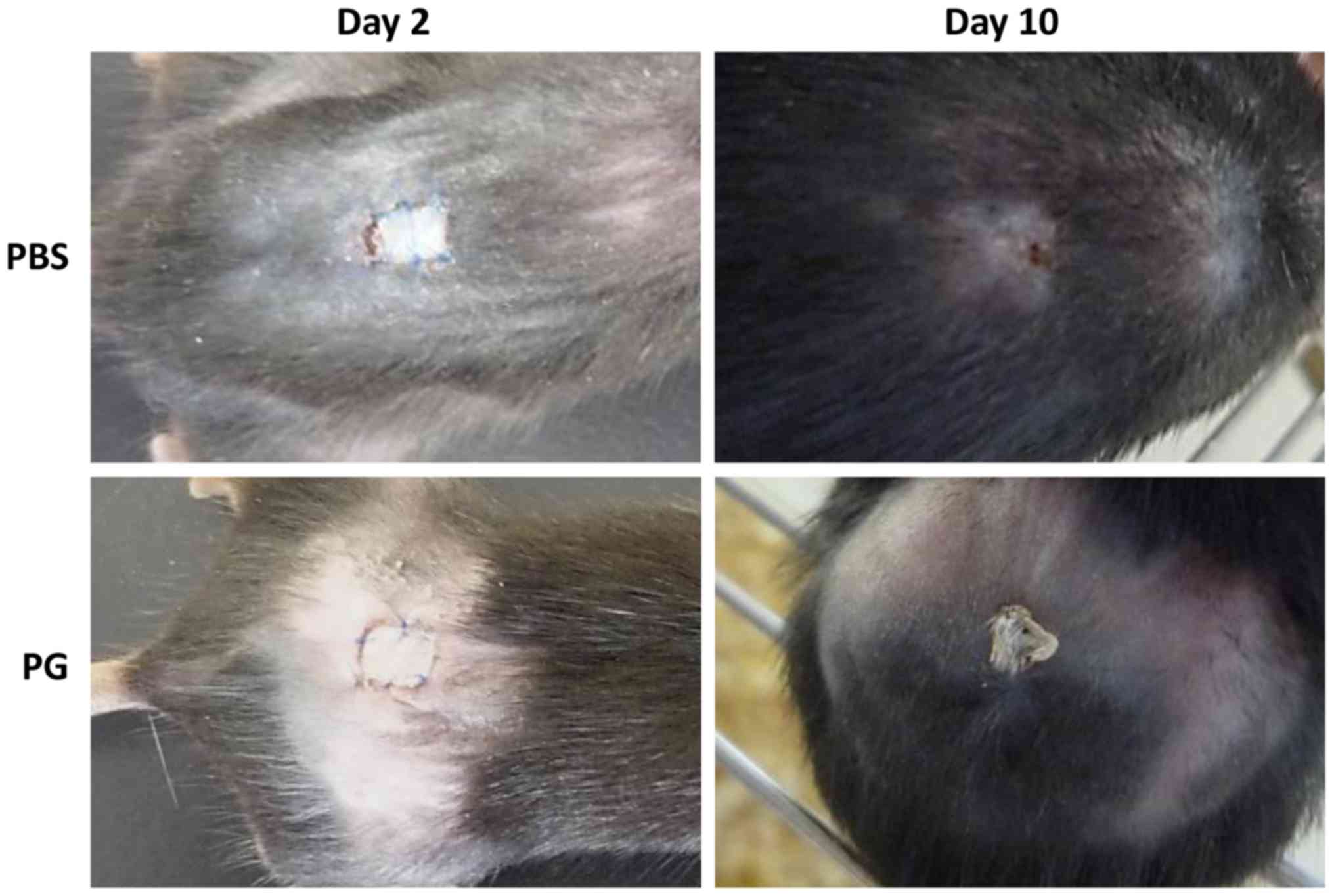
Macroscopic appearance skin
grafts
The macroscopic appearance of skin grafts was
observed (Fig. 2). On day 2 of
transplantation, the grafts were retained in the two groups and the
macroscopic appearance between the control and PG-administered mice
was not significantly different. From day 3 of transplantation, the
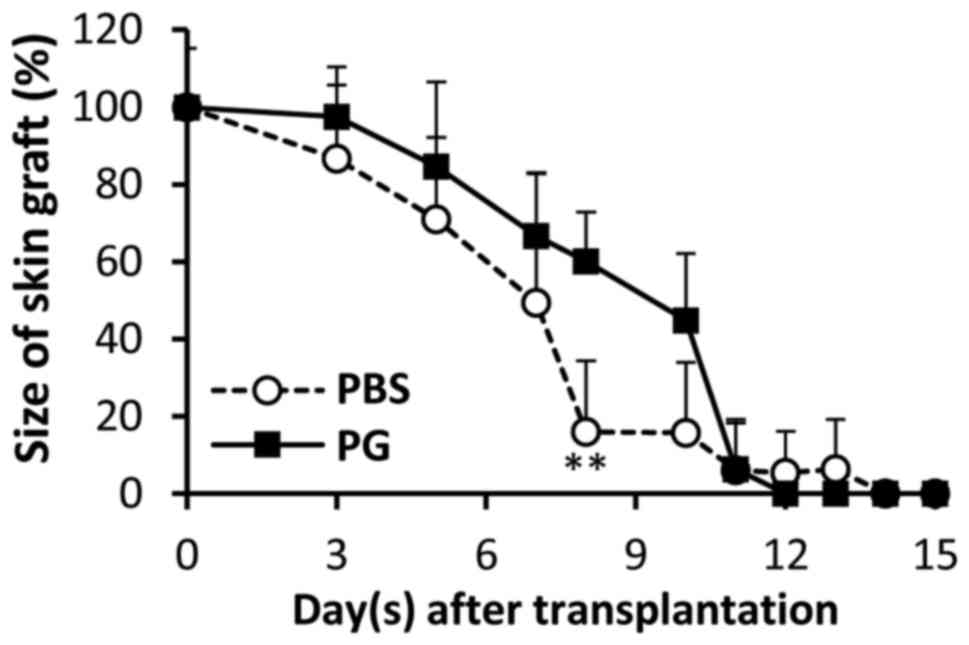
sizes of the grafts were gradually reduced and the remaining graft
sizes in the control mice were smaller than those in the
PG-administered mice. A significant difference between the graft
sizes was observed on day 8 (Fig. 3).
On day 10, the grafts of the control mice were rejected, whereas
the grafts of PG-administered mice were retained (Fig. 2). Although the edge of grafts in the
PG-administered mice dried, the middle region of grafts tightly
adhered to the recipient skin.
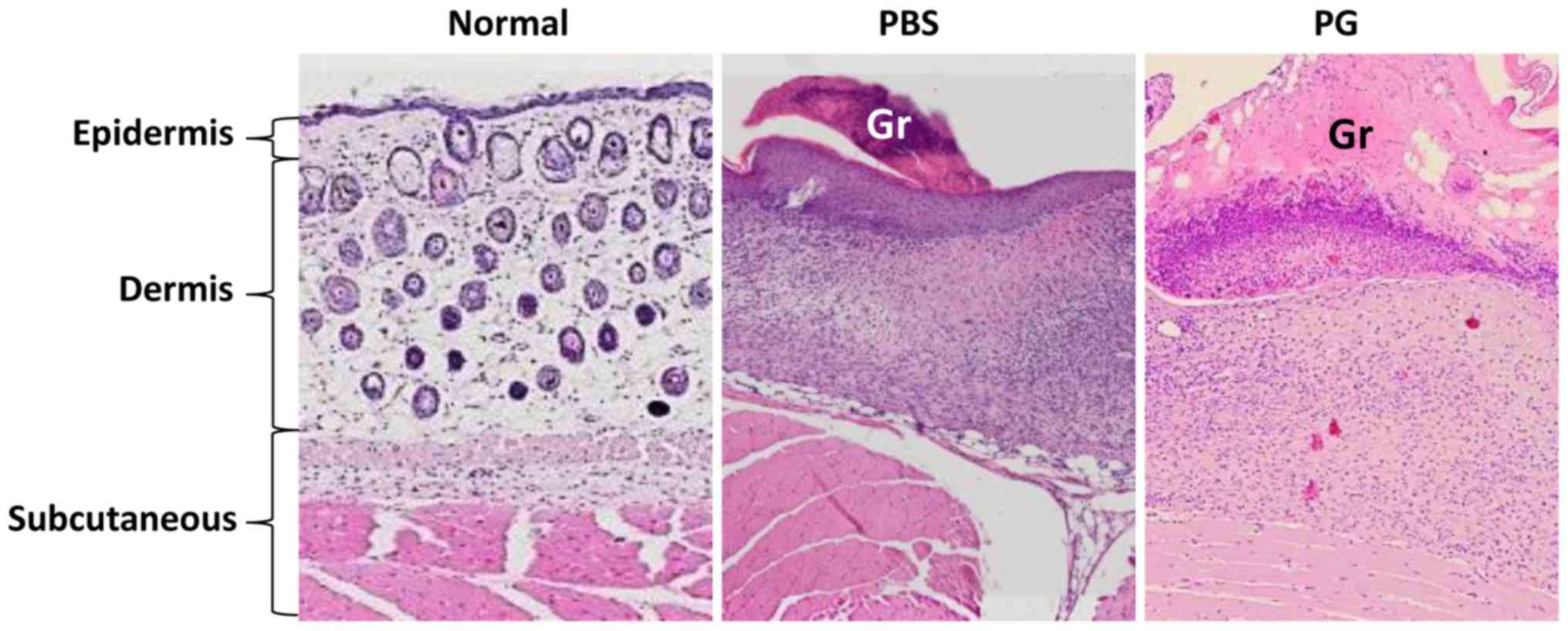
Histology at graft sites
The histology of skin grafts was observed (Fig. 4). The grafts in the control mice were
smaller than those in the PG-administered mice. They were loosely
attached to the recipient skin. In comparison to the normal skin,
the subcutaneous layer of skin graft was thickened. A reduction of
cell infiltration under the graft was observed in the
PG-administered mice.
Discussion
In the present study, the prophylactic effect of
salmon nasal cartilage PG on skin allograft rejection was
investigated. Oral administration of PG was demonstrated to
attenuate the progress of skin graft rejection, although allograft
rejection was not finally prevented.
Graft rejection was observed from day 4 in the
PBS-administered mice, whereas the rejection in PG-administered
mice was not observed until day 10. Significant differences in
graft survival between the control and PG-administered mice were
identified on days 8 and 9 (Fig. 1;
P=0.0177 and 0.0034, respectively). Reduction of graft size was
correlated with graft survival (Fig.
3). Th17 cells reportedly mediate acute allograft rejection by
recruiting neutrophils and monocytes into the graft, which then
contribute to transplant inflammation (5–7). It is
well known that interleukin (IL)-17A promotes the expression of
neutrophil- and monocyte-recruiting chemokines, and the produced
chemokines elicit recruitment of phagocytes (16,17). Our
previous studies demonstrated that oral administration of PG
inhibited recruitment of macrophages and neutrophils onto
inflammatory sites via suppression of IL-17-induced chemokines in
EAE (12) and collagen-induced
arthritis (13). In the present
study, reduction of cell infiltration under the graft was observed
in PG-administered mice (Fig. 4).
Therefore, prolonged survival of the grafts in PG-administered mice
may be due to inhibition of phagocyte recruitment through
suppression of IL-17-induced chemokines, as shown in other mouse
inflammatory models (11–14). To support this hypothesis, further
evaluations of phagocytes, Th17 cells, IL-17, as well as other
associated chemokines are required. In addition, molecular analysis
of nuclear factor-κB and Janus kinase/signal transducers and
activators of transcription signaling on skin transplantation would
be examined to provide an understanding of the molecular mechanism
of PG.
To date, various immunosuppressant agents have been
developed and used to prevent the rejection of transplanted organs
or tissues (18). The majority of
immunosuppressive agents act non-selectively, resulting in common
side effects, including increased susceptibility to infections and
decreased cancer immunosurveillance. Calcineurin inhibitors are
associated with nephrotoxicity, cardiotoxicity and neurotoxicity
(18), while everolimus induces
stomatitis (19). These side effects
affect the treatment course, such as discontinuation of therapy or
dose reduction (19). To prolong the
time before graft rejection during these irregular treatment
courses, administration of mild immunosuppressive agents becomes an
attractive option. Numerous natural compounds, such as
non-digestible saccharides have been shown to suppress the immune
response and promote health homeostasis (20). Fructooligosaccharides are found in
various fruits and vegetables, and dietary supplementation with
fructooligosaccharides attenuates allergic airway inflammation
(21). However, to the best of our
knowledge, their actions on graft rejection have not been
examined.
In conclusion, in the present study, the suppressive
effect of allograft rejection by PG was presented. Its action was
not exhaustive, as the skin grafts were finally rejected in the
PG-administered mice (Fig. 1), which
indicates that PG is a mild anti-inflammatory substance. Therefore,
salmon PG may present as a useful adjunct agent for
immunosuppressant drugs. According to our previous studies,
although PG demonstrates an anti-inflammatory effect, daily
administration of PG did not affect bacterial infections or tumor
growth in mice (unpublished data). Therefore, this biopolymer is
considered to be a safe agent for prevention of graft
rejection.
Acknowledgements
The present study was supported by the Ministry of
Education, Culture, Sports, Science and Technology City Area
Program for the Promotion of Science and Technology in Regional
Areas (Japan).
Glossary
Abbreviations
Abbreviations:
|
PG
|
proteoglycan
|
|
Tregs
|
regulatory T cells
|
|
EAE
|
autoimmune encephalomyelitis
|
|
PBS
|
phosphate-buffered saline
|
|
IL-17A
|
interleukin-17A
|
References
|
1
|
Winsett R, Stratta RJ, Alloway R, Wicks MN
and Hathaway DK: Immunosuppressant side effect profile does not
differ between organ transplant types. Clin Transplant. 15:46–50.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nankivell BJ, Borrows RJ, Fung CL-S,
O'Connell PJ, Chapman JR and Allen RDM: Calcineurin inhibitor
nephrotoxicity: longitudinal assessment by protocol histology.
Transplantation. 78:557–565. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schweer T, Gwinner W, Scheffner I, Schwarz
A, Haller H and Blume C: High impact of rejection therapy on the
incidence of post-transplant diabetes mellitus after kidney
transplantation. Clin Transplant. 28:512–519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang S, Herrera O and Lechler RI: New
spectrum of allorecognition pathways: implications for graft
rejection and transplantation tolerance. Curr Opin Immunol.
16:550–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan X, Paez-Cortez J, Schmitt-Knosalla I,
D'Addio F, Mifarrej B, Donnarumma M, Habicht A, Clarkson MR,
Iacomini J, Glimcher LH, et al: A novel role of CD4 Th17 cells in
mediating cardiac allograft rejection and vasculopathy. J Exp Med.
205:3133–3144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gorbaxheva V, Fan R, Li X and Valujskikh
A: Interleukin-17 promotes early allograft inflammation. Am J
Pathol. 177:1265–1273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itoh S, Nakae S, Axtell RC, Velotta JB,
Kimura N, Kajiwara N, Iwakura Y, Saito H, Adachi H, Steinman L, et
al: IL-17 contributes to the development of chronic rejection in a
murine heart transplant model. J Clin Immunol. 30:235–240. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wood KJ, Bushell A and Hester J:
Regulatory immune cells in transplantation. Nat Rev Immunol.
12:417–430. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Danen EH and Yamada KM: Fibronectin,
integrins, and growth control. J Cell Physiol. 189:1–13. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sashinami H, Takagaki K and Nakane A:
Salmon cartilage proteoglycan modulates cytokine responses to
Escherichia coli in mouse macrophages. Biochem Biophys Res Commun.
351:1005–1010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitsui T, Sashinami H, Sato F, Kijima H,
Ishiguro Y, Fukuda S, Yoshihara S, Hakamada K and Nakane A: Salmon
cartilage proteoglycan suppresses mouse experimental colitis
through induction of Foxp3+ regulatory T cells. Biochem Biophys Res
Commun. 402:209–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sashinami H, Asano K, Yoshimura S, Mori F,
Wakabayashi K and Nakane A: Salmon proteoglycan suppresses
progression of mouse experimental autoimmune encephalomyelitis via
regulation of Th17 and Foxp3+ regulatory T cells. Life Sci.
91:1263–1269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshimura S, Asano K and Nakane A:
Attenuation of collagen-induced arthritis in mice by salmon
proteoglycan. Biomed Res Int. 2014:4064532014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirose S, Asano K and Nakane A:
Attenuation of obesity-induced inflammation in mice orally
administered with salmon cartilage proteoglycan, a prophylactic
agent. Biochem Biophys Res Commun. 484:480–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Billingham RE and Medawar PB: The
technique of free skin grafting in mammals. J Exp Biol. 28:385–402.
1951.
|
|
16
|
Ye P, Rodriguez FH, Kanaly S, Stocking KL,
Schurr J, Schwarzenberger P, Oliva P, Huang W, Zhang P, Zhang J, et
al: Requirement of interleukin 17 receptor signaling for lung CXC
chemokine and granulocyte colony-stimulating factor expression,
neutrophil recruitment, and host defense. J Exp Med. 194:519–527.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaffen SL: An overview of IL-17 function
and signaling. Cytokine. 43:402–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mika A and Stepnowski P: Current methods
of the analysis of immunosuppressive agents in clinical materials.
J Pharm Biomed Anal. 127:207–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji YD, Aboalela A and Villa A:
Everolimus-associated stomatitis in a patient who had renal
transplant. BMJ Case Rep. 2016:bcr20162175132016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kerperien J, Jeurink PV, Wehkamp T, van
der Veer A, van de Kant HJ, Hofman GA, van Esch EC, Garssen J,
Willemsen LE and Knippels LM: Non-digestible oligosaccharides
modulate intestinal immune activation and suppress cow's milk
allergic symptoms. Pediatr Allergy Immunol. 25:747–754. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yasuda A, Inoue KI, Sanbongi C, Yanagisawa
R, Ichinose T, Yoshikawa T and Takano H: Dietary supplementation
with fructooligosaccharides attenuates airway inflammation related
to house dust mite allergen in mice. Int J Immunopathol Pharmacol.
23:727–735. 2010. View Article : Google Scholar : PubMed/NCBI
|