Introduction
The epidermal growth factor receptor (EGFR) is a
member of the erbB family of four related cell membrane receptors,
including EGFR (HER1 or erbB1), HER2 (erbB2), HER3 (erbB3) and HER4
(erbB4). These receptors possess an extracellular N-terminal
ligand-binding domain, a hydrophobic transmembrane domain involved
in interactions between receptors within the cell membrane, and a
C-terminal intracellular domain and multiple phosphorylation sites.
EGFR can be activated through a series of events initiated by
binding to its ligands, such as EGF, TGF-α, amphiregulin,
epiregulin and hemiregulin. By binding to its ligands, EGFR
triggers the homodimerization of EGFR or heterodimerization of EGFR
with other ErbBs, resulting in the autophosphorylation of the
receptor. The activated receptor recruits signaling complexes and
activates the MEK, ERK, PI-3-K, STATS and PLC-γ pathways (1). These pathways are potent oncogenic
regulators of tumor cell growth, invasion, angiogenesis and
metastasis. EGFR is overexpressed in many human epithelial
carcinomas including colorectal, esophageal, prostate, ovarian,
head and neck, lung and kidney, and this overexpression may play a
role in tumorigenesis and poor patient prognosis and outcome
(2).
Principal strategies for EGFR target therapy include
the monoclonal antibody (mAb) and small molecule tyrosine kinase
inhibitors. mAb blocks the receptor extracellular domain inhibiting
ligand binding and receptor activation, and the small molecule
tyrosine kinase inhibitors do not permit autophosphorylation by
blocking the intracellular domain. Cetuximab (Erbitux) and
panitumumab (Vectibix) are monoclonal antibodies which bind
specifically to EGFR on both normal and tumor cells, and
competitively inhibit the binding of ligands leading to the
inhibition of cell proliferation, differentiation and survival.
Cetuximab is a chimeric mAb which normally contains
approximately 34% of murine proteins (3). It was first approved in 2004 for
colorectal cancer and later for head and neck cancers. Chimeric mAb
is less immunogenic than murine mAb and has improved therapeutic
utility. However, due to their murine sequences, both may cause
allergic reactions in a portion of the patients (4,5).
Panitumumab is a fully humanized mAb and was approved for the
treatment of EGFR-expressing metastatic colorectal carcinoma
(6). Fully humanized mAbs are
normally non-immunogenic and thus allow repeated administration
without a human anti-human antibody response (7).
Occasional hypomagnesemia, followed by secondary
hypokalemia, and hypocalcemia have been reported as side effects of
cetuximab and panitumumab. Soon after the approval of cetuximab for
colorectal cancer treatment, Schrag et al originally
reported the occurrence and clinical course of hypomagnesemia in 34
of 154 patients who were treated with cetuximab in their
institution (8). Out of 154
patients, 10 had grade 3 or 4 hypomagnesemia. Hypomagnesemia was
also reported in 38% patients treated with panitumumab, with grade
3 and 4 hypomagnesemia occurring in 3% of those treated with
panitumumab (9). Fakih et
al, in a retrospective study, reported the incidence of
hypomagnesemia in 27% of patients treated with cetuximab. Recently,
Tejpar et al prospectively measured magnesium concentrations
in a cohort of 98 patients treated with EGFR-targeting antibodies
with or without combined chemotherapy (10). Ninety-seven percent of the patients
had decreasing serum magnesium concentrations during EGFR-targeting
treatment compared to baseline measurements. The mean serum
magnesium slope during EGFR-targeting treatment (with or without
combined chemotherapy) was significantly lower compared to
chemotherapy alone.
In the present study, we aimed to retrospectively
assess the occurrence of electrolyte depletion in patients treated
with cetuximab or panitumumab with or without combined
chemotherapy. Moreover, we aimed to compare the electrolytic
changes caused by these two agents as well as to track any
noticeable changes in other routine laboratory parameters in these
patients.
Materials and methods
A study proposal was made to the Institutional
Review Board of M.D. Anderson Cancer Center Orlando, and a waiver
of authorization was obtained before initiating the study. Data
from records of all patients who received treatment with cetuximab
or panitumumab at the M.D. Anderson Cancer Center Orlando between
January 1, 2007 and January 1, 2008 were reviewed.
The data collection method consisted of reviewing
the records of the patients identified from the two patient
database systems used in this institution (Sunrise XA and HBOC data
base). Patient-specific data were securely de-identified and
entered into a master key log for analysis. The parameters
collected included the dose, route and date of drug administered,
and various laboratory parameters including hematology and blood
chemistry. Statistical analysis was performed using
SPSS®.
Results
During the study period, 58 patients received
treatment with cetuximab, with or without combined chemotherapy, of
which approximately 1/3 had head and neck cancer; the rest were
treated for colorectal cancer. Twenty-one patients received
panitumumab; all had colorectal cancer. The treatment duration
spanned from just over 2 weeks to more than 1 year.
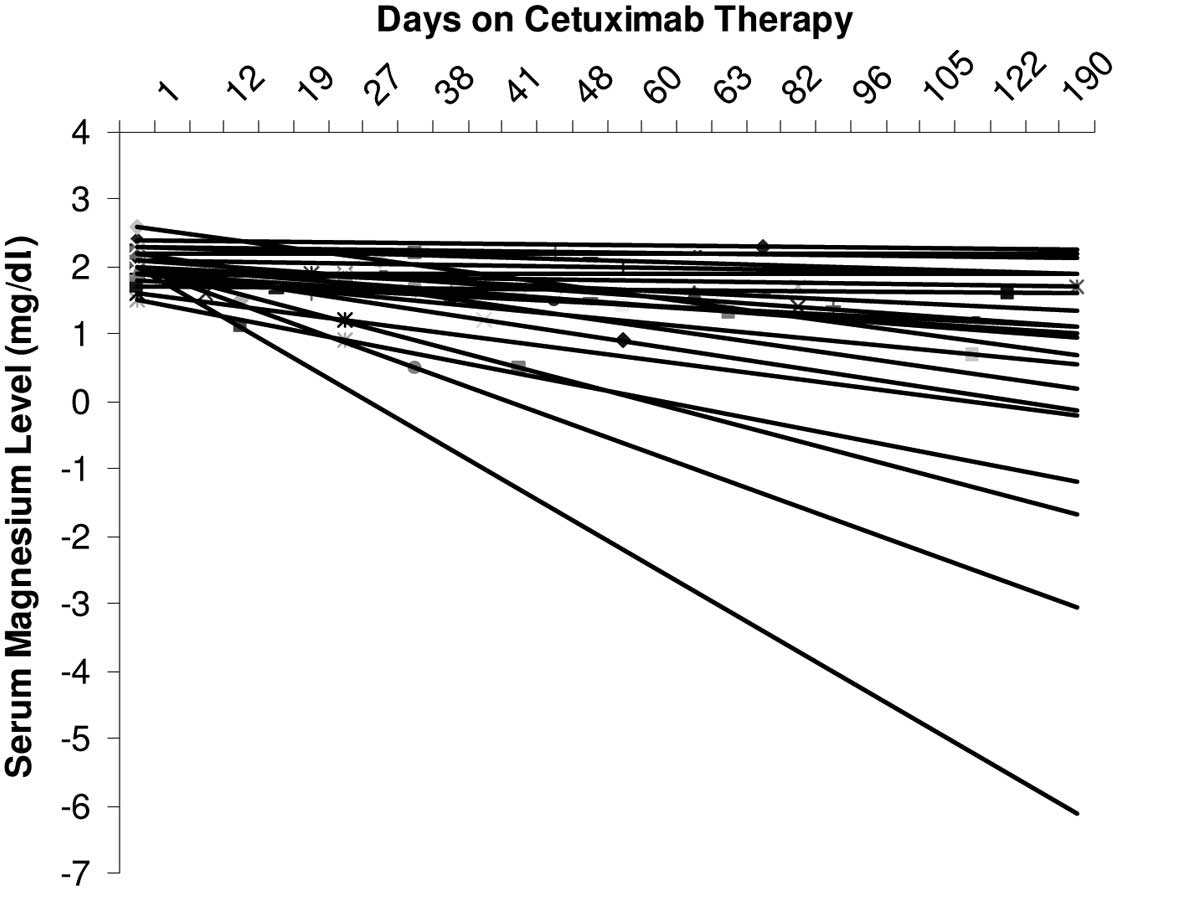
Of the 58 patients who received cetuximab, 32
experienced varying grades of hypomagnesemia during the treatment
period. Of these patients, 28 had grade 1 hypomagnesemia
(<1.7–1.2 mg/dl), 3 had grade 2 (<1.2–0.9 mg/dl) and 1 had
grade 3 hypomagnesemia (<0.9–0.7 mg/dl). The slopes of the
linear trend lines connecting the serum magnesium levels in these
patients were almost exclusively negative (Fig. 1). There were few patients whose
magnesium levels were not monitored. Thirty-eight patients showed
some grades of decrease in blood calcium level, of which 5 had
grade 2 and the remaining grade 1 hypocalcemia.
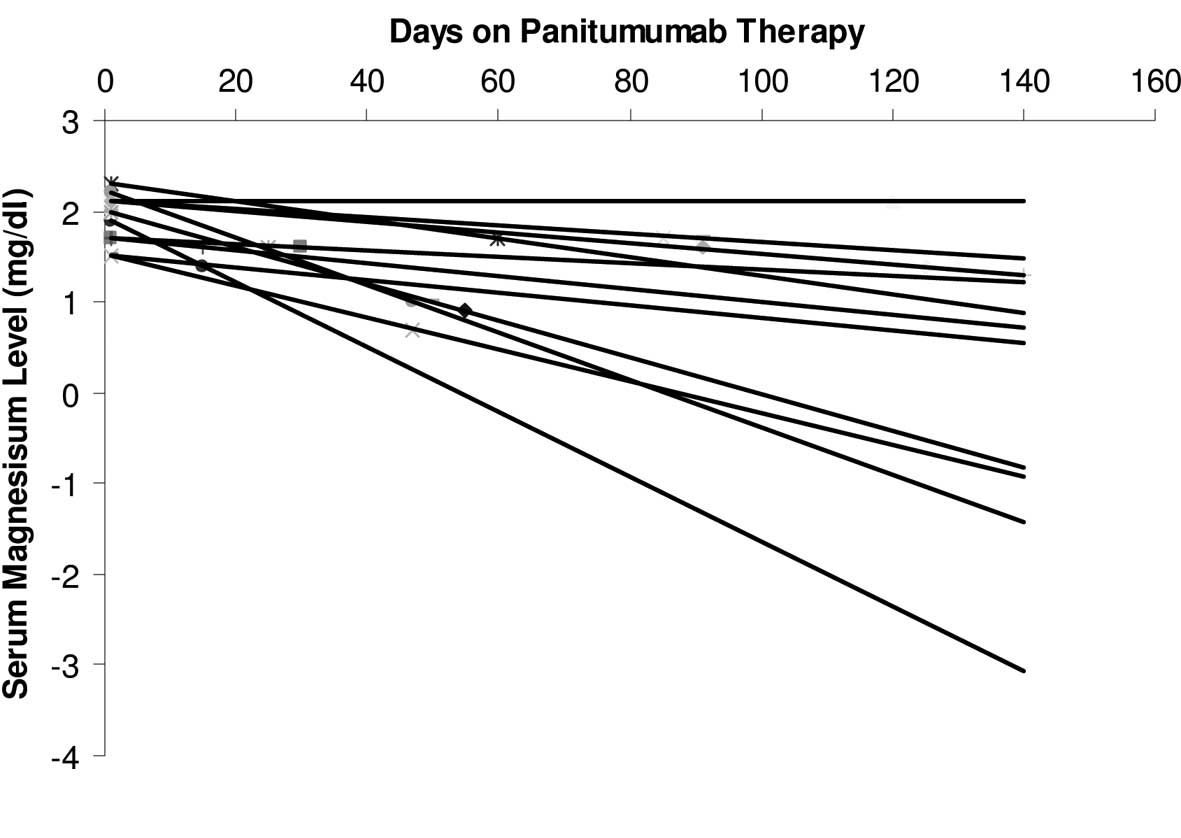
Of the 21 patients who received panitumumab, 18 had
a decrease in their serum magnesium levels to varying extents. The
slopes of the linear trend lines connecting the serum magnesium
levels in these patients were almost exclusively negative (Fig. 2). The serum magnesium level was not
monitored in 1 patient. Ten patients had grade 1 and 8 patients had
grade 2 hypomagnesemia. Table I
documents the comparison of hypomagnesemia and hypocalcemia
incidence rates in patients who received cetuximab vs. the patients
who received panitumumab.
 | Table I.Comparison of the rate of incidence of
hypomagnesemia and hypocalcemia in patients who received cetuximab
vs. panitumumab. |
Table I.
Comparison of the rate of incidence of
hypomagnesemia and hypocalcemia in patients who received cetuximab
vs. panitumumab.
| Anti-EGFR
antibody | Hypomagnesemia
incidence (%)
| Hypocalcemia
incidence (%)
|
|---|
| Grade 1a | Grade 2b | Grade 3/4c | Grade 1d | Grade 2e | Grade 3/4f |
|---|
| Cetuximab | 48 | 5 | 2 | 56 | 10 | 0 |
| Panitumumab | 52 | 38 | 0 | 52 | 14 | 0 |
The serum potassium level was also affected in many
of the patients who received both these anti-EGFR agents.
Surprisingly, in all the patients there was a considerable decrease
in the serum albumin levels. Initially it was thought that these
changes in the albumin levels were related to the disease itself.
However, careful observation showed a direct relationship of the
changes in albumin with the initiation or discontinuation of the
treatments with the two anti-EGFR antibodies. Thirty-six patients
who received cetuximab had varying severity of hypoalbuminemia
during anti-EGFR treatment. Among them 13 patients had grade 1, 21
had grade 2, and 2 had grade 3 hypoalbuminemia.
Patients who received panitumumab also exhibited a
similar pattern of hypoalbuminemia, with 15 out of 21 patients
showing different grades. Of the 15 patients, 9 had grade 2 and 2
had grade 3 hypoalbuminemia; the rest had grade 1. Table II compares the hypoalbuminemia
noted in patients receiving both agents.
 | Table II.Comparison of the rate of incidence of
hypoalbuminemia in patients who received cetuximab vs.
panitumumab. |
Table II.
Comparison of the rate of incidence of
hypoalbuminemia in patients who received cetuximab vs.
panitumumab.
| Anti-EGFR
antibody | Hypoalbuminemia
incidence (%)
|
|---|
| Grade 1a | Grade 2b | Grade 3c |
|---|
| Cetuximab | 22 | 36 | 3 |
| Panitumumab | 19 | 42 | 7 |
Seventeen patients required magnesium or/and calcium
supplementation during cetuximab therapy. Magnesium and calcium
supplementation was given as intravenous administration on the day
of the cetuximab therapy. However, in many of these patients the
serum magnesium levels continued to plunge before they were due for
the next dose of cetuximab. A higher proportion of patients (15
patients) on panitumumab had received magnesium and/or calcium
supplementation during the treatment period, given intravenously on
the day of treatment.
Discussion
In this study, the serum levels of magnesium,
calcium and albumin were monitored in patients who received the
anti-EGFR antibodies cetuximab and panitumumab. Since cetuximab was
approved for use in colorectal and head and neck cancers, there
have been a few studies, both prospective and retrospective,
investigating the decrease in the serum magnesium levels as an
adverse effect. There have been no studies comparing the changes in
blood electrolytes between populations of patients taking cetuximab
and panitumumab.
The result of this retrospective study shows an
approximate 60% rate of incidence of hypomagnesemia (grade 1–4) in
patients receiving cetuximab, which is comparable to previously
reported studies (11). We noted
that the incidence of hypomagnesemia was higher in the case of
patients treated with panitumumab compared to cetuximab. The
severity of hypomagnesemia was also greater in patients receiving
panitumumab. However, it should be noted that the sample sizes of
the patients receiving these two agents were different. Continuous
monitoring of magnesium levels was not mandated in some of the
clinical trials involving cetuximab, nor in the standard care
practices of some institutions (12,13).
The mandatory monitoring of serum magnesium levels would have
revealed more cases of hypomagnesemia in these patients.
Magnesium is critically important in maintaining
normal cell function, and symptomatic magnesium depletion is often
associated with multiple biochemical abnormalities including
hypokalemia, hypocalcemia and metabolic acidosis. As a result,
hypomagnesemia is sometimes difficult to attribute solely to
specific clinical manifestations. Severe hypomagnesemia is a
serious electrolyte abnormality, the clinical manifestations of
which include neuromuscular, cardiovascular and metabolic
manifestations.
The earliest symptoms of magnesium deficiency are
usually neuromuscular and neuropsychiatric disturbances, the most
common being hyperexcitability. Neuromuscular irritability
including tremor, fasciculation, tetany, Chvostek and Trousseau
signs and convulsions were reported when hypomagnesemia was induced
in volunteers. Other manifestations include convulsions, apathy,
muscle cramps, hyperflexia, acute organic brain syndromes,
depression, generalized weakness, anorexia and vomiting. The
cardiovascular effects of magnesium deficiency include effects on
electrical activity (prolonged QT and QU interval), myocardial
contractility (ventricular tachycardia, Torsade de pointes,
ventricular fibrillation), potentiation of digitalis effects and
vascular tone.
Metabolic manifestations may include hypokalemia and
hypocalcemia. Hypokalemia is a common event in patients with
hypomagnesemia, occurring in 40–60% of cases. This is partly due to
the underlying disorders that cause magnesium and potassium loss,
including diuretic therapy or diarrhea.
The classic sign of severe hypomagnesemia (<1.2
mg/dl) is hypocalcemia. The mechanism is multifactorial.
Parathyroid gland function is abnormal, largely due to impaired
release of PTH. Impaired magnesium-dependent adenyl cyclase
generation of cAMP mediates the decreased release of PTH. Skeletal
resistance to this hormone in magnesium deficiency has also been
implicated. Hypomagnesemia also alters the normal heteroionic
exchange of calcium and magnesium at the bone surface, leading to
an increased bone release of magnesium ions in exchange for an
increased skeletal uptake of calcium from the serum.
The details of the exact mechanism of hypomagnesemia
in patients receiving anti-EGFR antibodies remain unclear. However,
recently, there have been some insights on the matter. In healthy
individuals, serum magnesium concentrations are tightly controlled
and vary between 0.70 and 1.1 mmol/l (14). In the kidney, approximately 80% of
the serum magnesium is ultrafiltered in the glomeruli.
Approximately 15–20% is reabsorbed in the proximal tubule.
Subsequently, 70% of the magnesium is reabsorbed passively by a
paracellular transport process in the thick ascending loop (TAL)
driven by lumen-positive transepithelial voltage. However, the fine
tuning of magnesium excretion takes place in the distal convoluted
tubule (DCT), where 5–10% of the filtered magnesium is reabsorbed
via an active transcellular transport process (15). Apical entry into DCT cells is
mediated by the magnesium permeable channel TRPM6 (transient
receptor potential cation channel, subfamily M, member 6) driven by
a favorable transmembrane voltage (16). Magnesium entry into DCT cells
appears to be the rate limiting step and site of regulation.
Finally, 3–5% of the filtered magnesium is excreted in the urine
(14). TRPM6 is also expressed in
intestinal epithelia where it plays a role in magnesium uptake. In
the kidney, EGFR was detected in glomerular epithelial cells, TAL
and DCT (17). Thus, both EGFR and
TRPM6 are predominantly expressed in the DCT, the main site of
active renal Mg2+ reabsorption. Moreover, it has been
shown that the EGF dose dependently stimulated TRPM6 activity
(18), and cetuximab significantly
inhibited the activity of TRPM6 in human embryonic kidney cells
(19).
The management of anti-EGFR antibody-inducing
hypomagnesemia has always been challenging. In most patients the
toxicity has been grade 1 or 2 (0.9 mg/dl to lower limit of
normal), grade 3 (0.7–0.8 mg/dl) or grade 4 (≤0.6 mg/dl). Mostly
grade 1 hypomagnesemia patients are generally asymptomatic and do
not require magnesium supplementation. In patients with higher
grade hypomagnesemia, intravenous replacement (2–3 g of magnesium
sulfate) was administered mostly on the day of the anti-EGFR
therapy. This method of replenishment was not adequate in some
patients, in particular those with grade 3 hypomagnesemia. Many
patients who have higher grade hypomangnesemia often present
symptoms of fatigue, cramps or sleepiness. Patients who receive a
combination of chemotherapy and anti-EGFR antibodies do not
normally complain about these symptoms because they tend to
attribute them to cytotoxic chemotherapy. It has been shown that
the reversal of hypomagnesemia in patients of this category
resulted in improvements in energy level and performance status
(11). The management of
hypomagnesemia needs to be aggressive in patients with grade 3 or 4
hypomagnesemia. Therefore, very high doses of magnesium are needed
to achieve clinically significant reversal of these symptoms.
Weekly magnesium replacement is often inadequate and serum
magnesium levels fall back to low baseline level within 3–4 days.
There have been reports suggesting that these patients would
require daily to twice weekly treatment with intravenous magnesium
to a level up to 6–10 g/dose. There have also been cases of
continuing or worsening grade 4 hypomagnesemia despite the daily 10
g of magnesium replacement (11).
The possible reason for this is that as long as the EGFR in the
kidney and intestine is inhibited, the renal magnesium wasting
coupled with inadequate intestinal absorption will worsen. Since
EGFR inhibition is rapid and long lasting after anti-EGFR therapy,
the effectiveness of IV Mg2+ supplementation diminishes.
As long as the EGFR inhibition continues in the body, magnesium
wasting from the body is perpetuated. Thus, the alleviation of
symptoms is transient and the deficient symptoms recur soon before
the next dose of EGFR inhibitor. Since these agents are either
given as weekly or bi-weekly cycles, the management of
hypopmagnesemia and secondary hypocalcemia becomes difficult and
sometimes the therapy needs to be interupted or terminated.
Moreover, intravenous Mg2+ loading in these patients
causes a significant renal Mg2+ leak. A possible way to
control this would be through the use of oral magnesium
supplements. In oral magnesium transport, TRPM6 plays a role in its
absorption in the gut. Also, evidence suggests that autosomal
recessive hypomagnesemia with secondary hypocalcemia (HSH) is
caused by a mutation in TRPM6, which encodes the TRPM6
protein (20). In HSH patients,
substitution of high oral doses of magnesium achieved good serum
magnesium levels (14). However,
diarrhea and other gastrointestinal disturbances associated with
the use of magnesium supplementation, often limits its importance
in treating many patients receiving anti-EGFR therapy. The
selection of better tolerated magnesium supplements would benefit
these patients.
Observation of hypoalbuminemia was unexpected, as
there has been no documentation of this as an adverse effect of
anti-EGFR antibody therapy. Initially, it was thought that this
change was related to the disease itself rather than caused by the
anti-EGFR therapy. Surprisingly in this patient population, there
appeared to be a good correlation between the
initiation/discontinuation of the therapy and the changes in
albumin level. The normal serum concentration of albumin in healthy
adults is approximately 35–50 g/l. Hypoalbuminemia is common in
seriously ill patients. The increased likelihood of poor patient
outcomes such as mortality, morbidity and prolonged hospital stay
in acutely ill patients with hypoalbuminema is well recognized.
Because of its importance as an outcome predictor, serum albumin
level was included as one of the component parameters in the Acute
Physiology and Chronic Health Evaluation (APACHE) score (21). The medical literature documents
several examples of an inverse relationship between serum albumin
levels and survival in patients with advanced cancer (22). Many studies, spanning 40 years,
have demonstrated that subclinical cancer, particularly of the
breast, cervix and ovaries, may occur in more than 60% of pregnant
women, when albumin remains between 20–25 g/l, and spontaneous
remission normally is achieved postpartum when albumin returns to
more than 43 g/l (23). It is well
recognized that the restoration of albumin profiles usually leads
to full remission. When the albumin level falls it causes relapse.
This is clearly demonstrated in Hodgkin's disease (24).
Serum albumin plays a diverse, complex and important
role in maintaining physiological homeostasis. At reduced albumin
levels, these homeostatic functions may be impaired, resulting in
the development and/or progression of pathologic processes
underlying poor outcome. Albumin has an antioxidant and free
radical scavenging activity, a capacity to prevent apoptosis and an
affinity for binding lipids, drugs, toxic substances and other
ligands (25). Because of its high
frequency in a broad range of pathologic conditions,
hypoalbuminemia may plausibly be interpreted as a normal
compensatory mechanism not requiring intervention. For instance,
albumin redistribution into the interstitial space may provide
protection from oxidative stress affecting extravascular tissues
during disease states.
The mechanism of hypoalbuminemia in patients
receiving anti-EGFR therapy is unknown. Plasma volume expansion due
to overhydration, external albumin losses resulting from
albuminuria, malnutrition, defective albumin synthesis, catabolism
and distribution are some of the possible reasons for a decrease in
serum albumin levels. The primary cause of low albumin in cancer
patients has been identified by some researchers (26,27)
as the specific inhibition of albumin gene transcription by the
tumor necrosis factor (TNF).
Many chemotherapy agents, in particular those with
high lipid solubility, bind extensively to albumin. It is also
important to note that the relative concentrations of free or
albumin-bound fractions of a chemotherapeutic agent will influence
the severity of its side effects. Therefore, the maintenance of
adequate serum albumin levels in patients receiving anti-EGFR
therapy would play a key role in containing some of the adverse
effects of concurrently administered chemotherapeutic agents.
In the present study, serum levels of magnesium,
calcium and albumin in patients receiving cetuximab and panitumumab
were compared. The rate of incidence of hypomagnesemia was
comparable to previously reported studies, but was found to be
higher in patients treated with panitumumab compared to cetuximab.
The severity of hypomagnesemia was also greater in patients
receiving panitumumab. The observation of hypoalbuminemia was an
unexpected adverse effect of the anti-EGFR antibody therapy.
However, the inclusion of case-matched controls in the study would
have added more value to the data.
References
|
1.
|
Hynes NE and Lane HA: ERBB receptors and
cancer: the complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Di Fiore PP, Pierce JH, Fleming TP, et al:
Overexpression of the human EGF receptor confers an EGF-dependent
transformed phenotype to NIH 3T3 cells. Cell. 51:1063–1070.
1987.PubMed/NCBI
|
|
3.
|
Riechmann L, Clark M, Waldmann H and
Winter G: Reshaping human antibodies for therapy. Nature.
332:323–327. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Baselga J, Pfister D, Cooper MR, et al:
Phase I studies of anti-epidermal growth factor receptor chimeric
antibody C225 alone and in combination with cisplatin. J Clin
Oncol. 18:904–914. 2000.PubMed/NCBI
|
|
5.
|
Robert F, Ezekiel MP, Spencer SA, et al:
Phase I study of anti-epidermal growth factor receptor antibody
cetuximab in combination with radiation therapy in patients with
advanced head and neck cancer. J Clin Oncol. 19:3234–3243.
2001.PubMed/NCBI
|
|
6.
|
Peeters M, Balfour J and Arnold D: Review
article: panitumumab – a fully human anti-EGFR monoclonal antibody
for treatment of metastatic colorectal cancer. Aliment Pharmacol
Ther. 28:269–281. 2008.
|
|
7.
|
Lynch DH and Yang XD: Therapeutic
potential of ABX-EGF: a fully human anti-epidermal growth factor
receptor monoclonal antibody for cancer treatment. Semin Oncol.
29:47–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Schrag D, Chung KY, Flombaum C and Saltz
L: Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer
Inst. 97:1221–1224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Van Cutsem E, Siena S, Humblet Y, et al:
An open-label, single-arm study assessing safety and efficacy of
panitumumab in patients with metastatic colorectal cancer
refractory to standard chemotherapy. Ann Oncol. 19:92–98.
2008.PubMed/NCBI
|
|
10.
|
Tejpar S, Piessevaux H, Claes K, et al:
Magnesium wasting associated with epidermal-growth-factor
receptor-targeting antibodies in colorectal cancer: a prospective
study. Lancet Oncol. 8:387–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Fakih M: Management of anti-EGFR-targeting
monoclonal antibody-induced hypomagnesemia. Oncology. 22:74–76.
2008.PubMed/NCBI
|
|
12.
|
Sobrero A, Fehrenbacher L and Rivera F:
Randomized Phase III trial of cetuximab plus irinotecan versus
irinotecan alone for metastatic colorectal cancer in 1298 patients
who have failed prior oxaliplatin-based therapy. In: Program and
abstracts of the American Association for Cancer Research Annual
Meeting; Los Angeles. April 2007;
|
|
13.
|
Van Cutsem E, Nowacki M and Lang I:
Randomized Phase III study of irinotecan and 5-FU/FA with or
without cetuximab in the first-line treatment of patients with
metastatic colorectal cancer (mCRC): the CRYSTAL trial. In: Program
and abstracts of the American Association for Cancer Research
Annual Meeting; Los Angeles. April 2007;
|
|
14.
|
Konrad M, Schlingmann KP and Gudermann T:
Insights into the molecular nature of magnesium homeostasis. Am J
Physiol Renal Physiol. 286:F599–F605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hoenderop JG and Bindels RJ: Epithelial
Ca2+ and Mg2+ channels in health and disease.
J Am Soc Nephrol. 16:15–26. 2005.
|
|
16.
|
Voets T, Nilius B, Hoefs S, et al: TRPM6
forms the Mg2+ influx channel involved in intestinal and
renal Mg2+ absorption. J Biol Chem. 279:19–25.
2004.PubMed/NCBI
|
|
17.
|
Gesualdo L, Di Paolo S, Calabro A, et al:
Expression of epidermal growth factor and its receptor in normal
and diseased human kidney: an immunohistochemical and in situ
hybridization study. Kidney Int. 49:656–665. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sack E and Talor Z: High affinity binding
sites for epidermal growth factor (EGF) in renal membranes. Biochem
Biophys Res Commun. 154:312–317. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Groenestege WM, Thebault S, van der Wijst
J, et al: Impaired basolateral sorting of pro-EGF causes isolated
recessive renal hypomagnesemia. J Clin Invest. 117:2260–2267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Schlingmann KP, Weber S, Peters M, et al:
Hypomagnesemia with secondary hypocalcemia is caused by mutations
in TRPM6, a new member of the TRPM gene family. Nat Genet.
31:166–170. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Knaus WA, Wagner DP, Draper EA, et al: The
APACHE III prognostic system. Risk prediction of hospital mortality
for critically ill hospitalized adults. Chest. 100:1619–1636. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lis CG, Grutsch JF, Vashi PG and
Lammersfeld CA: Is serum albumin an independent predictor of
survival in patients with breast cancer? JPEN J Parenter Enteral
Nutr. 27:10–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Seaton K: Albumin, cancer and pregnancy. J
Natl Med Assoc. 94:629–630. 2002.PubMed/NCBI
|
|
24.
|
Gobbi PG, Gendarini A, Crema A, et al:
Serum albumin in Hodgkin's disease. Cancer. 55:389–393. 1985.
|
|
25.
|
Emerson TE Jr: Unique features of albumin:
a brief review. Crit Care Med. 17:690–694. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Peters T: All About Albumin. New York
Academic Press; New York: 1996
|
|
27.
|
Seaton K: Albumin concentration controls
cancer. J Natl Med Assoc. 93:490–493. 2001.PubMed/NCBI
|