Introduction
Carcinomas are one of the most detrimental human
diseases, and malignant tumors reduce the quality of life and are
the leading cause of mortality in China (1). Liver cancer is one of the most common
types of cancer, with the highest mortality rate among malignant
tumors (2,3). Liver cancer is considered a digestive
system tumor with a high malignant potential and poor prognosis,
and the majority of patients succumb within a few weeks or months
following diagnosis (4). The
prevention, control, diagnosis and treatment of liver cancer have
become important subjects within healthcare research, and replacing
or improving conventional treatment methods for liver cancer is
vital. Gene therapy, as an efficient, specific and highly targeted
treatment, has received increasing attention (5,6).
Taurine (Tau) is a sulfur-containing acid with an
amino group, that has the ability to scavenge oxygen-free radicals,
regulate intracellular calcium homeostasis, maintain cell membrane
stability and protect cells (7). The
molecule has been found to be particularly effective in the
prevention of cardiovascular and cerebrovascular diseases (8). A previous study also demonstrated
antitumor properties of Tau (9).
Furthermore, the addition of a certain amount of Tau to drinking
water has been shown to extend the mean lifespan of mice with
transplanted tumors, with a tumor growth inhibition rate of 42.26%
(10). Additionally, serum Tau
levels in patients with breast cancer have been found to be
significantly lower compared with those in high-risk and control
groups. Thus, Tau may become a novel indicator for the early
diagnosis of breast and bladder cancers (11–13). The
study of tumor prevention and treatment with Tau is nascent.
However, research into the effect of Tau on tumors remains limited,
and the mechanism underlying the antitumor ability of Tau is yet to
be elucidated. Biochemical analysis has revealed that expression of
the proapoptotic protein, B-cell lymphoma-2-associated X (Bax), is
enhanced following treatment with various doses of Tau, whereas
expression of the antiapoptotic protein, B-cell lymphoma-2 (Bcl-2),
is inhibited in S180 transplanted tumor nude mice. Furthermore,
apoptotic cells can be observed morphologically following Tau
treatment (14). Tau can also
function as an antitumor agent by downregulating the expression of
matrix metalloproteinase-2, upregulating
N-acetylgalactosaminyltransferase, and inhibiting the potential
invasion and metastasis induced by ionizing radiation (13). In a previous study, Tau was shown to
induce the apoptosis of human colon cancer cells by upregulating
the expression of the p53 upregulated modulator of apoptosis (PUMA)
gene, independent of p53 (15).
However, the ability of Tau to prevent liver cancer has yet to be
documented.
In the present study, human hepatocellular carcinoma
(HHCC) HepG2 cells were used as target cells, in which the effect
and molecular mechanism of Tau on cell proliferation and apoptosis
was observed. The aim of the present study was to provide novel
targets and targeting drugs for the prevention of liver cancer.
Materials and methods
Cells and drug handling
HHCC HepG2 cells were obtained from the Cell Bank of
the Type Culture Collection of the Chinese Academy of Sciences
Committee (Shanghai, China). The cells were cultured in
vitro in RPMI 1640 culture medium containing 10% fetal bovine
serum (FBS; Gibco Life Technologies, New York, NY, USA) at 37°C and
5% CO2. Cells in the logarithmic phase were divided
randomly into a control group, Tau treatment groups (with
concentrations of 20, 40, 80 and 160 mM Tau) and a cisplatin (DDP)
treatment group (10 µg/ml DDP). The effect of Tau (Sigma-Aldrich,
St. Louis, MO, USA) on HepG2 cell proliferation was observed after
24, 48 and 72 h.
Survival rate detection
Logarithmic-phase cells were collected and dyed with
Trypan blue (Shanghai Biological Technology Co., Ltd., Shanghai,
China) to monitor and adjust the concentration of the cell
suspension. When cells are dyed with Trypan blue, living cells
appear transparent, whereas dead cells are dyed blue. A 200-µl cell
suspension was added to each well of a 96-well plate, and the cell
density was adjusted to 3,000–8,000 cells/well. Each group was
assessed in quadruplicate. The plates were incubated at 37°C and 5%
CO2 for 24 h. Following addition of the drugs, the
plates were incubated for 24, 48 and 72 h. At the end of each
incubation period, 20 µl CellTiter 96® AQueous One Solution Reagent
(Promega Corporation, Madison, WI, USA) was added to each well, and
the plates were incubated for an additional 4 h. A microplate
reader (xMark™; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to detect and record the optical density (OD) of each well
using a wavelength of 490 nm, and the inhibition rate was
calculated as follows: Inhibition rate (%) = (1 - OD of the
experimental group/OD of the control group) × 100.
Apoptosis evaluation
Cells were seeded in a 50-ml culture flask for 24 h,
and following treatment with the variable concentrations of the
drugs for 48 h, non-EDTA pancreatin (Sigma-Aldrich) was used to
digest and collect the cells. The cells were washed twice with cold
phosphate-buffered saline (PBS) and centrifuged at 1,000 × g for 5
min, after which 1–5×105 cells were collected. The cells
were resuspended in 400 µl 1X annexin-V binding buffer, and 5 µl
annexin V-fluorescein isothiocyanate (FITC) staining solution
(BestBio, Shanghai, China) was added and gently mixed. The cells
were incubated in the dark on ice for 15 min. Next, 10 µl propidium
iodide (PI) staining solution (BestBio) was added and mixed evenly
with the cells. Finally, the cells were incubated in the dark on
ice for 5 min, and within 1 h, the rate of cell apoptosis was
detected by flow cytometry (FCM) using a BD FACSCalibur (Becton
Dickinson Biosciences, Franklin Lakes, NJ, USA).
Western blotting
The cells were washed with PBS and lysed with
radioimmunoprecipitation assay cell lysis reagent, containing
proteinase and phosphatase inhibitors (Solarbio Science &
Technology Co., Ltd., Beijing, China) at 4°C for 30 min. The total
protein was collected for sodium dodecyl sulfate-polyacrylamide gel
electrophoresis. The protein concentrations were determined used
the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL,
USA) according to the manufacturer's instructions. The total
protein content of each well was 30 µg. A wet transfer method was
used to transfer the protein to the polyvinylidene fluoride
membrane (EMD Millipore Corporation, Billerica, MA, USA). The
membrane was incubated with polyclonal antibodies against PUMA
(ab9643; Abcam, Cambridge, UK), Bax (sc-526; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and Bcl-2 (sc-783; Santa Cruz
Biotechnology), and a monoclonal antibody against β-actin (TA-09;
Santa Cruz Biotechnology, Inc.). Following incubation overnight at
4°C, the membrane was complexed with a horseradish
peroxidase-conjugated secondary antibody (ZSGB-BIO, Beijing, China)
for 1.5 h. An enhanced chemiluminescence immunological method was
performed using a kit (Pierce) for detection in a dark room.
Image-Pro® Plus 6.0 gel image analysis software (Media Cybernetics,
Inc., Rockville, MD, USA) was used to scan the gray values of the
protein bands, and the β-actin internal reference bands were used
as a control to perform the semi-quantitative analysis.
Effect of exogenous PUMA transfection
on apoptosis
HepG2 cells were collected in the logarithmic phase
and randomly divided into six groups. In the control group, the
culture medium was removed and replaced with serum-free medium, and
the cells were cultivated for 6 h, after which the complete medium
was restored for a 48-h cultivation. In the negative control group
(HA group), the cells were transfected with the empty vector,
pCEP4-(HA)2-C1. In the positive control group (DDP
group), the cells were treated with 10 µg/ml DDP. The experimental
group was subdivided into the pCEP4-(HA)2-PUMA group
(plasmid was provided by Professor Yu Jian, University of
Pittsburgh Cancer Institute, Pittsburgh, PA, USA), the Tau 160 mM
group (treated with 160 mM Tau for 48 h), the
pCEP4-(HA)2-PUMA+Tau 160 mM group (transiently
transfected with recombinant vector pCEP4-(HA)2-PUMA for
6 h, after which the solution was changed and 160 mM Tau was
added.
The pCEP4-(HA)2-PUMA recombinant plasmid
and pCEP4-(HA)2-C1 empty vector plasmid were transfected
into the HHCC HepG2 cells using a Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA) in vitro DNA-mediated
transfection reagent.
Effect of PUMA-specific small
interfering (si)RNA on apoptosis
Based on human PUMA cDNA sequences, the siRNA
fragments, 5′-GGGUCCUGUACAAUCUCAUTT-3′ and
5′-AUGAGAUUGUACAGGACCCTT-3′, were designed and synthesized by
Shanghai GenePharma Technology Co., Ltd. (Shanghai, China).
Subsequent to the application of Lipofectamine 2000 for the
transfection of the PUMA siRNA (si-PUMA) into the HHCC HepG2 cells,
the effect of cell apoptosis and protein expression was observed.
The following four study groups were used in this experiment. In
the control group, the initial medium was discarded and replaced
with a serum-free medium, which was used for cell cultivation for 6
h. Subsequently, the cells were cultivated in complete medium for
an additional 48 h. In the negative control (NC) group, the cells
were transfected with NC siRNA, and then cultivated in the same
manner as the control group. In the si-PUMA group, the cells were
transfected with the si-PUMA fragment, after which the cells
underwent cultivation in the same manner as the control group. In
the si-PUMA + Tau 160 mM group, following transfection with the
si-PUMA fragment for 6 h, the cells were cultured for 48 h with
complete medium containing 160 mM Tau. In the Tau 160 mM group, the
cells were treated with complete medium containing 160 mM for 48
h.
Statistical analysis
Experimental results are presented as the mean ±
standard deviation, and statistical analysis was performed using
single factor analysis of variance with SPSS statistical software
(version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
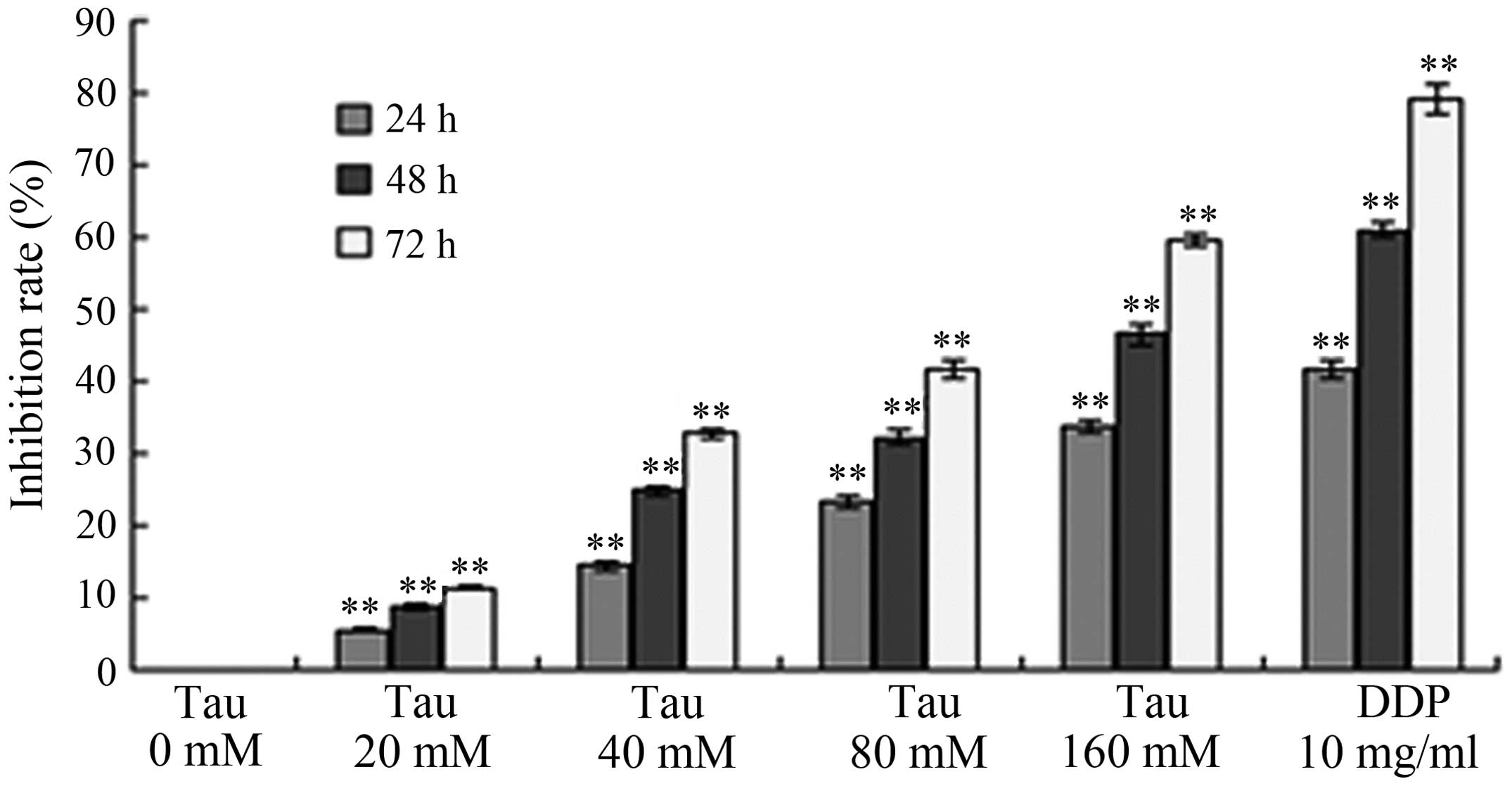
Inhibitory effect of Tau on cell
proliferation
HHCC HepG2 cells were treated with variable
concentrations of Tau, and the subsequent effect of this treatment
on tumor cell proliferation was observed. As shown in Fig. 1, increasing the concentration and
exposure time of Tau resulted in a gradual increase in the
inhibition of cell proliferation in the HHCC HepG2 cells. Thus, the
inhibitory effect of Tau on cell proliferation was demonstrated to
be time- and dose-dependent.
Effect of Tau on apoptosis
induction
HHCC HepG2 cells were cultured for 48 h in media
containing variable concentrations of Tau (0 mM for the control,
40, 80 and 160 mM for the experimental groups) and DDP (10 µg/ml).
Annexin V-FITC/PI was used to double-stain the cells. As the Tau
concentration increased, the apoptosis rate of HHCC HepG2 cells
(annexin V-FITC+/PI−, right lower limit) was
shown to gradually increase (Fig.
2). Statistically significant differences were observed between
the apoptotic rates of the experimental groups and the control
group (P<0.05 for the 40 mM group and P<0.01 for the 80 and
160 mM groups).
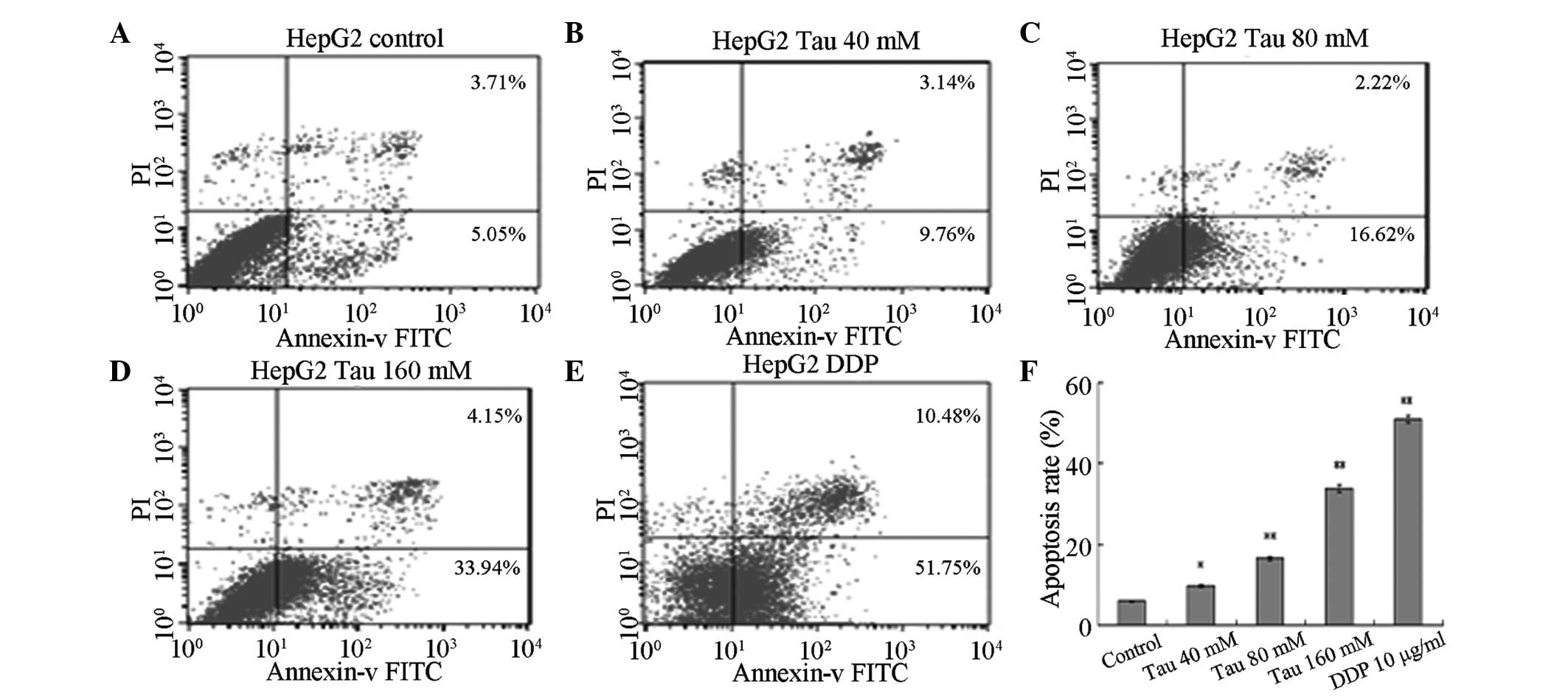 | Figure 2.Effect of Tau on apoptosis induction
in human hepatocellular carcinoma HepG2 cells. Effect of Tau on the
apoptosis induction in human hepatocellular carcinoma HepG2 cells.
HepG2 cells were treated with 40, 80, and 160 mM Tau and 10 µg/ml
DDP for 48 h, and apoptotic cells were analyzed by flow cytometry.
A, control group; B, 40 mM; C, 80 mM; D, 160 mM Tau; E, 10µg/ml DDP
and F, The statistical analysis of the apoptosis rate of HepG2
cells. Data from triplicate experiments were collected on a
histogram (n=4). *P<0.05 and **P<0.01, vs. control group.
Tau, taurine; PI, propidium iodide; FITC, fluorescein
isothiocyanate; DDP, cisplatin. |
Effect of Tau on the protein
expression levels of PUMA, Bax and Bcl-2
Western blotting results demonstrated that as the
Tau concentration increased, the protein expression levels of PUMA
and Bax significantly increased in the HHCC HepG2 cells, whereas
the protein expression levels of Bcl-2 significantly decreased
(Fig. 3). Thus, Tau appeared to have
a concentration-dependent effect on the changes in PUMA, Bax and
Bcl-2 protein expression levels, and these differences were
statistically significant (P<0.05).
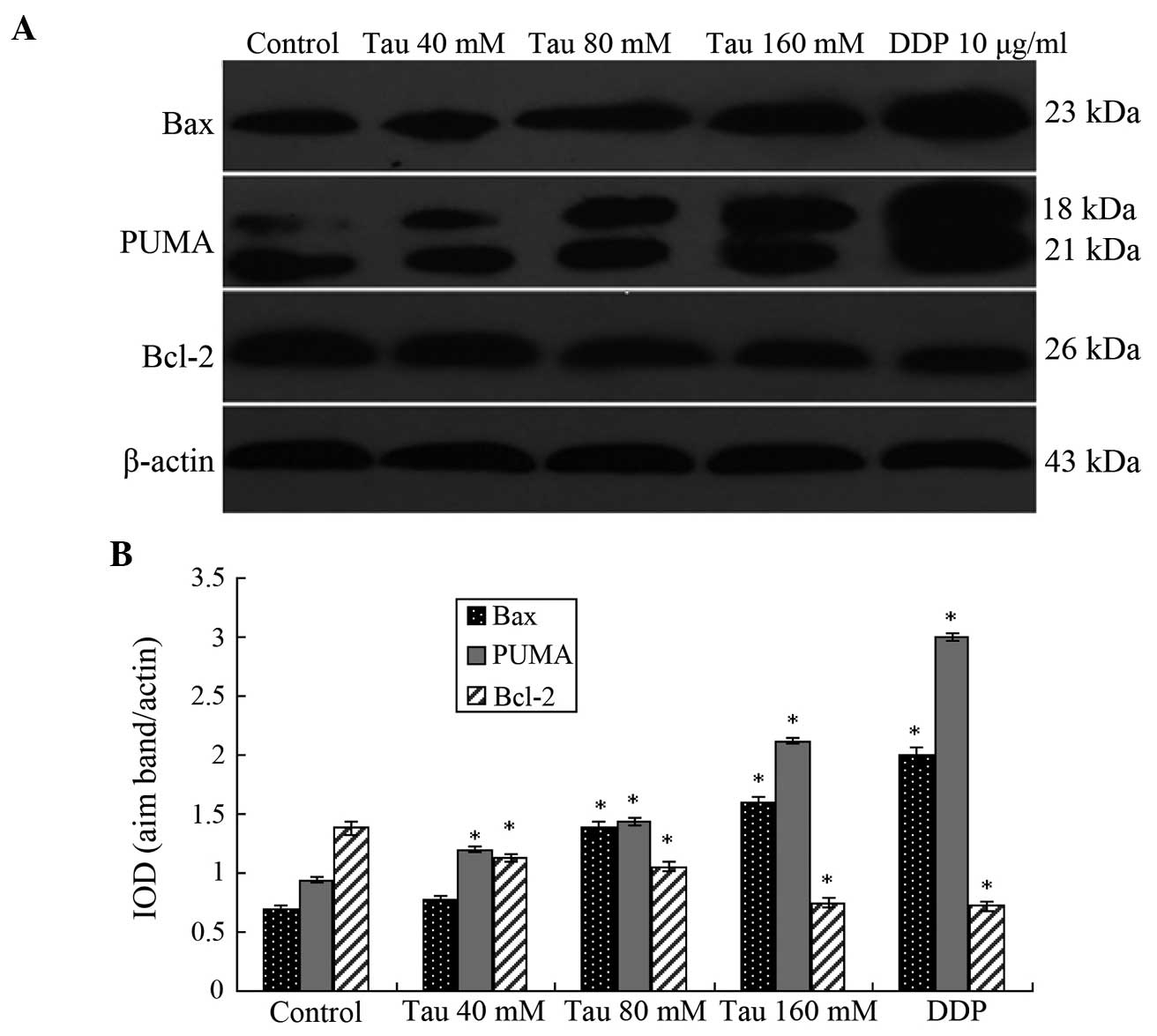 | Figure 3.Protein expression levels of PUMA, Bax
and Bcl-2 in human hepatocellular carcinoma HepG2 cells. (A)
Representative western blot showing the protein expression of PUMA,
Bax and Bcl-2. (B) Relative protein expression levels of PUMA, Bax
and Bcl-2, as assessed by the gray values. The PUMA antibody also
cross-reacted with an 18 kDa band of unknown origin. Expression
levels were normalized against the value obtained for β-actin
protein expression. Data are expressed as the mean ± standard
deviation (n=3). *P<0.05, vs. control group. Tau, taurine; DDP,
cisplatin; PUMA, p53 upregulated modulator of apoptosis; Bcl-2,
B-cell lymphoma-2; Bax, Bcl-2-associated X; IOD, optical
density. |
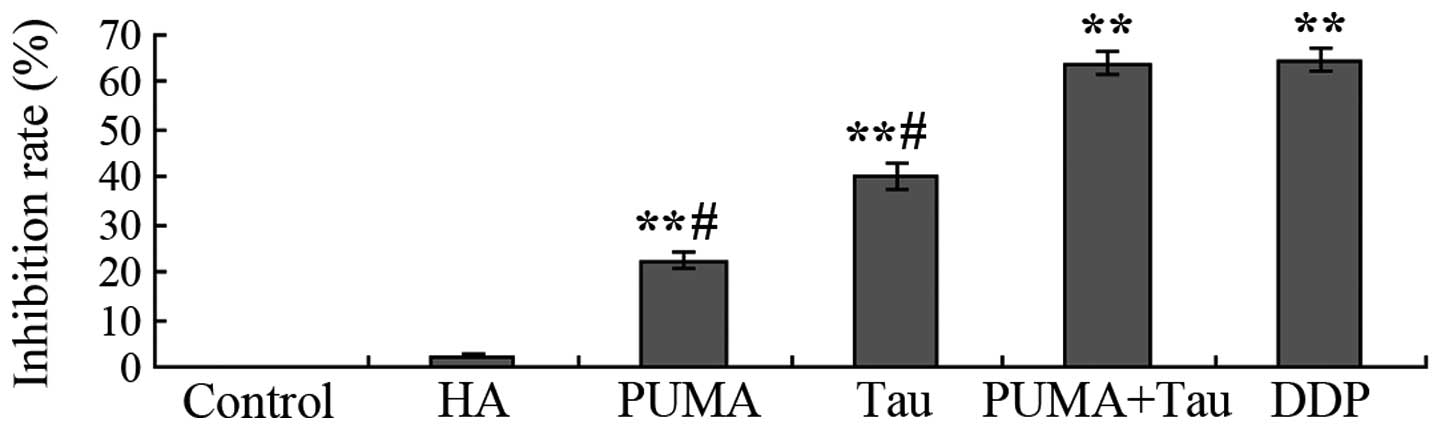
Effect of exogenous PUMA gene
transfection on the Tau-induced inhibition of cell
proliferation
Following transfection with the exogenous PUMA gene
or the combined effect of the PUMA gene and Tau on HepG2 cells over
48 h cell proliferation was assessed. The results demonstrated that
a significant in vitro inhibitory effect on HepG2 cell
growth was observed in the PUMA gene transfection group and the
PUMA + Tau group (P<0.01), with the inhibition rate of cell
proliferation considerably more significant in the PUMA + Tau group
(Fig. 4). No statistically
significant difference was observed in the growth rates between the
negative control (pCEP4-(HA)2-C1) group and the control
group (P>0.05). Thus, the results revealed that the inhibition
rate of Tau combined with PUMA was higher, as compared with PUMA
gene transfection or Tau treatment only.
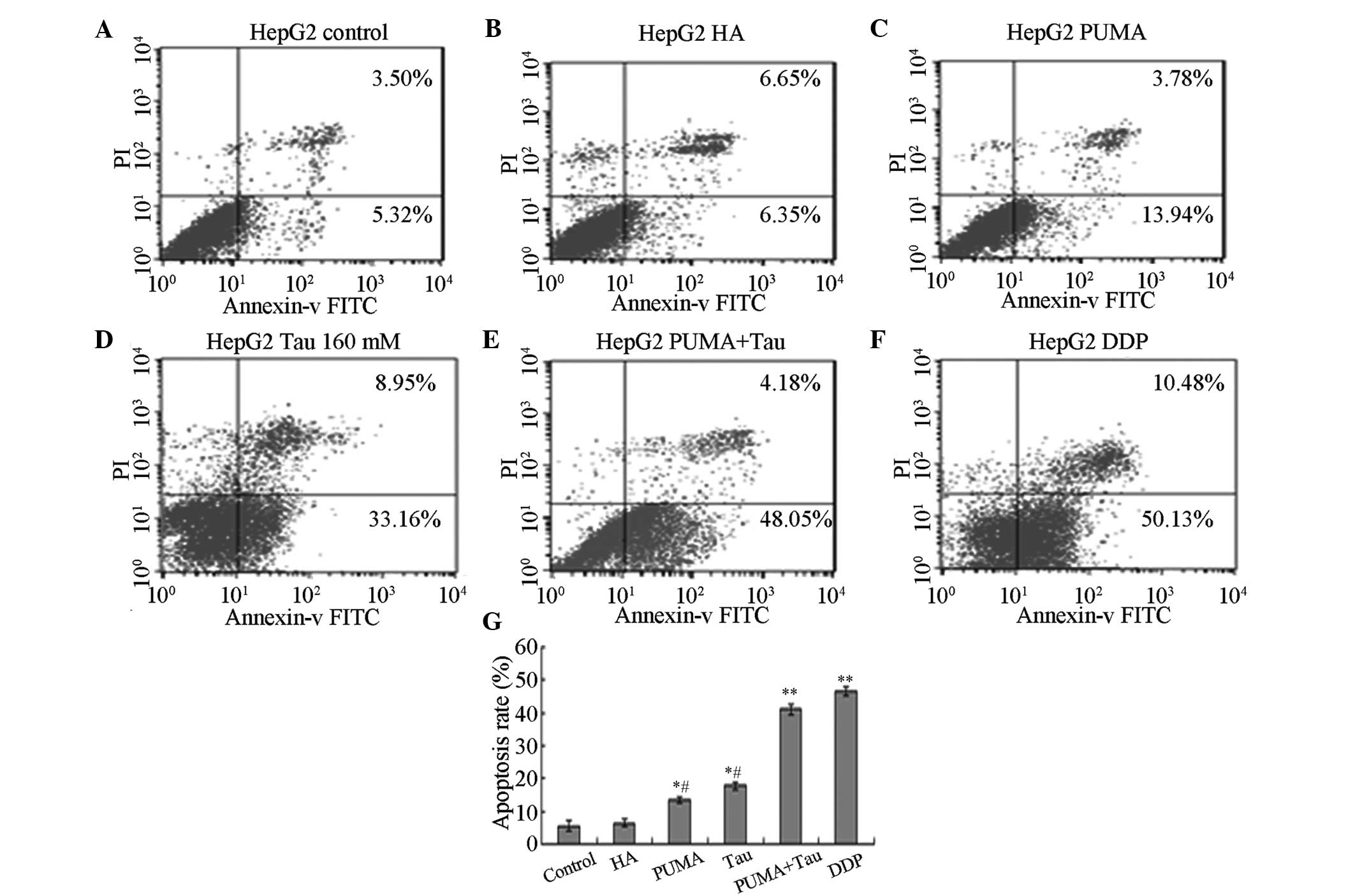
Effect of exogenous PUMA gene
transfection on HepG2 cell apoptosis
Following transfection with exogenous PUMA and
treatment with Tau for 48 h, annexin V-FITC/PI was used to
double-stain the cells, and FCM was used to detect the cell
apoptosis rate of each group. The results revealed that the
apoptosis rate in each experimental group was significantly
increased and statistically significant when compared with the
control and HA groups (P<0.05; Fig.
5). The apoptosis rate of PUMA + Tau cells was significantly
higher, as compared with the cells from the other groups
(P<0.01), however there was no obvious difference compared with
the DDP group. Thus, the results indicated that HepG2 cell
apoptosis can be induced following transfection with the PUMA gene,
and that a PUMA + Tau combination is more efficient in achieving
cell apoptosis compared with the solitary use of PUMA gene
transfection or treatment with Tau. The apoptosis rates of the
HepG2 cells treated with Tau or PUMA gene transfection alone were
significantly lower compared with that in the DDP group
(P<0.05).
Effect of PUMA-specific siRNA on
apoptosis induction
Following the specific silencing of PUMA gene
expression in the HepG2 cells, the HepG2 cells were treated with
160 mM Tau for 48 h and the cell apoptosis rate was determined
(Fig. 6). When compared with the
control group (6.86±0.22%), the apoptosis rate of the 160 mM Tau
group (31.16±1.86%) was significantly increased, whereas that of
the si-PUMA group (1.60±0.04%; P<0.01) was significantly
decreased. No statistically significant difference was observed in
the apoptosis rate between the NC group (7.15±0.27%) and the
si-PUMA + Tau group (7.02±0.25%; P>0.05). However, when compared
with the si-PUMA group (1.60±0.04%), the apoptosis rate of the
si-PUMA + Tau group (1.60±0.04%) was significantly increased
(P<0.01).
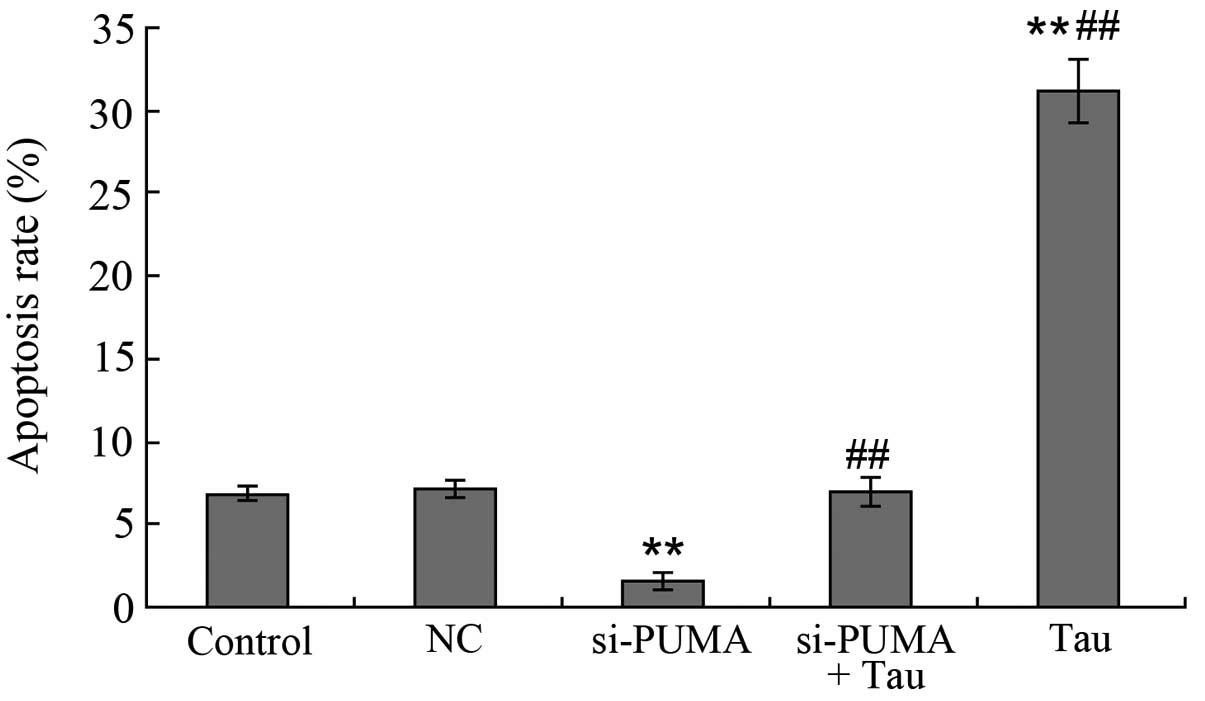 | Figure 6.Effect of PUMA-specific siRNA on
Tau-induced apoptosis of HepG2 cells. Apoptotic cells of HepG2 were
analyzed by flow cytometry. A, control; B, NC; C, si-PUMA; D,
si-PUMA + Tau and E, Tau group. Data from triplicate experiments
were collected on a histogram (n=4). **P<0.01, vs. control;
##P<0.01, vs. si-PUMA group. FITC, fluorescein
isothiocyanate; PUMA, p53 upregulated modulator of apoptosis; Tau,
taurine; PI, propidium iodide; NC, negative control; si, small
interfering. |
Effect of PUMA-specific siRNA on the
protein expression levels of Bax and Bcl-2
Following the specific silencing of PUMA expression
in the HHCC HepG2 cells, Bax and Bcl-2 protein expression levels in
the cells were shown to significantly change (Fig. 7). The results revealed that the
protein expression levels of PUMA and Bax in the si-PUMA group were
significantly downregulated, whereas Bcl-2 protein expression was
significantly upregulated. These changes were statistically
significant (P<0.05). When compared with the si-PUMA group, PUMA
and Bax protein expression levels in the si-PUMA + Tau group were
found to be significantly upregulated, whereas the expression of
Bcl-2 protein was significantly downregulated (P<0.05). The
protein expression levels of PUMA, Bax and Bcl-2 in the NC group
did not show a statistically significant difference from those in
the control group (P>0.05). These results indicated that Tau was
able to increase the protein expression levels of PUMA, which may
be a possible mechanism through which Tau is able to induce HHCC
HepG2 cell apoptosis.
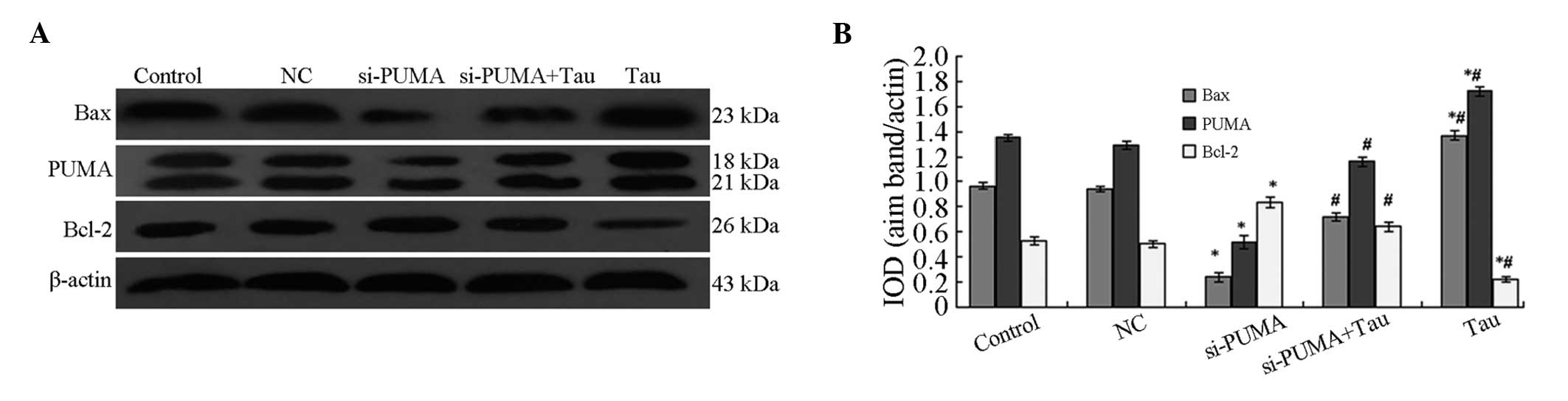 | Figure 7.Effect of PUMA-specific siRNA on the
protein expression levels of Bax and Bcl-2 in HepG2 cells. (A)
Representative western blot showing the protein expression of PUMA,
Bax and Bcl-2. (B) Relative protein expression levels of PUMA, Bax
and Bcl-2, as assessed by the gray values. The PUMA antibody also
cross-reacted with an 18 kDa band of unknown origin. Expression
levels were normalized against the value obtained for β-actin
protein expression. Data are expressed as the mean ± standard
deviation (n=3). *P<0.05, vs. control group;
#P<0.05, vs. si-PUMA group. PUMA, p53 upregulated
modulator of apoptosis; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; Tau, taurine; IOD, integrated optical
density; NC, negative control; si, small interfering. |
Discussion
Liver cancer is a digestive system neoplasm with
high mortality and recurrence rates. This cancer type is the fifth
most common malignancy and the third most common cause of cancer
mortality worldwide (16). Clinical
studies have shown that numerous chemotherapeutic drugs used to
treat liver cancer have strong toxic adverse effects. The
insensitivity of tumor cells to chemotherapeutic drugs and the
toxic adverse effects of these drugs have detrimental impacts on
clinical efficacy. Previous studies have found that Tau possesses
broad physiological and pharmacological functions, and previous
research has shown that changes in the in vivo concentration
of Tau may be a predictor of cancer (11,12). A
high concentration of Tau may appear in the urine of patients with
non-muscular invasive bladder cancer, suggesting that Tau may
become a new indicator for the diagnosis of bladder cancer.
Research by Nagy et al (17)
has shown that the Tau derivative, glutaurine, significantly
decreases the occurrence of MC29/l virus-induced HHCC and leukemia
in turkey chicks. In addition, McCourt et al (18) demonstrated that the Tau derivative,
taurolidine, can inhibit carcinoma growth in vitro and in
vivo. Research by Reddy et al (19) has shown that Tau can inhibit
colorectal cancer by increasing the activity of glutathione
transferase, NAD(P)H reductase and glucuronyl transferase.
The present study found that Tau, at a concentration
between 20 and 160 mM, exerts an inhibitory effect on HHCC HepG2
cell proliferation and apoptosis induction, with the effect time-
and dose-dependent (Figs. 1 and
2). With increasing Tau
concentrations, the protein expression levels of PUMA and Bax in
the HHCC HepG2 cells were found to be significantly upregulated,
whereas the protein expression of Bcl-2 was significantly
downregulated (Fig. 3). In addition,
the effect of Tau on the protein expression levels of PUMA, Bax and
Bcl-2 was found to be dose-dependent. Exogenous PUMA gene
transfection significantly increased the effect of Tau on HepG2
cell apoptosis and proliferation inhibition. Application of PUMA
gene transfection and Tau treatment was shown to be as effective as
treatment with DDP (with a final concentration of 10 mg/ml;
Figs. 4 and 5). However, following the targeted
silencing of PUMA expression in HepG2 cells and Tau treatment for
48 h, the apoptosis rate of the si-PUMA + Tau group was shown to be
significantly lower compared with that of the Tau group (Fig. 6). Furthermore, when compared with the
si-PUMA group, the protein expression levels of PUMA and Bax in the
si-PUMA + Tau group were significantly upregulated, while Bcl-2
protein expression was significantly downregulated (P<0.05).
These results demonstrated that Tau has the combined effect of
upregulating PUMA and Bax protein expression, while downregulating
Bcl-2 protein expression (Fig. 7).
Through this important mechanism, Tau can induce apoptosis and
inhibit the proliferation of HHCC HepG2 cells. Therefore, PUMA
plays an important role in the anti-HHCC effect of Tau, and may
become a novel target for use in gene therapy against liver
cancer.
Tau can inhibit tumor proliferation through several
mechanisms. Firstly, Tau has been hypothesized to enhance
antioxidation in the body through the removal of strong oxidizing
agents, which subsequently protects normal cells from oxidative
damage and induces tumor cell apoptosis (9,20,21). For
example, Tau can reverse the morphine-induced downregulation of
Bcl-2 and effectively control morphine-induced oxidative damage,
thus preventing nerve cell toxicity (22). In B16F10 mouse melanoma cells, Tau
functions by upregulating superoxide dismutase genes, glutathione
peroxidase and catalase, while inhibiting cell growth by decreasing
the concentration of reactive oxygen species in a dose-dependent
manner (23). In addition, following
treatment with Tau for liver fibrosis, the levels of hydrogen
peroxide lipids, transforming growth factor-β and hydroxyproline in
the blood and liver are significantly reduced, and liver damage and
fibrosis are decreased (24).
Secondly, Tau may inhibit tumor proliferation by
enhancing immunity. Abd-Rabou et al (25) found that application of Tau with
curcumin promotes immunity in an organism, which subsequently
resulted in the dissolution of Huh-7 carcinoma cells.
Thirdly, Tau may exert an antitumor function by
increasing the efficacy and decreasing the toxicity of
chemotherapeutic drugs. Following intravenous injection or gastric
perfusion of Tau and/or cyclophosphamide (CTX) in tumor-bearing
mice, the inhibition rate of the combination drug group was found
to be much higher compared with that of the CTX-only group,
indicating that Tau enhances the efficacy of CTX chemotherapeutic
drugs (15). The same method was
used to treat Lewis liver tumor-bearing mice, where Tau was
demonstrated to increase the white blood cell count, leading to the
alleviation of CTX toxicity (26).
Furthermore, the effect of Tau on the apoptosis of human cervical
carcinoma cells has been demonstrated to be time- and
dose-dependent, and the apoptosis rate has been found to be more
significant when combined with DDP.
In addition to modulating the expression of p53, Tau
can activate caspases 3, 6, 7 and 9 to activate the mitochondrial
pathway (27). Furthermore, Tau has
been shown to mitigate the oxidative stress and cell damage
produced by arsenic through mitochondrial-dependent and independent
pathways (28). Zhang et al
(29) found that downregulation of
nicotinamide N-methyltransferase leads to the upregulation of PUMA
gene expression and the induction of apoptosis in breast carcinoma
cells via the induction of mitochondrial apoptotic pathways. In
mouse carcinoma cell models, PUMA expression has been shown to
increase significantly following inactivation of human epidermal
growth factor receptor 2 (HER2). However, reduced PUMA expression
may result in a decrease in caspase activation, which leads to a
reduction in the apoptosis rates of mouse breast carcinoma cells
caused by HER2 inactivation, and lung carcinoma cells induced by a
mutation in the epidermal growth factor receptor (30). The results of the present study
demonstrated that Tau was able to downregulate the expression of
the apoptosis-inhibiting protein, Bcl-2, while upregulating the
expression of the apoptosis-inducing proteins, PUMA and Bax, to
subsequently alter the Bax/Bcl-2 ratio and induce apoptosis of
carcinoma cells through a mitochondrial-dependent pathway. PUMA was
also demonstrated to play a key role in this process.
In conclusion, the PUMA gene plays an important role
in mechanism underlying the effect of Tau on cell proliferation
inhibition and the induction of apoptosis in HHCC HepG2 cells.
Therefore, the PUMA gene may become a novel target for use in gene
therapy against liver cancer. However, whether PUMA is the only
Tau-induced apoptosis-associated gene requires further study.
References
|
1
|
Shen YM, Yu SF and Cui MY: The impact and
learning on the farmers’ health when conducting health education in
rural communities. Zhongguo NongCun Wei Sheng Shi Ye Guan Li.
27:603–605. 2007.
|
|
2
|
Subramaniam A, Shanmugam MK, Perumal E, et
al: Potential role of signal transducer and activator of
transcription (STAT)3 signaling pathway in inflammation, survival,
proliferation and invasion of hepatocellular carcinoma. Biochim
Biophys Acta. 1835:46–60. 2013.PubMed/NCBI
|
|
3
|
Sia D and Villanueva A: Signaling pathways
in hepatocellular carcinoma. Oncology. 81:18–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho M: Advances in liver cancer antibody
therapies: a focus on glypican-3 and mesothelin. BioDrugs.
25:275–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin J, Huang S, Wu S, et al: MicroRNA-423
promotes cell growth and regulates G(1)/S transition by targeting
p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis.
32:1641–1647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huxtable RJ: Physiological actions of
taurine. Physiol Rev. 72:101–163. 1992.PubMed/NCBI
|
|
8
|
Wójcik OP, Koenig KL, Zeleniuch-Jacquotte
A, Costa M and Chen Y: The potential protective effects of taurine
on coronary heart disease. Atherosclerosis. 208:19–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Zhao N, Zhang F, Yue W and Liang
M: Effect of taurine on leucocyte function. Eur J Pharmacol.
616:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu JS and Kim AK: Effect of combination of
taurine and azelaic acid on antimelanogenesis in murine melanoma
cells. J Biomed Sci. 17:(Suppl 1). S452010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El Agouza IM, Eissa SS, El Houseini MM,
El-Nashar DE and Abd El Hameed OM: Taurine: a novel tumor marker
for enhanced detection of breast cancer among female patients.
Angiogenesis. 14:321–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Srivastava S, Roy R, Singh S, et al:
Taurine - a possible fingerprint biomarker in non-muscle invasive
bladder cancer: A pilot study by 1H NMR spectroscopy. Cancer
Biomark. 6:11–20. 2010.PubMed/NCBI
|
|
13
|
Neary PM, Hallihan P, Wang JH, Pfirrmann
RW, Bouchier-Hayes DJ and Redmond HP: The evolving role of
taurolidine in cancer therapy. Ann Surg Oncol. 17:1135–1143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Sheng J, Zhang C, et al: Taurine
induces apoptosis in pulmonary artery smooth muscle cells. Zhongguo
Zhong Yao Za Zhi. 37:654–657. 2012.PubMed/NCBI
|
|
15
|
Zhang X, Tu S, Wang Y, Xu B and Wan F:
Mechanism of taurine-induced apoptosis in human colon cancer cells.
Acta Biochim Biophys Sin (Shanghai). 46:261–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giacomin A, Sergio A, Vanin V, Gazzola A,
Cazzagon N and Farinati F: Molecular targeted therapy in
hepatocellular carcinoma: present achievements and future
challenges. Dig Dis. 30:284–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagy K, Bátkai L, Tálas M and Feuer L:
Effect of glutaurine on liver tumour development and acute
leukaemia induced by MC29 virus in turkey poults. Acta Microbiol
Hung. 30:37–42. 1983.PubMed/NCBI
|
|
18
|
McCourt M, Wang JH, Sookhai S and Redmond
HP: Taurolidine inhibits tumor cell growth in vitro and in vivo.
Ann Surg Oncol. 7:685–691. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reddy BS, Rao CV, Rivenson A and Kelloff
G: Chemoprevention of colon carcinogenesis by organosulfur
compounds. Cancer Res. 53:3493–3498. 1993.PubMed/NCBI
|
|
20
|
Das J, Ghosh J, Manna P and Sil PC:
Taurine suppresses doxorubicin-triggered oxidative stress and
cardiac apoptosis in rat via up-regulation of PI3-K/Akt and
inhibition of p53, p38-JNK. Biochem Pharmacol. 81:891–909. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mates JM, Segura JA, Alonso FJ and Marquez
J: Sulphur-containing non enzymatic antioxidants: therapeutic tools
against cancer. Front Biosci (Schol Ed). 4:722–748. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J, Li Y, Yan G, et al: Protective
role of taurine against morphine-induced neurotoxicity in C6 cells
via inhibition of oxidative stress. Neurotox Res. 20:334–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J and Kim AK: Effect of taurine on
antioxidant enzyme system in B16F10 melanoma cells. Adv Exp Med
Biol. 643:491–499. 2009.PubMed/NCBI
|
|
24
|
Miyazaki T, Karube M, Matsuzaki Y, Ikegami
T, Doy M, Tanaka N and Bouscarel B: Taurine inhibits oxidative
damage and prevents fibrosis in carbon tetrachloride-induced
hepatic fibrosis. J Hepatol. 43:117–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abd-Rabou AA, Zoheir KM and Ahmed HH:
Potential impact of curcumin and taurine on human hepatoma cells
using Huh-7 cell line. Clin Biochem. 45:1519–1521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Zhao N, Zhang F, Yue W and Liang
M: Effect of taurine on leucocyte function. Eur J Pharmacol.
616:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim T and Kim AK: Taurine enhances
anticancer activity of cisplatin in human cervical cancer cells.
Adv Exp Med Biol. 776:189–198. 2013.PubMed/NCBI
|
|
28
|
Das J, Ghosh J, Manna P, Sinha M and Sil
PC: Taurine protects rat testes against NaAsO(2)-induced oxidative
stress and apoptosis via mitochondrial dependent and independent
pathways. Toxicol Lett. 187:201–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Wang Y, Li G, Yu H and Xie X:
Down-regulation of nicotinamide N-methyltransferase induces
apoptosis in human breast cancer cells via the
mitochondria-mediated pathway. PLoS One. 9:e892022014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bean GR, Ganesan YT, Dong Y, et al: PUMA
and BIM are required for oncogene inactivation-induced apoptosis.
Sci Signal. 6:ra202013. View Article : Google Scholar : PubMed/NCBI
|