Introduction
The functional p53 pathway is critical for
homeostasis and tumor inhibition. p53 mutations, which are detected
in more than half of human cancers, have been associated with tumor
development, poor prognosis and therapeutic resistance. Various
therapeutic strategies aiming to rescue or amplify p53 pathways are
being developed for cancer treatment (1–3). In
particular, p53AIP1 is emerging as a therapeutic target with great
potential.
The p53AIP1 gene is a p53 target gene located on
chromosome 11. Alternative splicing gives rise to three isoforms
(α, β and γ) of p53AIP1 proteins (4). Functional studies indicate that
p53AIP1 is a potent pro-apoptotic molecule. Overexpression of
p53AIP1 alone induces apoptosis (5). Blockage of p53AIP1 using specific
siRNA significantly attenuates p53-induced apoptosis (4). Available data suggest that p53AIP1
induces apoptosis through the mitochondrial pathway. By interacting
with Bcl-2, p53AIP1 decreases mitochondrial membrane potential and
induces cytochrome c release, which leads to caspase-9 activation
and the downstream activities (6).
Expression of p53AIP1 is primarily regulated by p53.
Activation of p53 by a number of factors, such as DNA damage,
induces the transcription of p53AIP1 (7–10).
In contrast to the p53-mediated regulation of p21 and MDM2, which
involves the phosphorylation of Ser-15 of p53 (11,12),
induction of p53AIP1 mRNA and its apoptotic function was uniquely
associated with the phosphorylation of Ser-46 of p53 (4). Substitution of Ser-46 impairs
p53-mediated apoptosis and its induction of p53AIP1
transcription.
The clinical relevance of p53AIP1 is supported by
reports on its association with prognosis and therapeutic
potential. Sawaya et al reported that p53AIP1 mRNA levels
were significantly lower in gastric carcinoma cells, as compared to
chronic gastritis samples (13).
Data by Yamashita et al revealed that p53AIP1 gene
expression in a lymph node metastasis-positive group was
significantly lower than in a negative group, and the overall
survival of a p53AIP1 low expression group was significantly worse
than that of a p53AIP1 high expression group (14). A study by Yoshida et al
indicated that Ad-p53AIP1 induced apoptosis in a number of cancer
cell lines. It appeared that cells with wild-type p53 were more
sensitive to Ad-p53AIP1 than cells with mutant p53 (5). However, the mechanism of p53-related
sensitivity was not elucidated.
In this study, we generated Ad-p53AIP1 and
established in vitro and in vivo models to evaluate
Ad-p53AIP1-induced tumor inhibition. We characterized
Ad-p53AIP1-induced apoptosis and cell cycle arrest in HepG2, HeLa
and 4T1 cells. Our data demonstrated that p53AIP1-induced
up-regulation of p53 through MDM2 down-regulation is an important
mechanism underlying Ad-p53AIP1-induced apoptosis and cell cycle
arrest.
Materials and methods
Reagents
Plasmids pDC316 and pBHGloxΔE1, 3Cre were purchased
from Fungenome Technology Co., Ltd. and Vector Gene Technology Co.,
Ltd., respectively. Ad-null viruses were a gift from Vector Gene
Technology Co., Ltd. Antibodies against p53AIP1, β-actin, p21, p53
and MDM2 were purchased from Santa Cruz Biotechnology, Inc.
Anti-cleaved PARP antibody was purchased from Cell Signaling
Technology, Inc. Anti-CDK4 antibody was purchased from Lab Vision
Inc., Fremont, CA. Rhodamine 123 was from Sigma Co., and the
reverse transcription kit was purchased from Jingmei Biotech Co.,
Ltd.
Construction, characterization and
amplification of p53AIP1-encoding adenovirus
The p53AIP1 gene was cloned from a cDNA library
using the following primers: forward, 5′ ATG GGA TCT TCC TCT GAG
GCG A 3′; reverse, 5′ TCA CTG CAA CCT CAA CGG TGC T 3′. The cloned
gene was verified by DNA sequencing. The p53AIP1 gene was then
subcloned into the pDC316 shuttle vector. For adenovirus packaging,
pDC316-p53AIP1 and pBHGloxΔE1, 3Cre (the backbone vector of the
AdMax adenoviral vector system) were co-transfected into HEK293
cells using Lipofectamine 2000 (Invitrogen). Twelve days after
transfection, individual plaques were chosen for DNA purification.
p53AIP1 integration was examined by PCR using the above mentioned
primers. Viruses derived from p53AIP1-positive clones were named
Ad-p53AIP1. This virus was then amplified and titrated as described
previously (15).
Cell culture and viral transduction
Human hepatocellular carcinoma cell line HepG2,
human embryonic kidney cell line HEK293 and murine BALB/c derived
mammary tumor cell line 4T1 cells were cultured in H-DMEM medium
supplemented with 10% FBS and antibiotics. For viral transduction,
unless specified, the cells were infected with Ad-null or
Ad-p53AIP1 at a MOI of 100 for 24 h, followed by specific
examinations.
RT-PCR
To examine the mRNA levels of p53AIP1, p53 and MDM2
in HepG2 cells, total RNA was extracted from the cells 6, 12 and 24
h after transduction. First-strand cDNA was synthesized using a kit
from Jingmei BioTech Co. Ltd., following the manufacturer's
protocol. The primer sequences for the PCR reactions were as
follows: p53 forward, 5′ AGC ACT GTC CAA CAA CAC CA 3′; reverse, 5′
TCC ATG TCA GTC TGA GTC AG 3′; MDM2 forward, 5′ CCC TGG TTA GAC CAA
AGC CAT 3′; reverse, 5′ GGC ACG CCA AAC AAA TCT CC 3′. The PCR
conditions were 94°C/5 min, (94°C/30 sec, 53°C/30 sec, 72°C/30 sec)
32 cycles, 72°C/7 min. PCR products (20 μl) were
electrophoresed on a 2% agarose gel.
Western blotting
Cell lysates were extracted from the treated cells
at indicated time-points after viral transduction, followed by
protein concentration determination. The protein lysate (50
μg) from each sample was separated using 10% SDS-PAGE gel
and transferred to a nitrocellulose membrane. The membrane was
blocked with 5% milk and probed with the primary antibody against
the protein to be detected. The specific bands were visualized with
ECL.
Effect of p53AIP1 on cell survival/growth
by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium
bromide (MTT) assay
Two thousand cells were plated into each well of
96-well plates one day prior to viral transduction. The cells were
then transduced with Ad-null or Ad-p53AIP1 virus at a MOI of 100
for 4 h, followed by medium replacement. Using the MTT assay, cell
survival/proliferation was determined daily after treatment for 5
days. For MTT assay, the cells were incubated with MTT (500
μg/ml) for 3 h. Incorporated MTT was dissolved and measured
at Dnm 570. Each data set was based on six parallel
samples.
Flow cytometric detection of cell cycle
distribution and mitochondrial membrane potential
For cell cycle analysis, the treated cells were
trypsinized and collected at indicated times, followed by fixation
with paraformaldehyde. Fixed cells were washed with PBS containing
0.1% Triton X-100 and treated with RNase A for 30 min and stained
with 50 μg/ml propidium iodide. Cells were analyzed using a
FACS Calibur flow cytometer. Cell cycle distribution was analyzed
using MultiFit software. To measure the mitochondrial membrane
potential, treated cells at indicated time points were typsinized,
pelleted and washed with PBS. Cells in suspension were then stained
with Rhodamine 123 (5 μg/ml) in the dark for 30 min,
followed by flow cytometric analysis.
Transmission electron microscopy
Non-treated control and Ad-null or Ad-p53AIP1
transduced (100 MOI for 96 h) HepG2 cells were fixed in 2.5%
glutaraldehyde for 4 h then treated with 1% OsO4. The
samples were then dehydrated through a graded ethanol series and
embedded, followed by ultra-thin sectioning, staining and viewing
under an electron microscope.
Hoechst staining
The cells treated with either Ad-null or Ad-p53AIP1
at 100 MOI for 48 h were collected (including floating and
trypsinized cells) and washed with PBS followed by fixation with 2%
paraformaldehyde at 4°C for 30 min. The cells were stained with 0.5
μg/ml Hoechst 33258 for 30 min. Stained cells were then
washed and mounted on slides using a cytospinner. Apoptotic cells
with nuclear condensation or fragmentation were counted under an
Olympus fluorescence microscope.
In vivo tumor xenograft study
Female BALB/c mice (6 weeks old, 18–22 g) and murine
mammary tumor cell line 4T1 were used for this study. The first set
of experiments was designed to examine in vivo tumor
development of 4T1 cells transduced with Ad-p53AIP1 prior to
implantation. With 7 mice in each group, the groups included PBS
treatment, Ad-null transduction and Ad-p53AIP1 transduction. After
exposure to PBS or Ad-null or Ad-p53AIP1 virus in vitro for
24 h, 4T1 cells from each group were injected s.c. into the flanks
of the mice (1×106 cells/100 μl/mouse). After
transplantation, tumor size was measured using calipers, and tumor
volume was estimated according to the following formula: Tumor
volume (mm3) = (A × B2)/2, where A is the
length and B is the tumor width. Tumor inhibition rate (%) =
[(average of control tumor volume – average of Ad-p53AIP1 tumor
volume)/ average of control tumor volume] × 100%. The second set of
experiments involved examining the effect of in vivo
delivery of Ad-p53AIP1 on 4T1 tumor development. To this end,
untreated 4T1 cells were implanted into 21 mice at a dose of
1×106 cells/100 μl/mouse. The mice were randomly
divided into three groups when tumor volumes reached 4–5 mm in
diameter. With 7 mice in each group, the animals were treated with
a multi-spot intra tumor injection of PBS, Ad-null or Ad-p53AIP1 at
2×108 PFU/100 μl/mouse. The injections were
repeated two more times with an interval of 7 days. Tumor
development and documentation were performed as above.
Statistical analysis
Data were presented as the mean ± SD. Statistical
analysis was performed using SAS 8.0 software package.
Results
Expression of p53AIP1 in
Ad-p53AIP1-transduced HepG2 cells
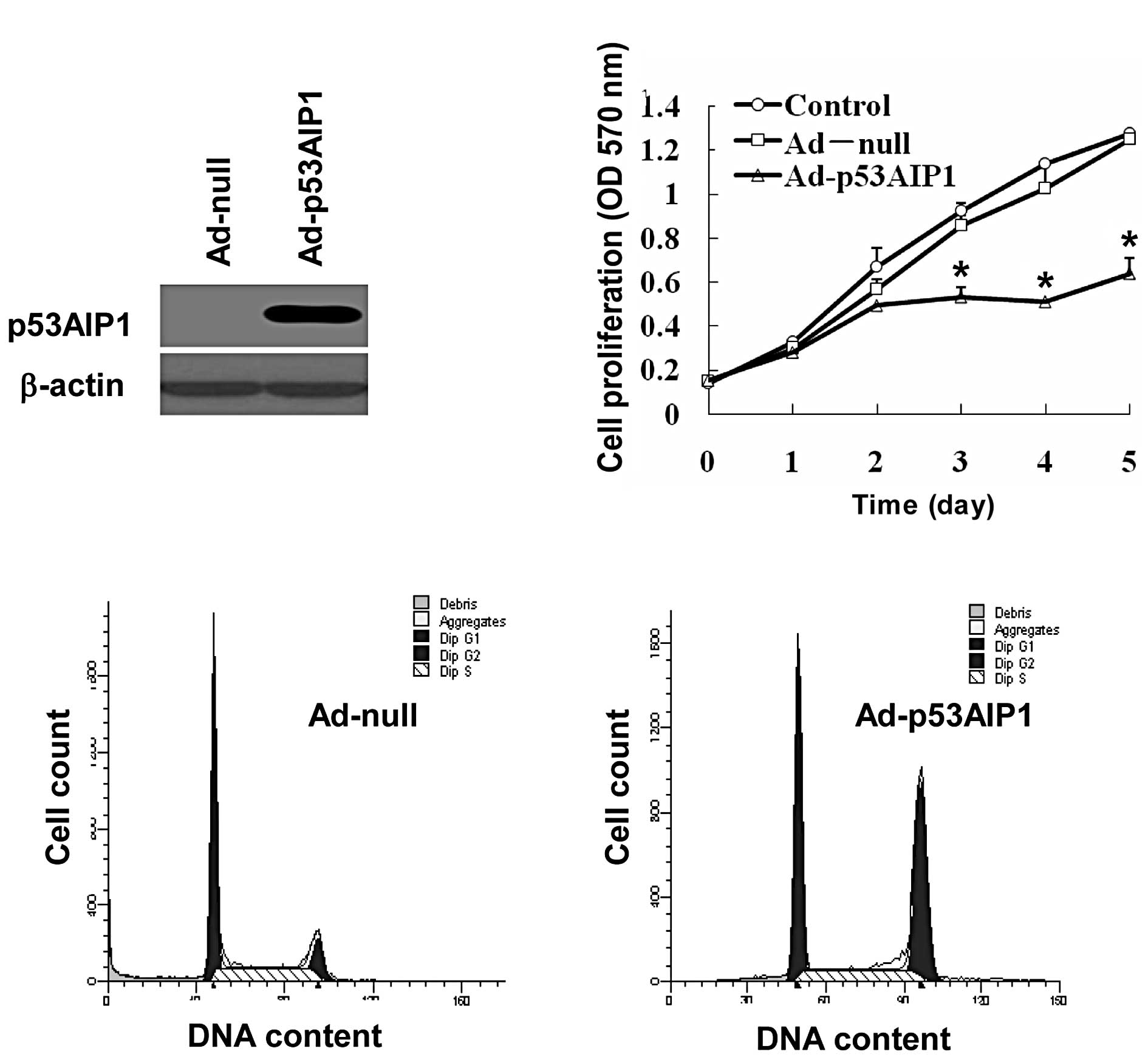
We first constructed the Ad-p53AIP1 virus to deliver
p53AIP1 expression in cancer cells. We then examined the protein
levels of p53AIP1 in HepG2 cells transduced with Ad-null or
Ad-p53AIP1. Compared to non-detectable p53AIP1 in mock-transduced
cells, the cells transduced with Ad-p53AIP1 expressed high levels
of p53AIP1 (Fig. 1A), which
provided a solid foundation for further functional studies.
To test the effect of Ad-p53AIP1 transduction on
cell proliferation, we treated HepG2 cells with 100 MOI of Ad-null
or Ad-p53AIP1 virus and compared their proliferation rates with the
non-treatment control. As shown in Fig. 1B, the proliferation of
Ad-p53AIP1-transduced cells was significantly inhibited, which was
approximately one half of the control cells on day 5. To test
whether Ad-p53AIP1 has any effect on cell cycle progression, we
analyzed the cell cycle distribution of HepG2 cells infected with
Ad-p53AIP1 for 24 h. We found that p53AIP1 expression increased the
number of HepG2 cells in G2/M phases. Since this was accompanied by
decreased proliferation, the data suggest that Ad-p53AIP1 induced
G2/M arrest in HepG2 cells, which contributed to its growth
inhibition effect (Fig. 1C).
p53AIP1 expression induces apoptosis in
HepG2 cells
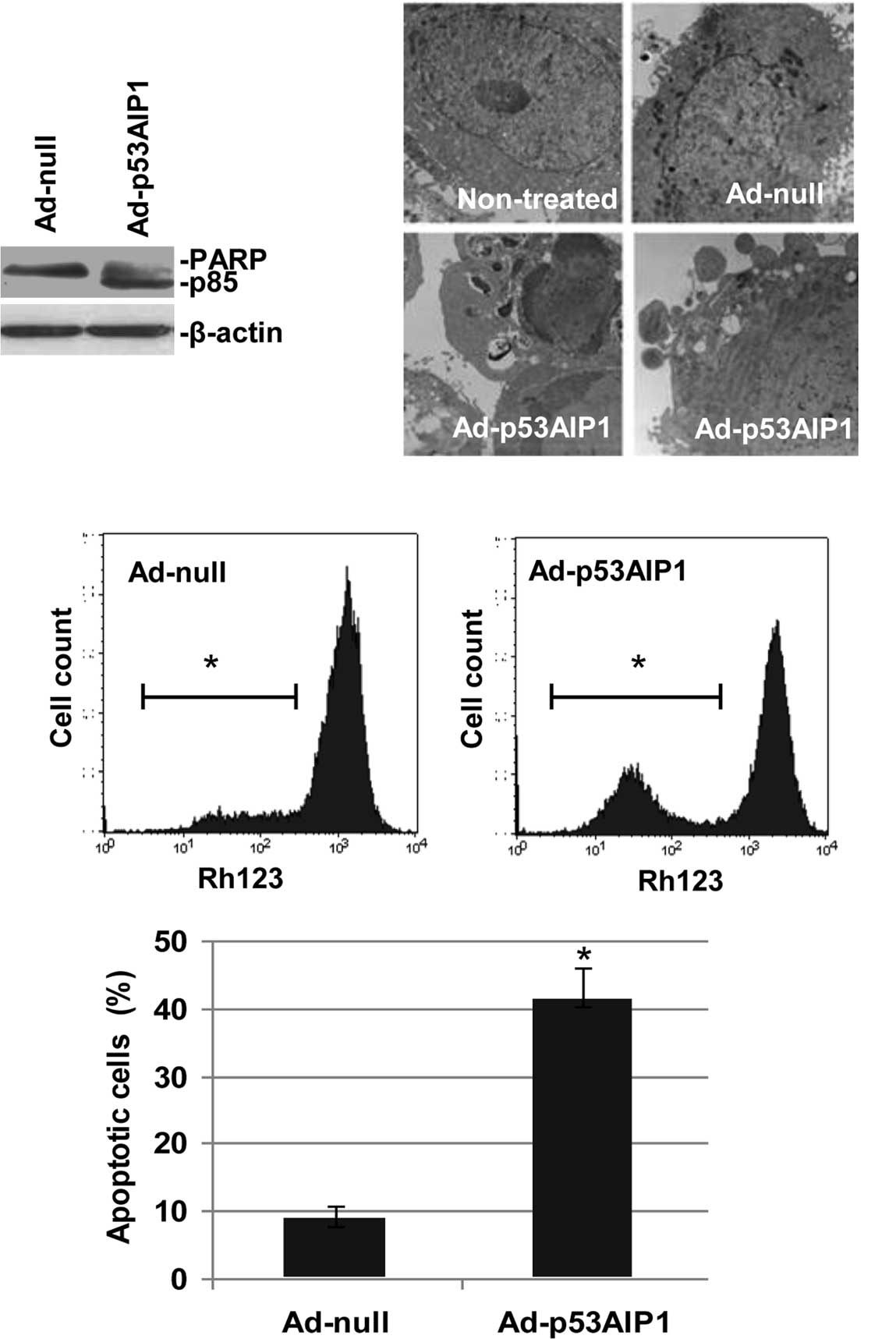
As p53AIP1 is a pro-apoptotic molecule, we
characterized Ad-p53AIP1-induced apoptosis in HepG2 cells by
poly(ADP-ribose) polymerase (PARP) cleavage, electron microscopy,
mitochondrial membrane potential and Hoechst staining. As shown in
Fig. 2A, in contrast to
non-detectable cleavage on Ad-null infected cells, PARP cleavage
was readily detected in HepG2 cells infected with Ad-p53AIP1 for 48
h. Under a transmission electron microscope (Fig. 2B), apoptotic HepG2 cells induced by
Ad-p53AIP1 infection for 96 h displayed chromatin condensation with
clumped perinuclear distribution and nuclear fragmentation. Other
features included membrane blebbing, loss of microvilli and
appearance of apoptotic bodies.
Flow cytometric analysis of mitochondrial membrane
potential with Rhodamine 123 revealed that the fluorescent
intensity in Ad-p53AIP1-infected HepG2 cells decreased as infection
time increased. In contrast to a geometric mean value of 889.40 in
Ad-null-infected cells, the fluorescent intensity decreased to
502.64 in the Ad-p53AIP1-infected cells 72 h after infection,
suggesting that p53AIP1-induced apoptosis in HepG2 cells involves
mitochondrial membrane potential collapse (Fig. 2C). Moreover, nuclear staining with
Hoechst 33258 showed that nuclear condensation and/or fragmentation
were detected in 41% of Ad-p53AIP1-induced cells, as compared to a
9% change in Ad-null-infected cells (Fig. 2D). In addition, we also observed
similar apoptotic patterns in HeLa cells infected with Ad-p53AIP1
(data not shown). Taken together, these data demonstrated that
Ad-p53AIP1 induced potent apoptotic activity in the infected
cells.
Ad-p53AIP1 inhibits the development and
growth of 4T1 breast tumors in vivo
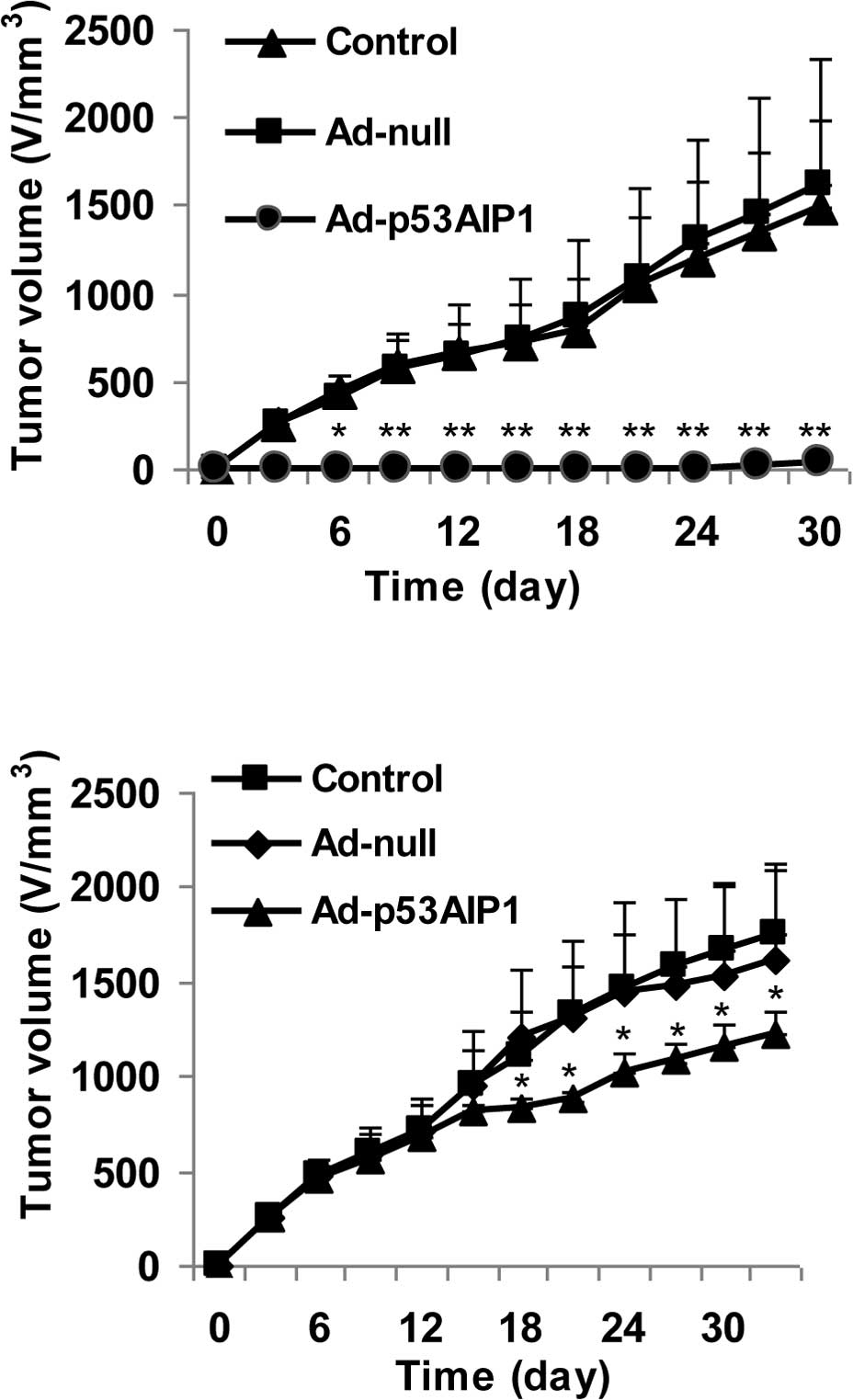
We next examined the efficacy of Ad-p53AIP1 on tumor
growth in vivo using a 4T1 breast tumor model. In the first
set of experiments, 4T1 tumor cells were treated with control
(PBS), Ad-null or Ad-p53AIP1 virus for 24 h before implantation.
The results showed that the tumors in the control and Ad-null
groups grew progressively, reaching ∼1,700 mm3 by day
30. In contrast, tumor growth was minimal in the group treated with
Ad-p53AIP1. The tumors were not palpable until day 21 (Fig. 3A). The striking difference between
the control and Ad-p53AIP1-treated tumors indicates that Ad-p53AIP1
has a potent tumor inhibition effect. In the second set of
experiments, we tested the effect of Ad-p53AIP1 on the progression
of 4T1 tumors. After the tumors were palpable, 2×108 PFU
Ad-null or Ad-p53AIP1 viruses were administered to the tumors by
multi-spot intra-tumor injection. As shown in Fig. 3B, tumor growth in the
Ad-p53AIP1-treated group was significantly slower (p<0.05) than
in the control and the Ad-null groups.
Up-regulation of p53 in
Ad-p53AIP1-transduced cells
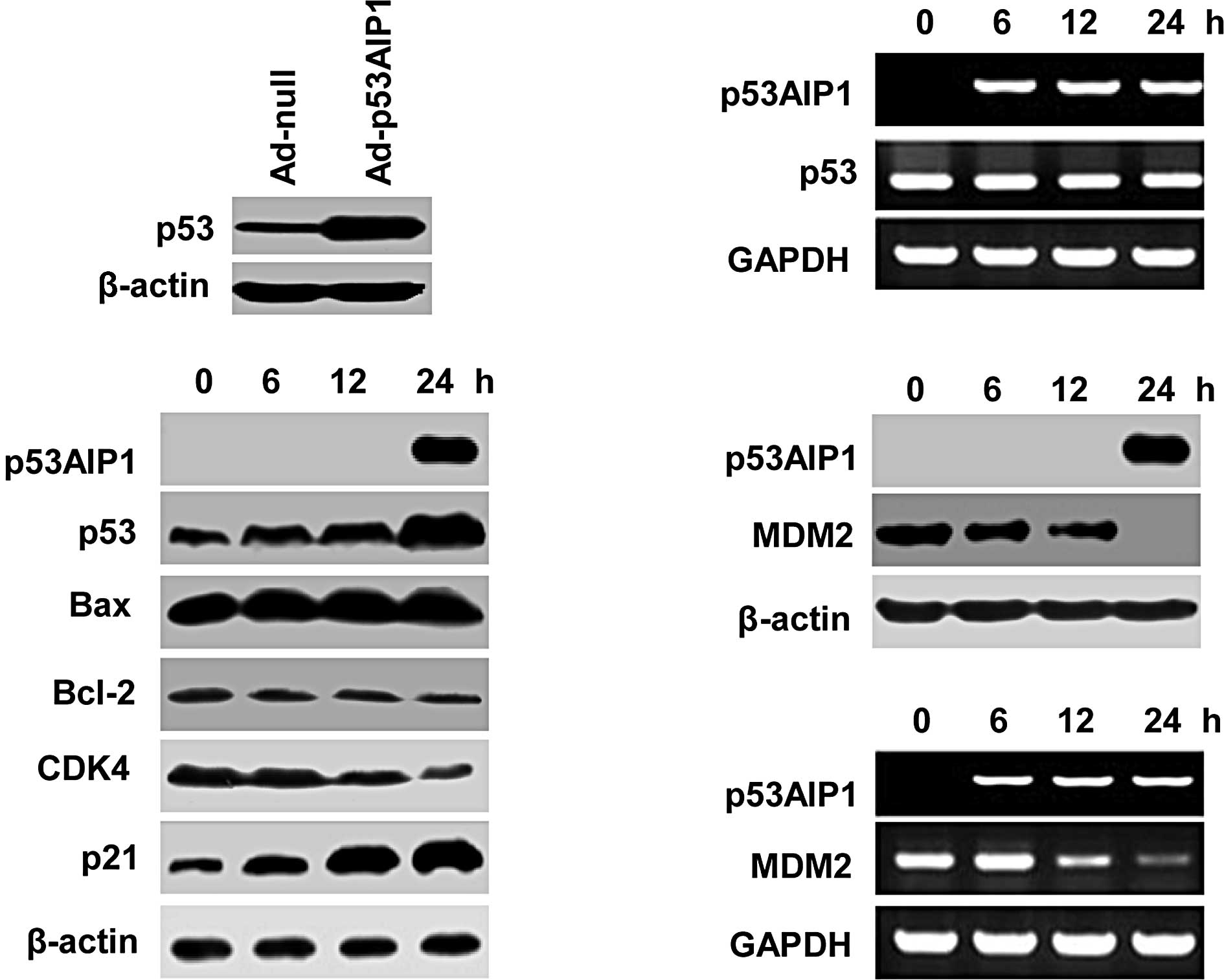
To study the underlying mechanisms of p53AIP1
overexpression-induced growth inhibition and apoptosis, we examined
the expression of a number of key factors involved in apoptosis and
cell cycle regulation, including p53, p21, Bax, Bcl-2 and CDK4 in
HepG2 cells. In contrast to minimal changes in Ad-null-infected
cells (data not shown), we found that p53 protein levels were
significantly up-regulated after Ad-p53AIP1 infection (Fig. 4A), which was accompanied by a
significant increase in p21, a moderate increase in Bax and
decrease in Bcl-2 and CDK4 (Fig.
4B). Since p53 mRNA levels in Ad-p53AIP1-transduced cells were
not increased (Fig. 4C),
p53AIP1-induced up-regulation of p53 appears to occur at the
protein level rather than the transcriptional level. These results
suggest that p53AIP1 not only functions as a target of p53 but also
has a feedback action on p53 expression.
Ad-p53AIP1 down-regulates MDM2
transcription
To elucidate the mechanism of p53AIP1-mediated
up-regulation of p53 protein levels, we examined whether the
expression of MDM2, a p53-specific E3 ubiquitin ligase, was
affected by p53AIP1 expression in HepG2 cells. We found that both
MDM2 protein and mRNA levels were decreased in
Ad-p53AIP1-transduced cells (Fig. 4D
and E). A decrease in MDM2 and a corresponding increase in p53
expression in p53AIP1-overexpressing cells suggest that p53AIP1
up-regulates p53 through down-regulation of MDM2, which leads to
increased p53 stability.
Discussion
Gene therapy targeting p53 pathways is a promising
new approach in cancer treatment. Adenovirus-mediated p53 gene
therapy for human cancers has been approved for clinical
application in China (16).
However, direct delivery of p53 might be compromised by certain p53
antagonists in cancer cells, such as MDM2 overexpression (17,18).
Thus, identifying novel targets to rescue or augment the function
of the p53 pathway has been actively pursued. In this study, we
tested the effects of Ad-p53AIP1 on cancer cell proliferation and
apoptosis in both in vitro and in vivo settings. We
also discovered a novel mechanism of p53AIP1-induced tumor
regression.
p53AIP1 is a p53 target gene and a potent
pro-apoptotic molecule. Since it is downstream of p53 and can
circumvent p53 compromising factors, these characteristics make it
a good candidate for a p53 alternative in cancer gene therapy.
Recent association between low levels of p53AIP1 in human cancer
tissues and a poor prognosis further supports its implication in
cancer therapy (4). Previously,
Yoshida et al examined the Ad-p53AIP1-mediated apoptotic
effect on a number of cancer cell lines and found that p53AIP1
alone was able to induce marked apoptosis in many cell lines.
Ad-p53AIP1 appeared to be more effective in p53+/+
cells, although the mechanism was not clear (5).
In this study, we constructed Ad-p53AIP1 and
established its in vitro and in vivo models for
further studies, especially in mechanistic investigation. Using
HepG2 cells, we demonstrated that Ad-p53AIP1, not only stimulates
apoptosis, but also induces cell cycle arrest in G2/M phases.
p53AIP1-induced cell cycle arrest was accompanied by p21
up-regulation and CDK4 down-regulation. This novel finding suggests
that p53AIP1 may function beyond apoptotic induction and that its
cell cycle regulation may also contribute to p53AIP1-mediated tumor
regression. Moreover, data from in vivo experiments
demonstrated that Ad-p53AIP1 effectively inhibited 4T1 tumor
development and progression. Ad-p53AIP1-mediated tumor regression
was more effective using pre-inoculation treatment than intra-tumor
injection. This can partially be explained by the low efficiency
associated with in vivo viral delivery.
One of the major findings of this study is that
p53AIP1 may up-regulate p53 protein levels in HepG2 cells. We
demonstrated that p53AIP1 expression up-regulated p53 protein
levels but not mRNA levels. We also found that p53AIP1
down-regulated MDM2 transcription. Since MDM2 is a ubiquitin ligase
that induces p53 degradation (19–21),
p53AIP1-mediated inhibition of MDM2 might lead to p53 protein
accumulation. This finding may partially explain why p53AIP1 is
more effective in cancer cells with wild-type p53 (5) and why cell cycle regulation was
modified in p53AIP1-overexpressing cells (Fig. 2). However, p53AIP1-induced
up-regulation of p53 and down-regulation of MDM2 in HeLa cells were
less evident when compared to HepG2 cells (data not shown). Hence,
the factors that determine p53AIP1-induced MDM2-p53 interaction and
cause cell line-dependent discrepancy require further
investigation.
In summary, we established in vitro and in
vivo models with which to study Ad-p53AIP1-mediated tumor
inhibition. These can be used to explore more specific factors that
may affect the efficacy of Ad-p53AIP1-induced apoptosis and cell
cycle arrest. Our findings related to p53AIP1-induced MDM2-p53
interaction in HepG2 cells are of great significance. They suggest
that gene therapy targeting Ad-p53AIP1 may be more advantageous for
tumor cells overexpressing oncoprotein MDM2 or having a mutation in
the MDM2 inhibitor p14ARF and p16INK4 (22,23).
Further studies along this line may open a new avenue for fighting
human cancers.
Acknowledgements
This study was supported by grants
from the Major State Basic Research Development Program of China
(to Dr Yinglin Lu, no. 2002CB513105).
References
|
1.
|
Shimada H, Matsubara H, Shiratori T, et
al: Phase I/II adenoviral p53 gene therapy for
chemoradiation-resistant advanced esophageal squamous cell
carcinoma. Cancer Sci. 97:554–561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Edelman J and Nemunaitis J: Adenoviral p53
gene therapy in squamous cell cancer of the head and neck region.
Curr Opin Mol Ther. 5:611–617. 2003.PubMed/NCBI
|
|
3.
|
Wolf JK, Bodurka DC, Gano JB, et al: A
phase I study of Adp53 (INGN 201; ADVEXIN) for patients with
platinum- and paclitaxel-resistant epithelial ovarian cancer.
Gynecol Oncol. 94:442–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Oda K, Arakawa H, Tanaka T, et al:
p53AIP1, a potential mediator of p53-dependent apoptosis, and its
regulation by Ser-46-phosphorylated p53. Cell. 102:849–862. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yoshida K, Monden M, Nakamura Y and
Arakawa H: Adenovirus-mediated p53AIP1 gene transfer as a new
strategy for treatment of p53-resistant tumors. Cancer Sci.
95:91–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Matsuda K, Yoshida K, Taya Y, Nakamura K,
Nakamura Y and Arakawa H: p53AIP1 regulates the mitochondrial
apoptotic pathway. Cancer Res. 62:2883–2889. 2002.PubMed/NCBI
|
|
7.
|
Lunghi P, Costanzo A, Levrero M and Bonati
A: Treatment with arsenic trioxide (ATO) and MEK1 inhibitor
activates the p73-p53AIP1 apoptotic pathway in leukemia cells.
Blood. 104:519–525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wesierska-Gadek J, Gueorguieva M and Horky
M: Roscovitine-induced up-regulation of p53AIP1 protein precedes
the onset of apoptosis in human MCF-7 breast cancer cells. Mol
Cancer Ther. 4:113–124. 2005.PubMed/NCBI
|
|
9.
|
Xie P, Tian C, An L, et al: Histone
methyltransferase protein SETD2 interacts with p53 and selectively
regulates its downstream genes. Cell Signal. 20:1671–1678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yagi S, Oda-Sato E, Uehara I, et al:
5-Aza-2′-deoxycytidine restores proapoptotic function of p53 in
cancer cells resistant to p53-induced apoptosis. Cancer Invest.
26:680–688. 2008.
|
|
11.
|
Gao C, Nakajima T, Taya Y and Tsuchida N:
Activation of p53 in MDM2-overexpressing cells through
phosphorylation. Biochem Biophys Res Commun. 264:860–864. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tegeder I, Grosch S, Schmidtko A, et al: G
protein-independent G1 cell cycle block and apoptosis with morphine
in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer
Res. 63:1846–1852. 2003.PubMed/NCBI
|
|
13.
|
Sawaya M, Yoshimura T, Shimoyama T,
Munakata A and Fukuda S: Difference of p53AIP1 mRNA expression in
gastric mucosa between patients with gastric cancer and chronic
gastritis infected with Helicobacter pylori. J Clin
Gastroenterol. 42:351–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yamashita SI, Masuda Y, Yoshida N, et al:
p53AIP1 expression can be a prognostic marker in non-small cell
lung cancer. Clin Oncol (R Coll Radiol). 20:148–151. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Li XY, Liu JH, Li ZJ, et al: Construction
of a recombinant adenoviral vector carrying integrin-alpha and the
influence of integrin-alpha on the adhesion characteristics of
SMMC-7721 cells. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 18:413–416.
2006.PubMed/NCBI
|
|
16.
|
Zhao M, Xiao SW, Yang JX, Zhang SW and Lu
YY: Detection of p53 gene change and serum antibody level in phase
II clinical trial of ad p53 gene therapy. Zhonghua Yi Xue Za Zhi.
85:3495–3498. 2005.PubMed/NCBI
|
|
17.
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kussie PH, Gorina S, Marechal V, et al:
Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor
transactivation domain. Science. 274:948–953. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bouska A, Lushnikova T, Plaza S and
Eischen CM: Mdm2 promotes genetic instability and transformation
independent of p53. Mol Cell Biol. 28:4862–4874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Clegg HV, Itahana K and Zhang Y: Unlocking
the Mdm2-p53 loop: ubiquitin is the key. Cell Cycle. 7:287–292.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhang Z and Zhang R: Proteasome activator
PA28 gamma regulates p53 by enhancing its MDM2-mediated
degradation. EMBO J. 27:852–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Weber JD, Taylor LJ, Roussel MF, Sherr CJ
and Bar-Sagi D: Nucleolar Arf sequesters Mdm2 and activates p53.
Nat Cell Biol. 1:20–26. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Honda R and Yasuda H: Association of
p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for
tumor suppressor p53. EMBO J. 18:22–27. 1999. View Article : Google Scholar : PubMed/NCBI
|