Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of cancers worldwide, particularly in Southeast Asia
(1). Japan has one of the highest
incidence rates of HCC among developed countries (2,3).
Worldwide and also in Japan, HCC is mainly caused by chronic
hepatitis B virus (HBV) and hepatitis C virus (HCV) infection
(4,5). Nearly 90% of patients with HCC in
Japan are chronically infected with HBV or HCV. Meanwhile, other
patients with HCC in Japan have no confirmed chronic viral
hepatitis, and the percentage of these patients is reportedly much
higher in Western countries (6–8).
Lately, the incidence of HCC without an underlying hepatitis virus
infection is increasing (9,10).
HCC cases without chronic viral hepatitis include patients who
suffer from other chronic liver diseases predisposing to HCC, such
as alcoholic liver disease (11),
hemochromatosis (12), Budd-Chiari
syndrome (13) and non-alcoholic
steatohepatitis (NASH) (14). In
addition, there is a subpopulation of patients with HCC that
develops from normal liver or liver tissue damaged from an unknown
cause at a constant rate.
Many recent reports suggest a close correlation of
oxidative stress and the carcinogenicity of HCC. Reactive oxygen
species are known to damage DNA and membranes, induce nucleotide
mutation and apoptosis, and are thought to induce carcinoma
(15). Oxidative stress is likely
to be a key component in the induction of HCC.
8-Hydroxydeoxyguanosine (8-OHdG) is a DNA
base-modified product generated by reactive oxygen species and is a
mutation prone to induce a G-C to T-A transversion during DNA
replication (16). Previous
studies have demonstrated that 8-OHdG is implicated in
carcinogenesis (17,18) and hepatocarcinogenesis (19). 8-OHdG is induced during liver DNA
damage (19) and expressed in
livers with chronic hepatitis C (CH-C) (20) and chronic hepatitis B (CH-B)
(21). 8-OHdG, a good marker of
oxidative DNA damage, is thought to be involved in
hepatocarcinogenesis in the setting of chronic viral hepatitis.
Free radical-mediated damage to cellular membranes
results in lipid peroxidation and generates a variety of
DNA-reactive aldehydes, including 4-hydroxynonenal (4-HNE). 4-HNE
interacts with proteins or DNA, yielding etheno (ɛ)-modified DNA
bases (22), and was reported to
form DNA adducts in the human p53 gene at a unique mutational hot
spot in HCC (23).
Superoxide dismutase (SOD) is a metalloenzyme and
catalyzes the dismutation of superoxide anions into molecular
oxygen and hydrogen peroxide. Thus, SOD plays an important role in
defending cells against oxygen radical toxicity. Hepatocytes
contain abundant mitochondria, and reactive oxygen species in
hepatocytes are mainly generated in the mitochondria. Manganese SOD
(MnSOD) is induced in the mitochondria by oxidative stress as well
as by various stimuli, such as viral infections (24,25).
Therefore, MnSOD is thought to be a reliable marker of oxidative
stress in hepatocytes.
In the present study, the expression levels of
8-OHdG, 4-HNE and MnSOD were measured in non-tumor liver tissue of
HCC patients without diagnosed disease predisposing to HCC, and
these indices were compared to those of HCC patients with CH-B or
CH-C. From these results, we propose the existence of different
mechanisms of carcinogenesis through oxidative stress between
normal livers or livers damaged by unknown etiologies and livers
with chronic viral hepatitis.
Materials and methods
Patients
Resected HCC and non-tumor liver tissues adjacent to
HCC were obtained from 24 patients negative for anti-HCV antibodies
(HCV-Ab) and hepatitis B surface antigen (HBsAg) in serum (Group
N). Patients diagnosed with chronic liver diseases, such as
alcoholic liver disease and NASH, were excluded from Group N. In
Group N, 11 patients were positive for the antibody to hepatitis B
core antigen (HBcAb) in serum, 11 were negative and 2 were unknown.
Tissue samples were also obtained from 21 patients positive for
HCV-Ab, negative for HBsAg and HBcAb (Group C), and 24 patients
negative for HCV-Ab and positive for HBsAg (Group B). Non-tumor
liver tissues were also obtained from 3 patients with metastatic
hepatic tumors (colon, gastric and maxillary sinus cancer) and
negative for HCV-Ab and HBsAg in serum. All patients, including 3
controls, underwent partial hepatectomy at the Department of
Surgery, Kurume University School of Medicine, from January 1995 to
September 2004. All patients fulfilled the following conditions. i)
Primary tumors, except for 3 metastatic tumors; ii) initial therapy
for hepatic tumors; iii) patients who abused alcohol (daily intake
>60 g of ethanol for male, >40 g for female) were excluded;
iv) patients with co-existing liver disease diagnosed by clinical
and histological examination, such as alcoholic liver disease,
NASH, hemochromatosis, Wilson disease, autoimmune hepatitis,
primary biliary cirrhosis, Budd-Chiari syndrome and
Schistosomiasis japonica, were excluded. Surgically resected
liver tissues were fixed in formalin and embedded in paraffin, and
serial sections (4-μm thick) were prepared. Informed consent was
obtained from each patient, and the study was approved by the
Ethics Committee of Kurume University. The study was carried out
according to the ethical guidelines of the 1975 Declaration of
Helsinki.
Clinical data and histological
assessment
Baseline data included the following
characteristics: age, gender, diagnosis of diabetes mellitus (DM),
body mass index (BMI), serum levels of aspartate aminotransferase
(AST), alanine aspartate aminotransferase (ALT), total bilirubin,
albumin and α-fetoprotein, prothrombin time, platelet count and
indocyanine green retention (ICG R15). BMI was calculated as weight
in kilograms/height in meters squared. Hepatic function before the
resection was evaluated using the Child-Pugh classification
(26) and the liver damage grade
of the Liver Cancer Study Group of Japan (27). Histopathological features of tumors
at the time of surgery, including maximal tumor diameter, tumor
number and tumor differentiation (28), were also assessed. Tumor staging of
HCC was determined using the tumor node metastasis (TMN)
classification (29).
H&E staining was performed for the histological
diagnosis. Assessment of liver fibrosis and inflammation was made
according to the classification of Desmet et al (30). Hepatic steatosis was also graded
according to Brunt et al (31). In short, steatosis observed in up
to 33, 33–66 and >66% of the liver histology was determined as
grade 1, 2 and 3, respectively. Hepatic steatosis, if not observed,
was graded as grade 0. The histological quantification of hepatic
iron was carried out according to Deugnier et al (32) using liver samples stained with
Berlin blue instead of Perl's Prussian blue. The total iron score
(TIS, 0–60) calculated by this scoring system was shown to
correlate highly with the biochemical hepatic iron index and
hepatic iron concentration as measured by atomic absorption
spectrophotometry in patients with chronic liver disease.
Immunohistochemistry
Each paraffin section was first deparaffinized. For
immunohistochemical staining of 8-OHdG, sections were heated in 10
mM sodium citrate buffer (pH 6.0) at 121°C for 10 min in an
autoclave and then treated with 0.3% hydrogen peroxide in methanol
for 15 min. After the sections were washed three times with
phosphate-buffered saline (PBS), they were incubated with Protein
Block (Dako Japan Inc., Kyoto, Japan) for 1 h at room temperature
and sequentially reacted with mouse monoclonal antibody against
8-OHdG (1:50 dilution; Japan Institute for the Control of Aging,
Shizuoka, Japan) overnight at 4°C. After the sections were rinsed
in PBS three times, they were incubated with a biotinylated
secondary antibody conjugated with avidinbiotin-horseradish
peroxidase (Dako Japan Inc.) and reacted with 3,3-diaminobenzidine
(DAB), and subsequently the sections were counterstained with
Mayer's hematoxylin for 1 min. For immunohistochemical staining of
4-HNE, sections were heated in 10 mM sodium citrate buffer (pH 6.0)
at 100°C for 5 min in a microwave and reacted with mouse monoclonal
antibody against 4-HNE (1:160 dilution; Japan Institute for the
Control of Aging) overnight at 4°C. For immunohistochemical
staining of MnSOD, sections were reacted with rabbit polyclonal
antibody against MnSOD (1:1,600 dilution; Abcam Inc., Cambridge,
MA, USA) overnight at 4°C. For the detection of the staining of
4-HNE and MnSOD, the ChemMate Envision method (Dako Japan Inc.) was
used with DAB as the chromagen.
Hepatocytes that stained positively for 8-OHdG were
counted in at least five different random fields at a x400
magnification, and the number of positive cells per 1,000
hepatocytes was calculated. Quantitation of 4-HNE-protein adducts
and MnSOD was performed using image analysis software (WinROOF;
Mitani Corp., Fukui, Japan) by evaluating five different random
fields (magnification x400) for positively stained hepatocytes and
expressed as a percentage of the total area. Stained inflammatory,
Kupffer and bile duct cells were eliminated before quantitation for
4-HNE-protein adducts and MnSOD using image analysis software.
Statistics
Statistical analysis was performed using SPSS 12.0J
(SPSS Inc., Chicago, IL, USA). The Chi-square test or Fisher's
exact probability test was used to compare categorical data.
Differences between two groups were evaluated using the
Mann-Whitney U test. A relationship between different continuous
variables was investigated by linear regression analysis. A p-value
<0.05 was considered statistically significant.
Results
Clinical characteristics
Comparison of the clinical characteristics among the
three groups is summarized in Table
I. Patients in Group B were significantly younger than patients
in Group C (p=0.003) and Group N (p<0.001). In Group N, 8
patients were diagnosed with DM and 12 were not, and 1 patient was
unknown. The proportion of patients with DM in Group N was
significantly higher than that in Group B (p=0.001) or Group C
(p=0.020). Serum levels of ALT in Group N were significantly lower
than those in Group C (p=0.001). Total bilirubin in Group N was
significantly lower than that in Group B (p=0.041), and platelet
counts in Group N were significantly higher than those in Group C
(p=0.003). Tumor size was significantly smaller in Group C than in
Groups B or N (p=0.009 and 0.001, respectively). Gender, BMI, serum
levels of AST, albumin, α-fetoprotein, prothrombin time, ICG R15
and number of tumors in Group N were not different from those in
the other two groups. There were no significant differences in
Child-Pugh classification, liver damage grade or tumor staging
among the three groups.
 | Table I.Comparison of clinical
characteristics among the groups based on viral markers. |
Table I.
Comparison of clinical
characteristics among the groups based on viral markers.
| Group Na | Group Ba | Group Ca | p-valueb
|
|---|
| N-B | N-C | B-C |
|---|
| Age (years)c | 67.1±10.1 | 54.9±11.6 | 65.2±8.2 |
<0.001 | NS | 0.003 |
| Gender
(male/female) | 20/4 | 16/8 | 16/5 | NS | NS | NS |
| Body mass
indexc | 22.6±3.30 | 22.9±3.0 | 23.4±2.6 | NS | NS | NS |
| DM (−/+) | 12/8 | 21/0 | 18/1 | 0.001 | 0.020 | NS |
| AST (IU/l)c | 41.5±21.2 | 78.8±149.1 | 55.9±32.8 | NS | NS | NS |
| ALT (IU/l)c | 33.6±49.6 | 46.6±33.0 | 64.5±46.5 | NS | 0.001 | NS |
| Total bilirubin
(mg/dl)c | 0.85±0.41 | 1.15±0.55 | 0.88±0.29 | 0.041 | NS | NS |
| Albumin
(g/dl)c | 3.8±0.3 | 3.9±0.5 | 3.8±0.4 | NS | NS | NS |
| Prothrombin time
(%)c | 93.4±15.8 | 86.6±11.7 | 89.1±10.3 | NS | NS | NS |
| ICG(15) (%)c | 14.0±6.20 | 11.8±9.8 | 20.0±12.1 | NS | NS | 0.010 |
| Platelet
(x104/ml)c | 19.9±10.3 | 15.5±5.8 | 12.7±4.40 | NS | 0.003 | NS |
| α-fetoprotein
(ng/ml)d | 58.3
(1.5–12,850) | 36.2
(2.4–559,791) | 33.9
(3.0–42,568) | NS | NS | NS |
| Liver damage
(A/B,C) | 18/4 | 16/7 | 16/4 | NS | NS | NS |
| Child-Pugh (score
5/6) | 15/9 | 16/7 | 17/4 | NS | NS | NS |
| Tumor size
(mm)c,e | 69.8±49.6 | 61.0±43.0 | 34.0±19.9 | NS | 0.001 | 0.009 |
| No. of tumors
(single/multiple) | 13/11 | 18/6 | 13/8 | NS | NS | NS |
| Tumor stage
(I,II/III,IV) | 7/17 | 10/14 | 9/10 | NS | NS | NS |
Histological features
Table II shows the
histological features of the study population. There were 4
patients in Group N with grade 0 for histological hepatic
inflammatory activity, as well as stage 0 for hepatic fibrosis.
When the patients with grades of hepatic inflammation were divided
into A0–1 and A2–3, the grades of histological inflammatory
activity in Group C were significantly higher than those in Group B
(p=0.006) and Group N (p<0.001). When the stages of hepatic
fibrosis were divided into F0–2 and F3–4, the histological fibrotic
stages in Group N were significantly lower than those in Group B
(p=0.008) and Group C (p=0.029). There was no patient with hepatic
steatosis assessed as grade 3 in this study and, when divided into
grade 0 and grade 1–2, there were no significant differences
between groups. There was also no difference in the TIS score
reflecting hepatic iron deposition between each group.
 | Table II.Comparison of the histological
characteristics of background liver tissue among the groups based
on viral markers. |
Table II.
Comparison of the histological
characteristics of background liver tissue among the groups based
on viral markers.
| Group N | Group B | Group C | p-valuea
|
|---|
| N-B | N-C | B-C |
|---|
| Grading
A0/1/2/3 | 5/19/0/0 | 1/19/4/0 | 0/9/12/0 | NS |
<0.001 | 0.006 |
| Staging
F0/1/2/3/4 | 8/9/5/1/1 | 1/11/1/6/5 | 0/6/7/5/3 | 0.008 | 0.029 | NS |
| Fatty deposit grade
0/1/2/3 | 8/16/0/0 | 9/14/1/0 | 3/17/1/0 | NS | NS | NS |
| Iron deposit (total
iron score)b | 11.3±7.7 | 10.2±7.0 | 7.7±7.1 | NS | NS | NS |
Immunohistochemical staining
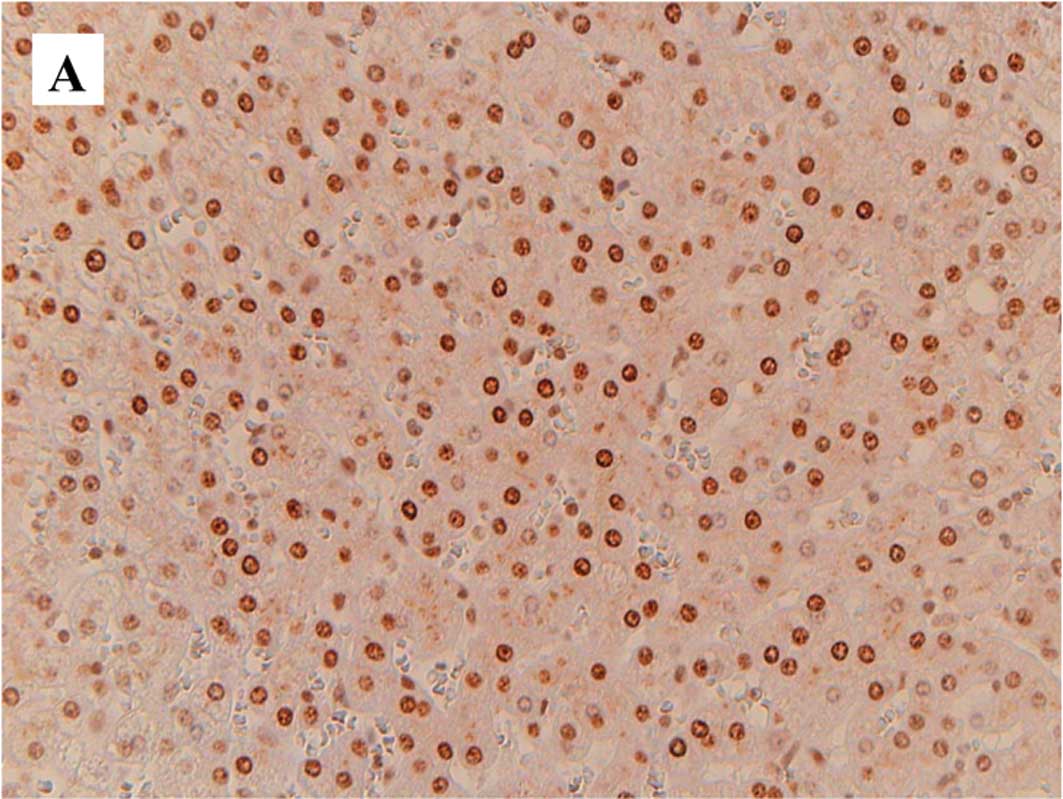
Fig. 1 is a
representative image of staining for 8-OHdG (A), 4-HNE (B) and
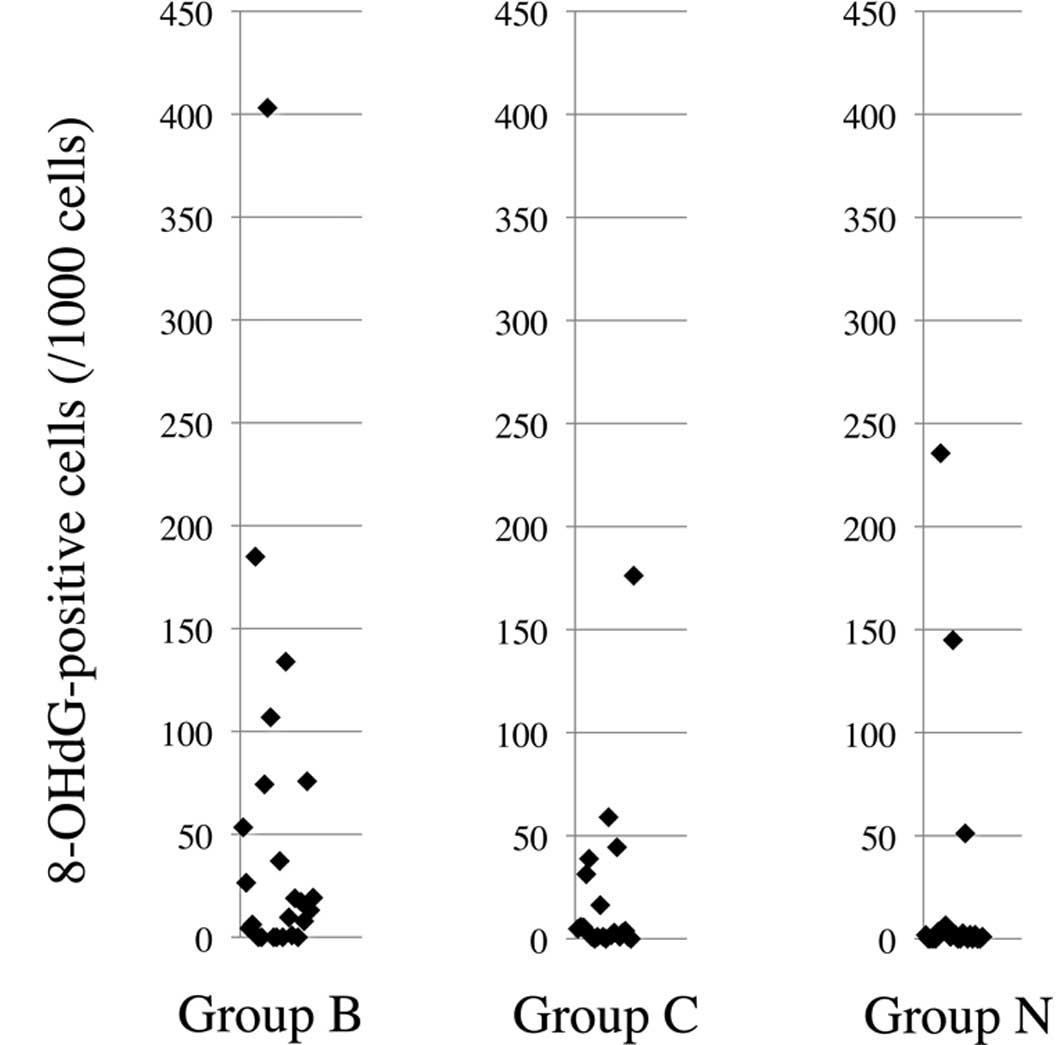
Mn-SOD (C) in the non-cancerous liver tissues. Table III and Fig. 2 show the results of the
immunohistochemical analysis for 8-OHdG, 4-HNE and MnSOD.
Immunohistochemical staining revealed that the number of
8-OHdG-positive hepatocytes per 1,000 cells in Group N (median 1.4,
range 0–235.6) was significantly lower than that in Group B (median
15.2, range 0–403.1) (p=0.014) and that in the combined Groups B
and C (median 5.75, range 0–403.1) (p=0.016). The number of
8-OHdG-positive cells in the three controls with metastatic hepatic
tumors (0, 0 and 26.1) was not significantly different from that in
the other three groups. Meanwhile, the ratio of 4-HNE-positive
cells to total area in Group N (median 1.96, range 0.06–26.46) was
significantly higher than that in Group B (median 0.62, range
0.07–4.68) (p<0.001) and that in Group C (median 0.36, range
0.01–8.50) (p<0.001). Additionally, the ratio of 4-HNE-positive
cells to total area in the three controls (0.6, 1.0 and 2.2) was
not significantly different from that in the other three groups.
The ratio of MnSOD-positive cells to total area in Group N (median
34.6; range 1.3–71.4) was not different from that in Group B
(median 25.8, range 3.8-69.0) or Group C (median 31.5, range
9.0–54.6). The ratio of MnSOD-positive cells to total area in the
three controls (26.2, 31.0 and 44.5) was not significantly
different from that in the other groups. There was no significant
difference in 8-OHdG, 4-HNE and MnSOD between the patients positive
and negative for HBcAb in Group N.
 | Table III.Comparison of the expression levels
for oxidative stress markers on liver tissue adjacent to
hepatocellular carcinoma among the groups based on viral
markers. |
Table III.
Comparison of the expression levels
for oxidative stress markers on liver tissue adjacent to
hepatocellular carcinoma among the groups based on viral
markers.
| Group N | Group B | Group C | p-valuea
|
|---|
| N-B | N-C | B-C |
|---|
| 8-OHdG | 1.40
(0–235.60) | 15.20
(0–403.10) | 2.99
(0–176.19) | 0.014 | 0.097 | 0.217 |
| 4-HNE | 1.96
(0.06–26.46) | 0.62
(0.07–4.68) | 0.36
(0.01–8.50) |
<0.001 |
<0.001 | 0.139 |
| MnSOD | 34.60
(1.3–71.40) | 25.80
(3.8–69.0) | 31.50
(5.7–100.0) | 0.433 | 0.733 | 0.453 |
For the total patients with HCC, including Groups B,
C and N, the number of 8-OHdG-positive cells, the ratio of
4-HNE-positive cells and the ratio of MnSOD-positive cells did not
correlate with each other. The number of 8-OHdG-positive cells and
the ratio of MnSOD-positive cells were not correlated with hepatic
inflammatory activity grade, fibrotic stage, grade of steatosis,
TIS score reflecting iron deposition, BMI or age. The percentage of
4-HNE-positive cells showed a weak negative correlation with
inflammatory activity grade (p=0.006, r=−0.329), fibrotic stage
(p=0.010, r=−0.308) and grade of fatty deposition (p=0.025,
r=−0.270).
Discussion
Previous reports have noted the high expression of
8-OHdG in the livers of chronic viral hepatitis patients (20,21),
and our results are consistent with these reports. Recent studies
have shown that high expression of 8-OHdG in livers with CH-C
predicted the development of primary HCC (33,34)
and also postoperative recurrence (35,36),
suggesting the association of 8-OHdG and carcinogenesis in livers
with CH-C. However, the expression levels of 8-OHdG in the livers
of Group N were low, suggesting that abundant expression of 8-OHdG
is not essential for developing HCC. Recent studies have
demonstrated that 8-OHdG is correlated with hepatic inflammation
and fibrosis in non-cancerous liver tissue of patients with HCV and
HCC (33–35). The lower expression of 8-OHdG in
Group N may reflect a lower grade of inflammation or a lower stage
of fibrosis in the livers of these patients compared to the livers
of patients with CH-B and CH-C.
The expression level of 4-HNE in livers of patients
with hepatitis is controversial. 4-HNE was reported to be a good
biomarker for predicting disease-free survival in patients with HCC
and CH-C; however, a weaker association with disease outcome when
compared to 8-OHdG was noted (36). Maki et al reported high
expression of 4-HNE in livers with CH-C and HCC compared to
controls (36), while in another
study, 4-HNE protein adducts were detected in only a few
hepatocytes in livers with CH-C (37). The expression level of 4-HNE
protein adducts in livers with CH-B was found to be lower than that
in livers with CH-C (38). In this
study, the expression levels of 4-HNE in the livers of Group N
subjects were higher than those in Groups B and C. Although the
number of controls was quite small, the average of the expression
levels of 4-HNE in the controls (0.61, 1.03 and 2.20) was 1.28, and
the expression levels of 4-HNE of 19 cases out of 24 in Group N
were higher than the average level in the controls. These results
suggest the possibility that 4-HNE plays a crucial role in
hepatocarcinogenesis in normal livers or cryptogenic liver damage.
Meanwhile, Coleman et al showed that 4-HNE is an agonist of
nuclear receptor peroxisome proliferator-activated receptors
(PPARs) and suggested that 4-HNE decreases oxidative
stress-associated liver damage through the activation of PPARs
(39). Lower expression of 4-HNE
in Groups B and C compared to Group N may suggest the suppression
of PPARs, and subsequently less attenuation of oxidative damage in
the livers of chronic viral hepatitis subjects. If so, oxidative
stress may be involved to a lesser extent in hepatocarcinogenesis
in normal livers or livers damaged by unknown etiologies. A weak
negative correlation of the expression levels of 4-HNE with the
grade of hepatic inflammatory activity, fibrotic stage and grade of
fatty deposition in our study might reflect the role of 4-HNE
against liver damage.
Several studies have shown that chronic hepatitis
virus infection may increase MnSOD expression and activity as an
adaptive response to increased superoxide ions (25,40),
and the elevation of serum levels and histological expression for
MnSOD was shown in patients with HCC (41–43).
Meanwhile, the association of gene polymorphisms in MnSOD with high
MnSOD activity and an increased risk for HCC has been reported
(44,45). These findings suggest that MnSOD is
not only an enzyme induced as an adaptive response to oxidative
stress, but also a key factor in the acceleration of tumor growth
through anti-apoptotic factors (43). In this study, the expression levels
of MnSOD in all three groups and controls were not significantly
different, which means that a specific role of MnSOD in
carcinogenesis or protection from oxidative stress in normal livers
or cryptogenic liver damage compared to livers with chronic viral
hepatitis was not found.
Varying expression levels of oxidative markers have
been reported in liver tissue in conditions known to cause HCC,
other than CH-B and CH-C. Several studies have found frequently
increased expression of 8-OHdG and strong staining of 4-HNE adducts
in livers with hemochromatosis and alcoholic liver disease
(37). In NASH, the expression of
8-OHdG in the liver has been frequently observed, and 4-HNE adducts
were found to be localized in hepatocytes with a predominance in
zone 3 (46). Criteria for
inclusion in Group N excluded cases with these liver diseases
associated with the development of HCC. For further analysis, it is
necessary to compare the oxidative stress marker expression levels
in livers of Group N patients and patients with HCC with such
diagnosed chronic liver diseases.
The clinical data shown in Table I, as well as lower grades of
hepatic inflammatory activity and lower fibrotic stages in the
livers of Group N shown in Table
II, are similar to results reported previously, as features of
HCC without chronic viral infection (47–50).
Significantly more patients were diagnosed with type 2 DM in Group
N than in Groups B or C. Davila et al showed that the
incidence of HCC was increased 2- to 3-fold in patients with DM,
regardless of the presence of other major HCC risk factors,
including HCV and HBV infection (10). DM is known to be associated with
NASH, and the development of HCC from DM-related NASH has been
reported (14). Cases diagnosed as
NASH by histological examination were excluded from this study;
however, one case with cirrhotic liver tissue might possibly have
been ‘burnt-out’ NASH. Metabolic disorders, including insulin
resistance, may be involved in hepatocarcinogenesis, even in livers
without NASH.
In conclusion, the expression patterns of oxidative
stress markers in normal livers or livers damaged by unknown
etiologies were considerably different from those in livers with
chronic viral hepatitis at the time of resection for HCC. 8-OHdG is
reported to be important in the development of HCC in patients with
CH-C and CH-B, but may not be critical in patients without known
liver disease predisposing to HCC. 4-HNE may possibly be associated
with carcinogenesis in the latter, although these cases may have
various unknown risk factors for HCC. To assess
hepatocarcinogenesis resulting from oxidative stress in detail,
further study with larger sample sizes is needed to determine the
specific associations of markers of oxidative stress and HCC in the
setting of different states of underlying hepatic disease. The
number of HCC cases with cryptogenic hepatitis and in normal livers
are expected to increase in the future. Thus, clarification of the
mechanism involved in the occurrence of HCC in normal livers or
livers damaged by unknown etiologies is critical for establishing
treatments, including vitamin C and E, which inhibit lipid
peroxidation, and for the prevention of HCC in patients with
DM.
Acknowledgements
This study was supported in part by
Health and Labour Sciences Research Grants for Research on
Hepatitis from the Ministry of Health, Labour and Welfare of
Japan.
References
|
1.
|
Wong F and Choi T: Primary liver cancer.
Asian experience. Surgery of Liver and Biliary Tract. Blumgart LH:
Churchill-Livingstone; London: pp. 1135–1151. 1988
|
|
2.
|
The Research Group for Population-based
Cancer Resistration in Japan: Cancer incidence and incidence rates
in Japan in 1995: estimates based on data from nine
population-based cancer registries. Jpn J Clin Oncol. 30:318–321.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Tanaka H and Tsukuma H: Hepatitis C virus.
Cancer Surveys: Infections and Human Cancer. Newton R, Beral V,
Weiss RA and Tooze J: Cold Spring Harbor Laboratory; New York: pp.
213–235. 1999
|
|
4.
|
Di Bisceglie AM, Goodman ZD, Ishak KG,
Hoofnagle JH, Melpolder JJ and Alter HJ: Long-term clinical and
histopathological follow-up of chronic posttransfusion hepatitis.
Hepatology. 14:969–974. 1991.PubMed/NCBI
|
|
5.
|
Kiyosawa K, Sodeyama T, Tanaka E, et al:
Interrelationship of blood transfusion, non-A, non-B hepatitis and
hepatocellular carcinoma: analysis by detection of antibody to
hepatitis C virus. Hepatology. 12:671–675. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Marrero JA, Fontana RJ, Su GL, Conjeevaram
HS, Emick DM and Lok AS: NAFLD may be a common underlying liver
disease in patients with hepatocellular carcinoma in the United
States. Hepatology. 36:1349–1354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Davila JA, Morgan RO, Shaib Y, McGlynn KA
and El-Serag HB: Hepatitis C infection and the increasing incidence
of hepatocellular carcinoma: a population-based study.
Gastroenterology. 127:1372–1380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Seeff LB and Hoofnagle JH: Epidemiology of
hepatocellular carcinoma in areas of low hepatitis B and hepatitis
C endemicity. Oncogene. 25:3771–3777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hatanaka K, Kudo M, Fukunaga T, et al:
Clinical characteristics of NonBNonC-HCC: Comparison with HBV and
HCV related HCC. Intervirology. 50:24–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Davila JA, Morgan RO, Shaib Y, McGlynn KA
and El-Serag HB: Diabetes increases the risk of hepatocellular
carcinoma in the United States: a population based case control
study. Gut. 54:533–539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gerber M, Thung S and Popper H: Pathology
of alcoholic liver injury. Update 1981 and problems. New Trends in
Hepatology. Oda T and Okuda K: Medical Tokyo Co Ltd; Tokyo: pp.
8–12. 1986
|
|
12.
|
Haddow JE, Palomaki GE, McClain M and
Craig W: Hereditary haemochromatosis and hepatocellular carcinoma
in males: a strategy for estimating the potential for primary
prevention. J Med Screen. 10:11–13. 2003. View Article : Google Scholar
|
|
13.
|
Moucari R, Rautou PE, Cazals-Hatem D, et
al: Hepatocellular carcinoma in Budd-Chiari syndrome:
characteristics and risk factors. Gut. 57:828–835. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hashimoto E, Yatsuji S, Kaneda H, et al:
The characteristics and natural history of Japanese patients with
nonalcoholic fatty liver disease. Hepatol Res. 33:72–76. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sasaki Y: Does oxidative stress
participate in the development of hepatocellular carcinoma? J
Gastroenterol. 41:1135–1148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shibutani S, Takeshita M and Grollman AP:
Insertion of specific bases during DNA synthesis past the
oxidation-damaged base 8-oxodG. Nature. 349:431–434. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hussain SP and Harris CC: Molecular
epidemiology of human cancer: contribution of mutation spectra
studies of tumor suppressor genes. Cancer Res. 58:4023–4037.
1998.
|
|
18.
|
Zhang H, Xu Y, Kamendulis LM and Klaunig
JE: Morphological transformation by 8-hydroxy-2′-deoxyguanosine in
Syrian hamster embryo (SHE) cells. Toxicol Sci. 56:303–312.
2000.
|
|
19.
|
Nakae D, Kobayashi Y, Akai H, et al:
Involvement of 8-hydroxyguanine formation in the initiation of rat
liver carcinogenesis by low dose levels of N-nitrosodiethylamine.
Cancer Res. 57:1281–1287. 1997.PubMed/NCBI
|
|
20.
|
Kitada T, Seki S, Iwai S, Yamada T,
Sakaguchi H and Wakasa K: In situ detection of oxidative DNA
damage, 8-hydroxydeoxyguanosine, in chronic human liver disease. J
Hepatol. 35:613–618. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ichiba M, Maeta Y, Mukoyama T, et al:
Expression of 8-hydroxy-2′-deoxyguanosine in chronic liver disease
and hepatocellular carcinoma. Liver Int. 23:338–345. 2003.
|
|
22.
|
Bartsch H and Nair J: Oxidative stress and
lipid peroxidation-derived DNA-lesions in inflammation driven
carcinogenesis. Cancer Detect Prev. 28:385–391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hu W, Feng Z, Eveleigh J, et al: The major
lipid peroxidation product, trans-4-hydroxy-2-nonenal,
preferentially forms DNA adducts at codon 249 of human p53 gene, a
unique mutational hotspot in hepatocellular carcinoma.
Carcinogenesis. 23:1781–1789. 2002. View Article : Google Scholar
|
|
24.
|
Liu R, Buettner GR and Oberley LW: Oxygen
free radicals mediate the induction of manganese superoxide
dismutase gene expression by TNF-alpha. Free Radic Biol Med.
28:1197–1205. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Qadri I, Iwahashi M, Capasso JM, et al:
Induced oxidative stress and activated expression of manganese
superoxide dismutase during hepatitis C virus replication: role of
JNK, p38 MAPK and AP-1. Biochem J. 378:919–928. 2004. View Article : Google Scholar
|
|
26.
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Nanashima A, Sumida Y, Morino S, et al:
The Japanese integrated staging score using liver damage grade for
hepatocellular carcinoma in patients after hepatectomy. Eur J Surg
Oncol. 30:765–770. 2004. View Article : Google Scholar
|
|
28.
|
Liver Cancer Study Group of Japan: General
Rules for the Clinical and Pathological Study of Primary Liver
Cancer. 2nd English edition. Kanehara; Tokyo: 2003
|
|
29.
|
Sobin L and Witteking C: International
Union Against Cancer Union TMN Classification of malignant tumors.
5th edition. John Wiley & Sons, Inc; New York: 1997
|
|
30.
|
Desmet VJ, Gerber M, Hoofnagle JH, Manns M
and Scheuer PJ: Classification of chronic hepatitis: diagnosis,
grading and staging. Hepatology. 19:1513–1520. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Brunt EM, Janney CG, Di Bisceglie AM,
Neuschwander-Tetri BA and Bacon BR: Nonalcoholic steatohepatitis: a
proposal for grading and staging the histological lesions. Am J
Gastroenterol. 94:2467–2474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Deugnier YM, Loreal O, Turlin B, et al:
Liver pathology in genetic hemochromatosis: a review of 135
homozygous cases and their bioclinical correlations.
Gastroenterology. 102:2050–2059. 1992.PubMed/NCBI
|
|
33.
|
Chuma M, Hige S, Nakanishi M, et al:
8-Hydroxy-2′-deoxyguanosine is a risk factor for development of
hepatocellular carcinoma in patients with chronic hepatitis C virus
infection. J Gastroenterol Hepatol. 23:1431–1436. 2008.
|
|
34.
|
Tanaka H, Fujita N, Sugimoto R, et al:
Hepatic oxidative DNA damage is associated with increased risk for
hepatocellular carcinoma in chronic hepatitis C. Br J Cancer.
98:580–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Matsumoto K, Satoh Y, Sugo H, et al:
Immunohistochemical study of the relationship between
8-hydroxy-2′-deoxyguanosine levels in noncancerous region and
postoperative recurrence of hepatocellular carcinoma in remnant
liver. Hepatol Res. 25:435–441. 2003.
|
|
36.
|
Maki A, Kono H, Gupta M, et al: Predictive
power of biomarkers of oxidative stress and inflammation in
patients with hepatitis C virus-associated hepatocellular
carcinoma. Ann Surg Oncol. 14:1182–1190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Paradis V, Kollinger M, Fabre M, Holstege
A, Poynard T and Bedossa P: In situ detection of lipid peroxidation
by-products in chronic liver diseases. Hepatology. 26:135–142.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Kageyama F, Kobayashi Y, Kawasaki T,
Toyokuni S, Uchida K and Nakamura H: Successful interferon therapy
reverses enhanced hepatic iron accumulation and lipid peroxidation
in chronic hepatitis C. Am J Gastroenterol. 95:1041–1050. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Coleman JD, Prabhu KS, Thompson JT, et al:
The oxidative stress mediator 4-hydroxynonenal is an intracellular
agonist of the nuclear receptor peroxisome proliferator-activated
receptor-beta/delta (PPARbeta/delta). Free Radic Biol Med.
42:1155–1164. 2007. View Article : Google Scholar
|
|
40.
|
Boya P, de la Pena A, Beloqui O, et al:
Antioxidant status and glutathione metabolism in peripheral blood
mononuclear cells from patients with chronic hepatitis C. J
Hepatol. 31:808–814. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Kawaguchi T, Suzuki K, Matsuda Y, et al:
Serum-manganesesuperoxide dismutase: normal values and increased
levels in patients with acute myocardial infarction and several
malignant diseases determined by an enzyme-linked immunosorbent
assay using a monoclonal antibody. J Immunol Methods. 127:249–254.
1990. View Article : Google Scholar
|
|
42.
|
Aida Y, Maeyama S, Takakuwa T, et al:
Immunohistochemical expression of manganese superoxide dismutase in
hepatocellular carcinoma, using a specific monoclonal antibody. J
Gastroenterol. 29:443–449. 1994. View Article : Google Scholar
|
|
43.
|
Clemente C, Elba S, Buongiorno G, et al:
Manganese superoxide dismutase activity and incidence of
hepatocellular carcinoma in patients with Child-Pugh class A liver
cirrhosis: a 7-year follow-up study. Liver Int. 27:791–797.
2007.
|
|
44.
|
Sutton A, Nahon P, Pessayre D, et al:
Genetic polymorphisms in antioxidant enzymes modulate hepatic iron
accumulation and hepatocellular carcinoma development in patients
with alcohol-induced cirrhosis. Cancer Res. 66:2844–2852. 2006.
View Article : Google Scholar
|
|
45.
|
Ezzikouri S, El Feydi AE, Chafik A, et al:
Genetic polymorphism in the manganese superoxide dismutase gene is
associated with an increased risk for hepatocellular carcinoma in
HCV-infected Moroccan patients. Mutat Res. 649:1–6. 2008.
View Article : Google Scholar
|
|
46.
|
Seki S, Kitada T, Yamada T, Sakaguchi H,
Nakatani K and Wakasa K: In situ detection of lipid peroxidation
and oxidative DNA damage in non-alcoholic fatty liver diseases. J
Hepatol. 37:56–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Toyoda H, Kumada T, Kiriyama S, et al:
Characteristics and prognosis of patients in Japan with viral
marker-negative hepatocellular carcinoma. J Gastroenterol Hepatol.
23:459–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Higashi Y, Tada S, Miyase S, et al:
Correlation of clinical characteristics with detection of hepatitis
B virus X gene in liver tissue in HBsAg-negative, and HCV-negative
hepatocellular carcinoma patients. Liver. 22:374–379. 2002.
View Article : Google Scholar
|
|
49.
|
Yokoi Y, Suzuki S, Baba S, Inaba K, Konno
H and Nakamura S: Clinicopathological features of hepatocellular
carcinomas (HCCs) arising in patients without chronic viral
infection or alcohol abuse: a retrospective study of patients
undergoing hepatic resection. J Gastroenterol. 40:274–282. 2005.
View Article : Google Scholar
|
|
50.
|
Watabe H, Shiratori Y, Tateishi R, et al:
Clinical features of patients with HCC who are negative for both
HBV and HCV markers. Hepatogastroenterology. 50:2157–2160.
2003.PubMed/NCBI
|