Introduction
Psoriasis is an inflammatory, T-cell-mediated skin
disease possessing a variable distribution, severity and course
from patient to patient (1). It
affects ~3% of the world population, although the regional
prevalence may differ (2).
Immunologically it is characterized by the intense proliferation
and aberrant differentiation of keratinocytes, and the infiltration
of the epidermis with lymphocytes and neutrophils wherein T-cells,
dendritic cells and certain inflammatory cytokines act as the
principal actors (3–6). The major inflammatory molecules
characteristic for psoriasis are tumor necrosis factor-α (TNF-α),
interferon-γ (IFN-γ), transforming growth factor-β (TGF-β) and
interleukins, including interleukin (IL)-1, IL-17 and IL-22
(4,5).
In addition to immunological involvement, psoriasis has been shown
to possess genetic susceptibility and is susceptible to
environmental triggers (3,7). The key role of microRNAs (miRNAs) in
regulating the hyperproliferation, differentiation of
keratinocytes, apoptosis and atypical immune activation in
psoriasis has been widely discussed (5,7). miRNAs
are small non-coding RNAs derived from larger primary RNA
transcripts in the human genome, with significant roles in
post-transcriptional gene expression regulation (8). miRNAs are transcribed by polymerase II
or III into primary precursor transcripts, which are first
processed in the nucleus by Drosha and DiGeorge syndrome critical
region 8 enzymes (9). Following
nuclear processing, a precursor miRNA is then transported by the
exportin 5-Ran GTPase shuttle system into the cytoplasm for final
processing by Dicer and RNase III-like endonuclease in order to
obtain mature miRNAs (10).
Subsequently, an RNA-induced silencing complex is formed, which
regulates gene expression by causing target mRNA degradation or
translation repression (9) (Fig. 1). Previous studies have shown that
there are multiple dysregulated miRNAs in psoriasis (3).
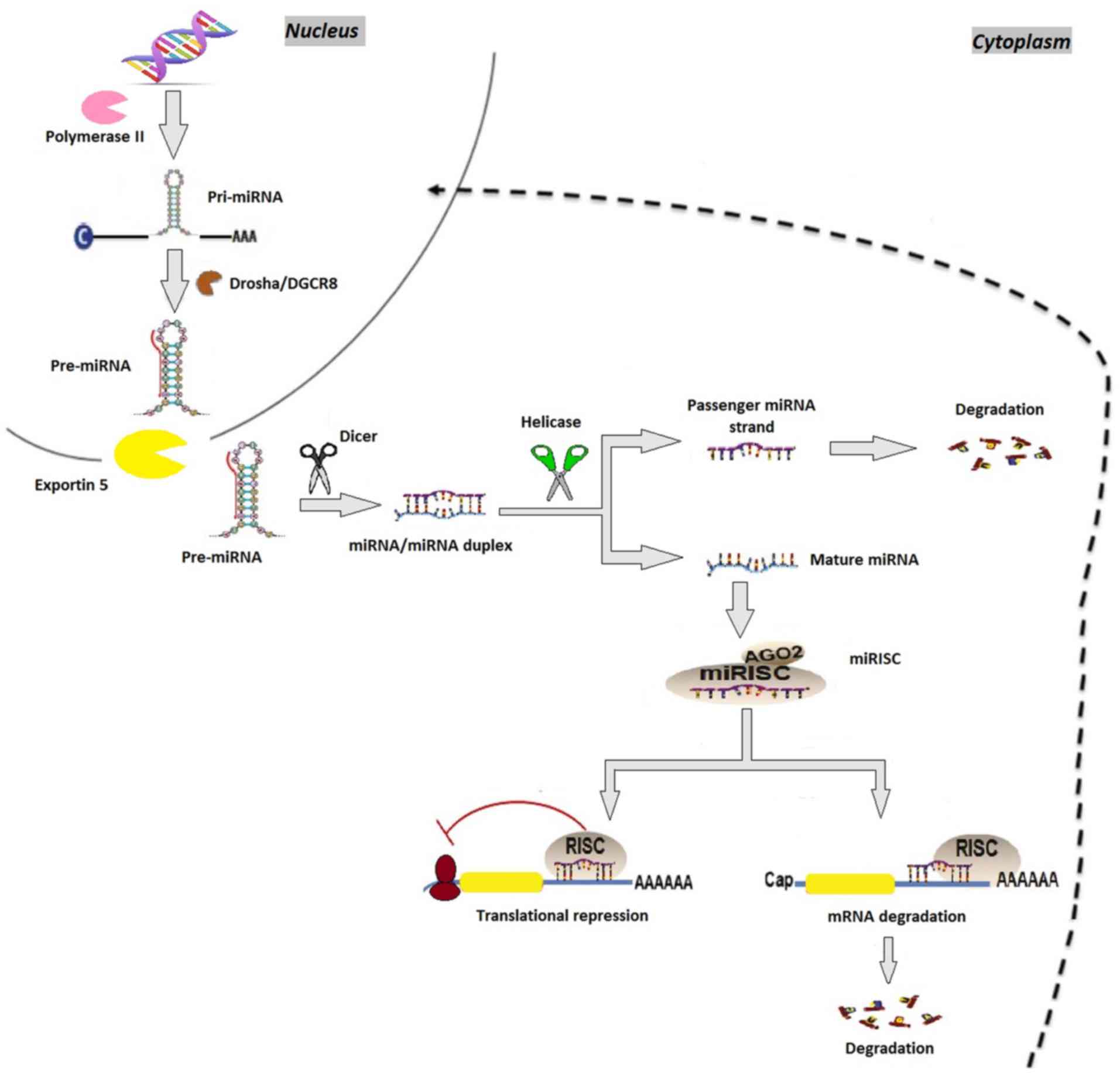 | Figure 1.miRNA biosynthesis. miRNA genes are
transcribed by RNA polymerase II as pri-miRNAs, which are
subsequently processed by Drosha, resulting in pre-miRNA.
Pre-miRNAs are then transported by the exportin 5-Ran GTPase
shuttle system into the cytoplasm where they are cleaved by another
RNase family enzyme (Dicer), resulting in the miRNA/miRNA duplex.
During this phase, Dicer interacts with AGO2 proteins generating
the RISC. Immediately following this, one strand of the miRNA
duplex is removed while the single stranded miRNA remains in the
complex and interacts with the 3′ untranslated region of target
mRNA genes, inducing posttranscriptional silencing and
translational repression. miRNA, microRNA; pri-miRNA, primary RNA;
pre-miRNA, miRNA precursor; DGCR8, DiGeorge syndrome critical
region 8; AGO2, argonaute 2; RISC, RNA-induced silencing
complex. |
In the following sections, the miRNAs most
frequently associated with psoriasis are presented, according to
their tendency to be either upregulated or downregulated, and their
presence within the blood or diseased tissue samples. These miRNAs
include: miR-21, which maintains skin inflammation in psoriasis
patients; miR-31, which enhances the production inflammatory
cytokines and chemokines via TNF-α; miR-146, which has a marked
correlation with the expression of IL-17; miR-155, leading to the
production of TNF-α; miR-203, which induces epithelial
differentiation and supresses skin immune responses; miR-99, which
inhibits keratinocyte differentiation by targeting insulin-like
growth factor-1 receptor (IGF-1R); miR-125, which supresses cell
proliferation; miR-197, which decreases the proliferation and
migration of keratinocytes; and miR-520, which supresses the
mitotic entry and proliferation of keratinocytes (3).
MicroRNAs involved in psoriasis
miR-21
miR-21 is overexpressed in psoriasis, is found in
psoriatic skin lesions, psoriatic epidermal cells, dermal T cells
and in blood samples, and it has a major role in psoriasis
(11). It activates mothers against
decapentaplegic homolog 7, which is an antagonist of the TGF-β1
signaling pathway (11,12). In psoriatic patients, the expression
of TGF-β is higher than normal, and is correlated with skin
inflammation (13). It also triggers
the transcription of miR-21 in epidermal keratinocytes. (11). miR-21-5p is known to downregulate
metalloproteinase inhibitor-3 (TIMP-3) in keratinocytes, which is
the main tissue inhibitor of the metalloproteinase gene family
(14). TIMP-3 inhibits the
TNF-converting enzyme, a disintegrin and metalloprotease 17
(ADAM17), which converts the inactive form of TNF into its soluble,
activated TNF configuration (15).
Binding to the TNF-receptor, the soluble form of TNF, activates the
signal transducer and activator of transcription 3, the
transcriptional activator of miR-21 (14,15). The
inhibition of miR-21 has been shown to have a beneficial effect in
the treatment of psoriasis (15)
(Fig. 2).
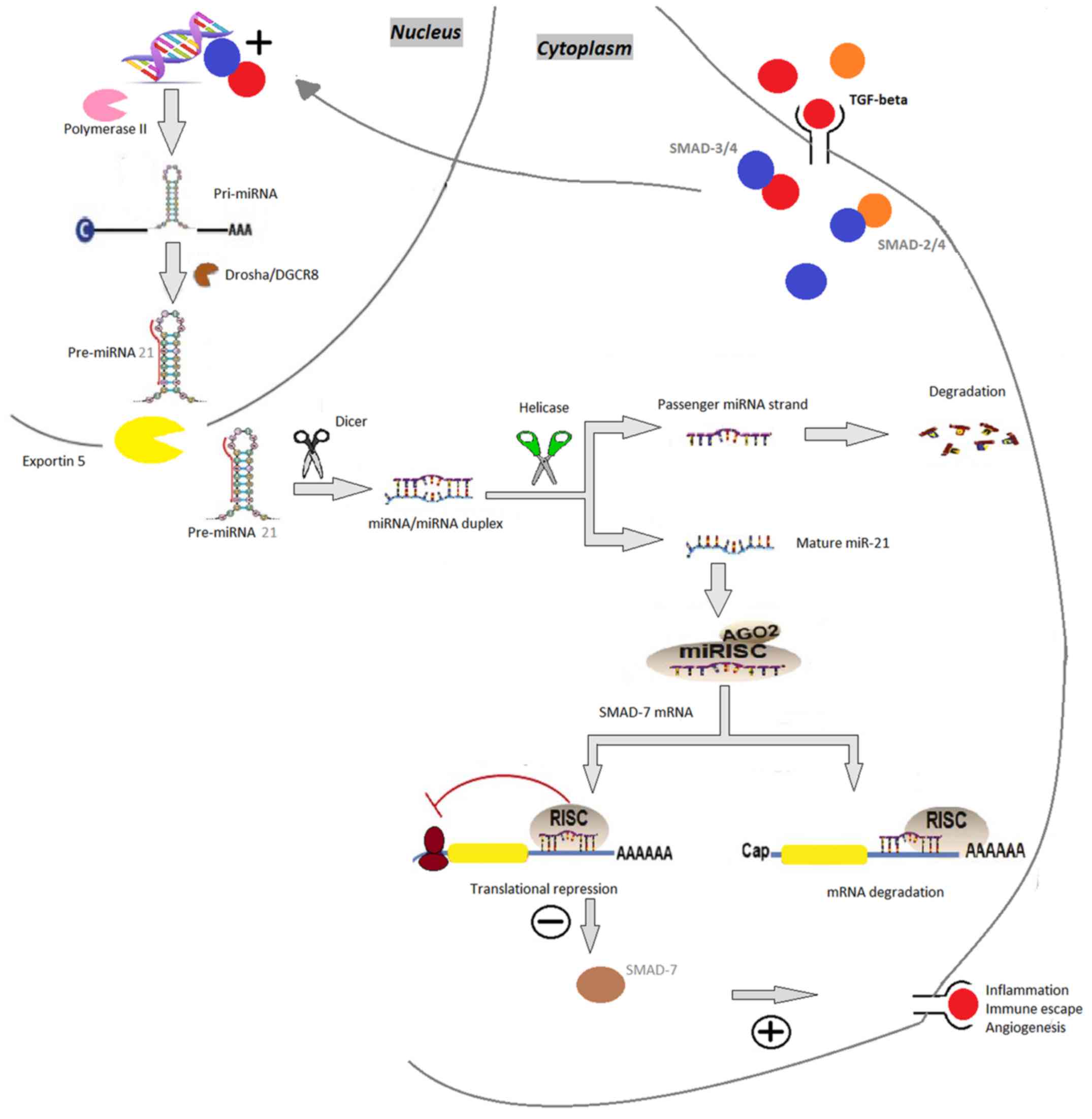 | Figure 2.miR-21. TGF-β binds to the receptors
leading to SMAD-2 or SMAD-3 phosphorylation, followed by
aggregation with SMAD-4. SMAD 3/4 appears to induce the
transcriptional induction of the synthesis of pri-miR-21, genes
which are subsequently processed by Drosha, resulting in
pre-miR-21. Pre-miR-21 is then transported by the exportin 5-Ran
GTPase shuttle system into the cytoplasm where it is cleaved by
another RNase family enzyme (Dicer), resulting miRNA/miRNA duplex.
During this phase, Dicer interacts with AGO2 proteins generating
the RISC. Immediately following this, one strand of the miRNA
duplex is removed while the single stranded miRNA remains in the
complex and interacts with the 3′ untranslated region of target
mRNA genes, inducing posttranscriptional silencing and repressing
translation of the inhibitory SMAD-7, thereby eliminating the
negative feedback mechanism of TGF-β signaling. miRNA/miR,
microRNA; pri-miR, primary RNA; pre-miR, miRNA precursor; DGCR8,
DiGeorge syndrome critical region 8; AGO2, argonaute 2; RISC,
RNA-induced silencing complex; TGF-β, transforming growth factor-β;
SMAD, mothers against decapentaplegic homolog 7. |
miR-31
The expression of miR-31 is increased in psoriatic
blood and skin samples (16). It
enhances nuclear factor κ-light-chain-enhancer of activated B cells
(NF-κB) signaling; NF-κB is a key mediator in the pathogenesis of
psoriasis, involved in various pathways, including inflammation,
keratinocyte proliferation, differentiation and apoptosis (17). miR-31 regulates the production of
inflammatory mediators (TNF-α, IL-1, IL-6, IL-17 and IL-22) and
stimulates leukocyte chemotaxis, thus inhibiting miR-31 may be a
therapeutic option in psoriasis (18). In this event, the suppression of
miR-31 decreases the expression of inflammatory cytokines and
chemokines and reduces keratinocyte hyperproliferation (15) (Fig.
3).
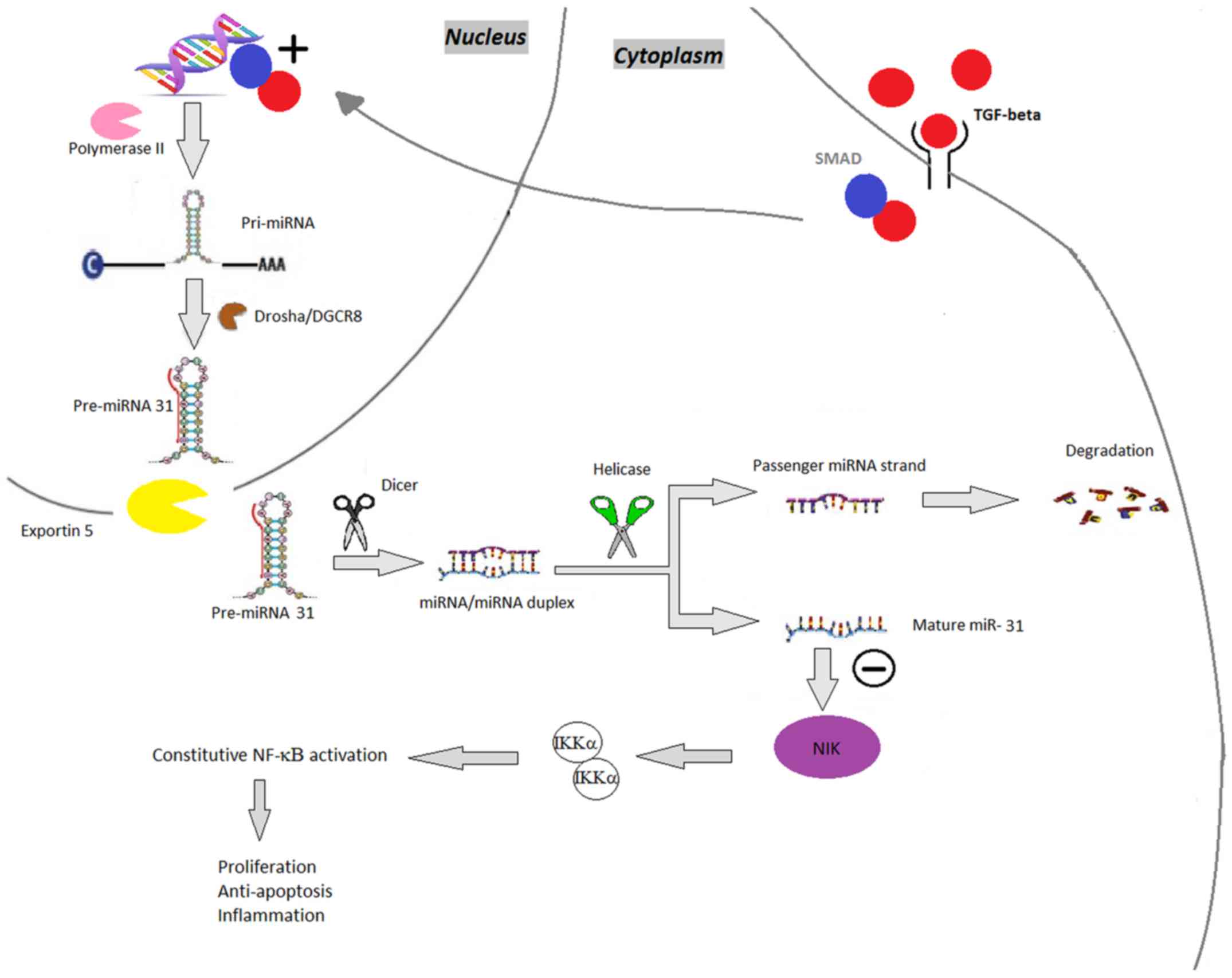 | Figure 3.miR-31. TGF-β binds to the receptors
leading to SMAD phosphorylation, followed by aggregation with
SMAD-4, which induces the synthesis of pri-miR-31, which is
subsequently processed by Drosha, resulting in pre-miR-31.
Pre-miR-31 is then transported by the exportin 5-Ran GTPase shuttle
system into the cytoplasm where it is cleaved by another RNase
family enzyme (Dicer), resulting in the miRNA/miRNA duplex and,
finally, mature miR-31. miR-31 negatively regulates NIK. NIK is
involved in phosphorylating IKKα and therefore in controlling the
NF-κB pathway. miRNA/miR, microRNA; pri-miR, primary RNA; pre-miR,
miRNA precursor; DGCR8, DiGeorge syndrome critical region 8; TGF-β,
transforming growth factor-β; SMAD, mothers against decapentaplegic
homolog 7; NF-κB, nuclear factor κ-light-chain-enhancer of
activated B cells; NIK, NF-κB-inducing kinase; IKK, inhibitor of
NF-κB subunit α. |
miR-146a
miR-146a is increased in psoriatic lesions and
peripheral blood mononuclear cells, and during chronic
inflammation; it appears to be involved in suppressing the innate
immune response in keratinocytes (19–21).
Reduced levels of miR-146a cause several effects, among which the
early onset of psoriasis, exacerbation of skin inflammation,
overexpression of IL-17, and hyperproliferation can be accounted
(20,22). According to Pivarsci et al, due
to Toll-like receptor (TLR) ligands, miR-146 is persistently
increased in keratinocytes, downregulating the expression of
inflammatory chemokines including IL-8 and C-C motif chemokine
ligand 20 (23). Consequently,
miR-146a decreases TLR-dependent epidermal inflammation via the
IL-1 receptor-associated kinase-1 and TNF receptor-associated
factor 6 pathways, which mediate the IL-17A signaling to NF-κB and
the recruitment of inflammatory cells (24–27)
(Fig. 4).
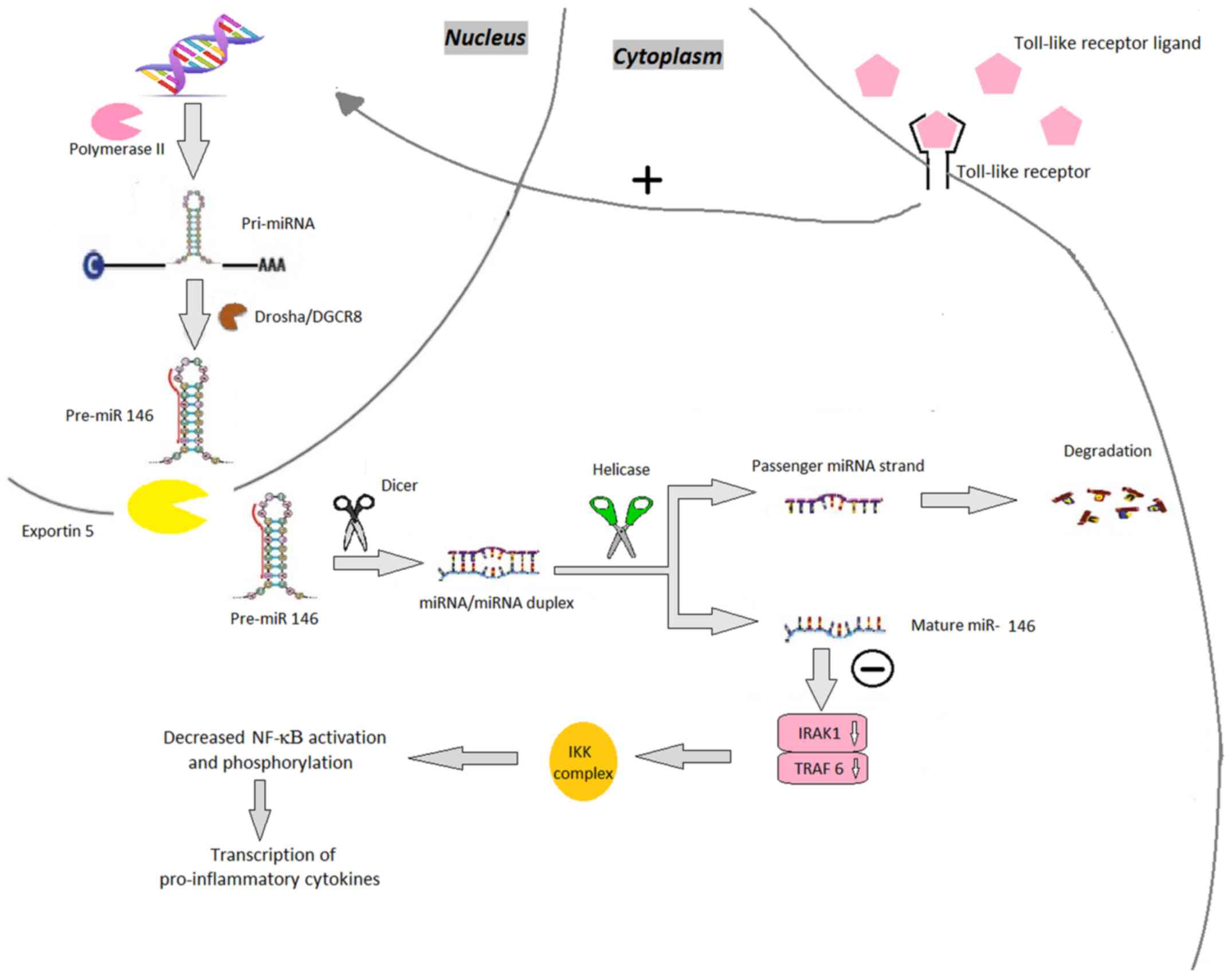 | Figure 4.miR-146. Toll-like receptor ligands
bind to their receptor increasing the production of pri-miR-146.
miR-146 genes are transcribed by RNA polymerase II as pri-miR-146,
which are subsequently processed by Drosha, resulting in
pre-miR-146. The pre-miR-146 is then transported by the exportin
5-Ran GTPase shuttle system into the cytoplasm where it is cleaved
by another RNase family enzyme (Dicer), resulting in the
miRNA/miRNA duplex and in the final step, mature miR-146. miR-146
downregulates the expression of TRAF6 and IRAK1. The decreased
expression of these modulators leads to decreased phosphorylation
of the IKK complex and thereby decreased activation of the NF-κB
pathway. miRNA/miR, microRNA; pri-miR, primary RNA; pre-miR, miRNA
precursor; DGCR8, DiGeorge syndrome critical region 8; TRAF6, TNF
receptor-associated factor 6; IRAK1, interleukin 1
receptor-associated kinase 1; NF-κB, nuclear factor
κ-light-chain-enhancer of activated B cells; IKK, inhibitor of
NF-κB. |
miR-155
The expression of miR-155 is upregulated in
psoriatic biopsy samples (15). It is
important in processes including cell growth and proliferation
(5). By decreasing the expression of
IL-4, a cytokine that characterizes the T helper (Th)2 phenotype,
miR-155 promotes differentiation towards a Th1 phenotype (3,28,29). Under such circumstances, during T-cell
activation, the expression of miR-155 increases, possibly leading
to the abnormal differentiation of CD4+ cells into
several T-cells subsets in chronic skin inflammation (3). In keratinocytes, miR-155 is induced by
TNF-α and IFN-γ (15). As a
proinflammatory miRNA, via positive feedback, miR-155 increases the
production of TNF-α (3). It also
targets phosphatase and tensin homolog, which inhibits
phosphoinositide 3-kinase (PI3K)/α-serine/threonine-protein kinase
(AKT) signaling, a novel pathway recently identified in association
with psoriasis, subsequently enhancing its own effect and
maintaining the inflammation in psoriasis (30) (Fig.
5).
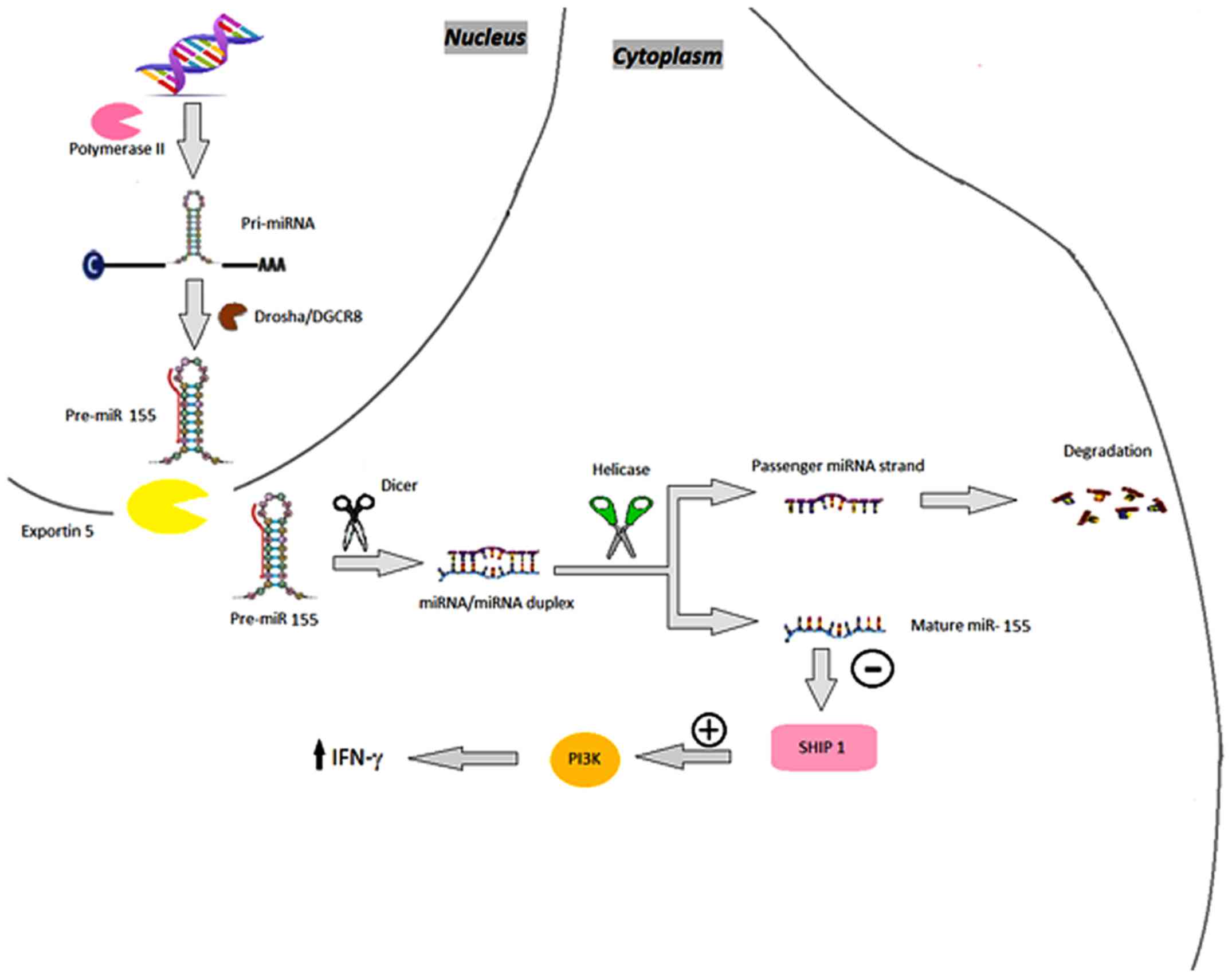 | Figure 5.miR-155. miR-155 genes are
transcribed by RNA polymerase II as pri-miR-155, which are
subsequently processed by Drosha, resulting in pre-miR-155.
Pre-miR-155 is then transported by the exportin 5-Ran GTPase
shuttle system into the cytoplasm where it is cleaved by another
RNase family enzyme (Dicer), resulting in the miRNA/miRNA duplex
and, finally, mature miR-155 that targets the 5′ inositol
phosphatase SHIP1, which downregulates its expression and, in turn,
promotes sustained activation of the PI3K pathway, consequently
enhancing the production of IFN-γ. miRNA/miR, microRNA; pri-miR,
primary RNA; pre-miR, miRNA precursor; DGCR8, DiGeorge syndrome
critical region 8; SHIP1, SH-2 containing inositol polyphosphatase
1; PI3K, phosphoinositide 3-kinase; IFN-γ; interferon-γ. |
miR-203
miR-203 is a skin-specific miRNA, which is
exclusively overexpressed in psoriatic keratinocytes and is
involved in angiogenesis in psoriasis and keratinocyte
differentiation (3). Although its
role in IL-17-induced vascular endothelial growth factor (VEGF)
remains to be fully elucidated, Xu et al showed that
upregulated IL-17 induced the expression of miR-203, which
activated the Janus kinase signaling pathway; this promoted the
secretion of VEGF in immortalized nontumorigenic human epidermal
cells (HaCaT) cells (31,32).
miR-125
miR-125 is found in the blood and skin lesions of
patients with psoriasis, and is involved in regulating fibroblast
growth factor receptor 2, which suppresses cellular proliferation
and prolongs the cellular differentiation of psoriatic
keratinocytes (33). In the serum of
patients with psoriasis, it appears to be downregulated (5). It appears that inhibiting miR-125 in
human keratinocytes may lead to hyperproliferation and delayed
cellular differentiation (34).
Narrow band UVB phototherapy can increase miR-125 levels
significantly (3).
miR-99
miR-99 is specifically downregulated in psoriasis,
particularly in keratinocytes and the upper layer of the epidermis
(3). It targets IGF-1R, which
enhances the proliferation of basal layer cells in patients with
psoriasis, stimulating hyperplasia and hyperkeratosis (15). Targeting the IGF-1R mRNA 3′
untranslated region (3′UTR), miR-99 decreases the protein levels of
IGF-1R, consequently inhibiting keratinocyte proliferation
(35). This also causes the cells to
differentiate, possibly explaining the higher level of miR-99a
detected in the superficial layers of the epidermis (36).
miR-197
Although downregulated in psoriatic skin samples,
miR-197 is involved in decreasing proliferation and migration, and
driving the differentiation process via normal keratinocytes
(5). It binds onto the IL-22RA1
subunit of IL-22, leading to hyperproliferation (37). It can also bind to the IL-17RA subunit
of the IL-17R, thus promoting normal proliferation and decreasing
the process of abnormal differentiation (38). By inhibiting IL-17RA in keratinocytes,
miR-197 reduces the expression of CCL, a chemoattractant for
dendritic cells and T lymphocytes (37).
miR-520
miR-520 is found in psoriatic keratinocytes and is
markedly downregulated (10). In
vitro experiments on HaCaT cells have confirmed the importance
of miR-520 in the proliferation and mitosis of human keratinocytes
(39). In vitro, it markedly
suppressed the proliferation and mitotic entry of HaCaT cells by
inhibiting AKT (40). miR-520a
downregulates the transcription factor E2F, which suppresses cell
cycle progression and proliferation (39). It also binds to the 3′UTR of AKT1
mRNA, thus inhibiting keratinocyte proliferation (40). Although utilizing miR-520 as a
treatment option for psoriatic patients remains a challenge, the
problem may be solved by using mimics of miRNA-520 (40).
Conclusion
In the present review, various examples of miRNA
involvement in psoriasis are described (Table I). miRNAs can be detected in small
volume blood samples or skin samples using quantitative real-time
polymerase chain reaction (41). A
promising characteristic is that miRNAs can be used in psoriasis
for diagnosis, prognosis or as a treatment option (5). Multiple interactions between epidermal
keratinocytes and immunocytes generally lead to the development of
this disease (15). A considerable
number of miRNAs have been described to be upregulated in
psoriasis, thus their inhibition may offer a revolutionary
treatment method (42). Therefore,
the increased miRNAs require downregulation using miRNA inhibitors,
whereas miRNAs that are decreased in psoriasis require
supplementation using miRNA mimics (43).
 | Table I.Characteristics of miRNAs in
psoriasis. |
Table I.
Characteristics of miRNAs in
psoriasis.
| miRNA | Level | Sites found | Effects |
|---|
| miR-21 | Upregulated | Skin lesions,
psoriasis epidermal cells, dermal T cells Blood samples | Inflammation Immune
evasion Angiogenesis |
| miR-31 | Upregulated | Skin samples Blood
samples | Enhances the
production of inflammatory cytokines and chemokines |
| miR-146 | Upregulated | Psoriasis lesions
Peripheral blood mononuclear cells | Maintains chronic
inflammation Recruitment of inflammatory cells |
| miR-155 | Upregulated | Biopsy samples | Pro-inflammatory,
increases the production of tumor necrosis factor-α |
| miR-203 | Upregulated | Psoriasis
lesions | Induces epithelial
differentiation and suppresses skin immune responses |
| miR-125 | Downregulated | Skin lesions Blood
samples | Via fibroblast
growth factor receptor 2, suppresses cell proliferation Prolongs
differentiation of psoriatic keratinocytes |
| miR-99 | Downregulated | Keratinocytes,
upper layer of epidermis | By targeting
insulin-like growth factor 1 receptor, inhibits keratinocyte
proliferation and drives them towards differentiation |
| miR-197 | Downregulated | Skin samples | Decreases the
proliferation and migration of keratinocytes |
| miR-520 | Downregulated | Keratinocytes Cell
cultures | Suppresses the
mitotic entry and proliferation of keratinocytes |
In conclusion, further investigations are likely to
confirm the advantages of using miRNAs in psoriasis as a biomarker,
prognostic marker or novel treatment option.
Acknowledgements
The authors would like to acknowledge Dr Ioan
Alexandru Florian at the Department of Neurosurgery, Cluj County
Emergency Hospital, Cluj-Napoca, Romania, for his assistance in
editing and revising the manuscript.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Both authors were equally involved in the conception
and design of the article, as well as in writing and revising the
manuscript. Both authors gave final approval of the version to be
published and have agreed to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. TLT gathered data and drafted the manuscript
regarding the upregulated miRNAs and provided the illustrations and
table. RIO gathered data and drafted the manuscript regarding the
downregulated miRNAs.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TNF-α
|
tumor necrosis factor-α
|
|
IFN-γ
|
interferon-γ
|
|
TGF-β
|
transforming growth factor-β
|
|
IL
|
interleukin
|
|
miRNA
|
microRNA
|
|
pri-miRNA
|
primary miRNA
|
|
SMAD7
|
mothers against decapentaplegic
homolog 7
|
|
TIMP-3
|
metalloproteinase inhibitor 3
|
|
ADAM17
|
a disintegrin and metalloprotease
17
|
|
NF-κB
|
nuclear factor κ-light-chain-enhancer
of activated B cells
|
|
IGF-1R
|
insulin-like growth factor 1
receptor
|
|
TLR
|
Toll-like receptor
|
|
CCL
|
chemokine ligand
|
|
IRAK1
|
interleukin 1 receptor-associated
kinase 1
|
|
TRAF6
|
TNF receptor-associated factor 6
|
|
PTEN
|
phosphatase and tensin homolog
|
|
VEGF
|
vascular endothelial factor
|
|
AKT
|
α-serine/threonine-protein kinase
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
HaCaT
|
immortalized nontumorigenic human
epidermal cells
|
References
|
1
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gudjonsson JE and Elder JT: Psoriasis:
Epidemiology. Clin Dermatol. 25:535–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang RY, Li L, Wang MJ, Chen XM, Huang QC
and Lu CJ: An Exploration of the role of MicroRNAs in psoriasis: A
systematic review of the literature. Medicine (Baltimore).
94:e20302015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ortega C, Fernández-A S, Carrillo JM,
Romero P, Molina IJ, Moreno JC and Santamaría M: IL-17-producing
CD8+ T lymphocytes from psoriasis skin plaques are
cytotoxic effector cells that secrete Th17-related cytokines. J
Leukoc Biol. 86:435–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ross K: Towards topical microRNA-directed
therapy for epidermal disorders. J Control Release. 269:136–147.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soltanzadeh-Yamchi M, Shahbazi M, Aslani S
and Mohammadnia-Afrouzi M: MicroRNA signature of regulatory T cells
in health and autoimmunity. Biomed Pharmacother. 100:316–323. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruksha TG, Komina AV and Palkina NV:
MicroRNA in skin diseases. Eur J Dermatol. 27:343–352.
2017.PubMed/NCBI
|
|
8
|
Liu Y and Liu Q: MicroRNAs as regulatory
elements in psoriasis. Open Med (Wars). 11:336–340. 2016.PubMed/NCBI
|
|
9
|
Barca-Mayo O and Lu QR: Fine-tuning
oligodendrocyte development by microRNAs. Front Neurosci. 6:132012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hawkes JE, Nguyen GH, Fujita M, Florell
SR, Callis Duffin K, Krueger GG and O'Connell RM: microRNAs in
psoriasis. J Invest Dermatol. 136:365–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang M, Sun Z, Dang E, Li B, Fang H, Li
J, Gao L, Zhang K and Wang G: TGFβ/SMAD/microRNA-486-3p signaling
axis mediates keratin 17 expression and keratinocyte
hyperproliferation in psoriasis. J Invest Dermatol. 137:2177–2186.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Degueurce G, D'Errico I, Pich C, Ibberson
M, Schütz F, Montagner A, Sgandurra M, Mury L, Jafari P, Boda A, et
al: Identification of a novel PPARβ/δ/miR-21-3p axis in UV-induced
skin inflammation. EMBO Mol Med. 8:919–936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boele J, Persson H, Shin JW, Ishizu Y,
Newie IS, Søkilde R, Hawkins SM, Coarfa C, Ikeda K, Takayama K, et
al: PAPD5-mediated 3 adenylation and subsequent degradation of
miR-21 is disrupted in proliferative disease. Proc Natl Acad Sci
USA. 111:11467–11472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guinea-Viniegra J, Jiménez M, Schonthaler
HB, Navarro R, Delgado Y, Concha-Garzón MJ, Tschachler E, Obad S,
Daudén E and Wagner EF: Targeting miR-21 to treat psoriasis. Sci
Transl Med. 6:225re12014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masalha M, Sidi Y and Avni D: The
contribution of feedback loops between miRNAs, cytokines and growth
factors to the pathogenesis of psoriasis. Exp Dermatol. 27:603–610.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stepicheva NA and Song JL: Function and
regulation of microRNA-31 in development and disease. Mol Reprod
Dev. 83:654–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan S, Xu Z, Lou F, Zhang L, Ke F, Bai J,
Liu Z, Liu J, Wang H, Zhu H, et al: NF-κB-induced microRNA-31
promotes epidermal hyperplasia by repressing protein phosphatase 6
in psoriasis. Nat Commun. 6:76522015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu N, Meisgen F, Butler LM, Han G, Wang
XJ, Söderberg-Nauclér C, Ståhle M, Pivarcsi A and Sonkoly E:
MicroRNA-31 is overexpressed in psoriasis and modulates
inflammatory cytokine and chemokine production in keratinocytes via
targeting serine/threonine kinase 40. J Immunol. 190:678–688. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hermann H, Runnel T, Aab A, Baurecht H,
Rodriguez E, Magilnick N, Urgard E, Šahmatova L, Prans E,
Maslovskaja J, et al: miR-146b probably assists miRNA-146a in the
suppression of keratinocyte proliferation and inflammatory
responses in psoriasis. J Invest Dermatol. 137:1945–1954. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ichihara A, Jinnin M, Yamane K, Fujisawa
A, Sakai K, Masuguchi S, Fukushima S, Maruo K and Ihn H:
microRNA-mediated keratinocyte hyperproliferation in psoriasis
vulgaris. Br J Dermatol. 165:1003–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zibert JR, Løvendorf MB, Litman T, Olsen
J, Kaczkowski B and Skov L: MicroRNAs and potential target
interactions in psoriasis. J Dermatol Sci. 58:177–185. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Srivastava A, Nikamo P, Lohcharoenkal W,
Li D, Meisgen F, Xu Landén N, Ståhle M, Pivarcsi A and Sonkoly E:
MicroRNA-146a suppresses IL-17-mediated skin inflammation and is
genetically associated with psoriasis. J Allergy Clin Immunol.
139:550–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pivarcsi A, Ståhle M and Sonkoly E:
Genetic polymorphisms altering microRNA activity in psoriasis - a
key to solve the puzzle of missing heritability? Exp Dermatol.
23:620–624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Q, Zhang J, Li J, Zou L, Zhang J, Xie
Z, Fu X, Jiang S, Chen G, Jia Q, et al: Forced miR-146a expression
causes autoimmune lymphoproliferative syndrome in mice via
downregulation of Fas in germinal center B cells. Blood.
121:4875–4883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia P, Fang X, Zhang ZH, Huang Q, Yan KX,
Kang KF, Han L and Zheng ZZ: Dysregulation of miRNA146a versus
IRAK1 induces IL-17 persistence in the psoriatic skin lesions.
Immunol Lett. 148:151–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chatzikyriakidou A, Voulgari PV, Georgiou
I and Drosos AA: The role of microRNA-146a (miR-146a) and its
target IL-1R-associated kinase (IRAK1) in psoriatic arthritis
susceptibility. Scand J Immunol. 71:382–385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shams K, Kurowska-Stolarska M, Schütte F,
Burden AD, McKimmie CS and Graham GJ: MicroRNA-146 and cell trauma
down-regulate expression of the psoriasis-associated atypical
chemokine receptor ACKR2. J Biol Chem. 293:3003–3012. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou RX, Liu RF, Zhao XC, Jia YR, An P, Hao
ZP, Li JQ, Li XH, Yin GH and Zhang KM: Increased miR-155-5p
expression in dermal mesenchymal stem cells of psoriatic patients:
Comparing the microRNA expression profile by microarray. Genet Mol
Res. Sep 2–2016.(Epub ahead of print). doi: 10.4238/gmr.15038631.
View Article : Google Scholar
|
|
29
|
García-Rodríguez S, Arias-Santiago S,
Blasco-Morente G, Orgaz-Molina J, Rosal-Vela A, Navarro P,
Magro-Checa C, Martínez-López A, Ruiz JC, Raya E, et al: Increased
expression of microRNA-155 in peripheral blood mononuclear cells
from psoriasis patients is related to disease activity. J Eur Acad
Dermatol Venereol. 31:312–322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu L and Leng H, Shi X, Ji J, Fu J and
Leng H: miR-155 promotes cell proliferation and inhibits apoptosis
by PTEN signaling pathway in the psoriasis. Biomed Pharmacother.
90:524–530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Y, Ji Y, Lan X, Gao X, Chen HD and Geng
L: miR 203 contributes to IL 17 induced VEGF secretion by targeting
SOCS3 in keratinocytes. Mol Med Rep. 16:8989–8996. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Primo MN, Bak RO, Schibler B and Mikkelsen
JG: Regulation of pro-inflammatory cytokines TNFα and IL24 by
microRNA-203 in primary keratinocytes. Cytokine. 60:741–748. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu N, Brodin P, Wei T, Meisgen F, Eidsmo
L, Nagy N, Kemeny L, Ståhle M, Sonkoly E and Pivarcsi A: miR-125b,
a microRNA downregulated in psoriasis, modulates keratinocyte
proliferation by targeting FGFR2. J Invest Dermatol. 131:1521–1529.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei T, Folkersen L, Biskup E, Xu N, Manfe
V, Niazi O and Gniadecki R: Ubiquitin-specific peptidase 2 as a
potential link between microRNA-125b and psoriasis. Br J Dermatol.
176:723–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang MJ, Xu YY, Huang RY, Chen XM, Chen
HM, Han L, Yan YH and Lu CJ: Role of an imbalanced miRNAs axis in
pathogenesis of psoriasis: Novel perspectives based on review of
the literature. Oncotarget. 8:5498–5507. 2017.PubMed/NCBI
|
|
36
|
Lerman G, Avivi C, Mardoukh C, Barzilai A,
Tessone A, Gradus B, Pavlotsky F, Barshack I, Polak-Charcon S,
Orenstein A, et al: MiRNA expression in psoriatic skin: Reciprocal
regulation of hsa-miR-99a and IGF-1R. PLoS One. 6:e209162011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lerman G, Sharon M, Leibowitz-Amit R, Sidi
Y and Avni D: The crosstalk between IL-22 signaling and miR-197 in
human keratinocytes. PLoS One. 9:e1074672014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elharrar E, Masalha M, Lerman G,
Leibowitz-Amit R, Kassem R, Harats M, Sidi Y and Avni D:
Positive-negative feedback loop between MiR-197 and IL-17A
signaling in human keratinocytes. Immunome Res. May 2–2016.(Epub
ahead of print). doi: 10.4172/1745-7580.10000111. View Article : Google Scholar
|
|
39
|
Wang R, Zhao Z, Zheng L, Xing X, Ba W,
Zhang J, Huang M, Zhu W, Liu B, Meng X, et al: MicroRNA-520a
suppresses the proliferation and mitosis of HaCaT cells by
inactivating protein kinase B. Exp Ther Med. 14:6207–6212.
2017.PubMed/NCBI
|
|
40
|
Wang X, Wang P, Zhu Y, Zhang Z, Zhang J
and Wang H: MicroRNA-520a attenuates proliferation of Raji cells
through inhibition of AKT1/NF-κB and PERK/eIF2α signaling pathway.
Oncol Rep. 36:1702–1708. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Van Gele M, Bracke S, de Medeiros Alves AK
and Lambert J: Exploring the feasibility of whole blood to identify
systemic miRNA biomarkers for patients with moderate to severe
psoriasis. Eur J Dermatol. 26:195–198. 2016.PubMed/NCBI
|
|
42
|
Liu Q, Wu DH, Han L, Deng JW, Zhou L, He
R, Lu CJ and Mi QS: Roles of microRNAs in psoriasis: Immunological
functions and potential biomarkers. Exp Dermatol. 26:359–367. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sonkoly E, Wei T, Janson PC, Sääf A,
Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B,
Scheynius A, et al: MicroRNAs: Novel regulators involved in the
pathogenesis of psoriasis? PLoS One. 2:e6102007. View Article : Google Scholar : PubMed/NCBI
|



















