Introduction
Cimicifugae Rhizoma, also known as Seungma in Korea,
Shengma in China and Shoma in Japanese is a traditional herbal
medicine that is used to treat various diseases in these countries
(1). Cimicifugae Rhizoma is
primarily derived from Cimicifuga heracleifolia Komarov or
Cimicifuga foetida Linnaeus (2). Cimicifugae Rhizoma has traditionally
been used as an anti-inflammatory, analgesic and antipyretic remedy
(3–6). It has been shown to induce alkaline
phosphatase synthesis in rat calvarial osteoblasts when tested
in vitro (7). Cimicifugae
Rhizoma has also been suggested to be useful for the treatment of
dental diseases, including periodontitis (8). It has demonstrated antimicrobial
activity against Porphyromonas gingivalis, a common
bacterium in oral biofilms, when tested in vitro (8), and has also shown chelating ability,
which may be applied for the prevention of oral calcium phosphate
precipitation (calculus formation) (9).
A limited study has been performed to evaluate the
effects of Cimicifugae Rhizoma on cell viability (10). Cimicifugae Rhizoma extract induced
G0/G1 cell cycle arrest of hepatocellular
carcinoma at a low concentration (25 µg/ml), triggered
G2/M arrest and apoptosis of the hepatocellular
carcinoma at higher concentrations (50 and 100 µg/ml) and inhibited
the growth of implanted mouse tumors in a dose-dependent manner,
with a growth inhibitory rate of 63.3% at 200 mg/kg (10). However, limited information is
currently available regarding the effects of Cimicifugae Rhizoma on
dental tissue, including mesenchymal stem cells derived from
gingiva.
The aim of the present study was to evaluate the
effects of extracts of Cimicifugae Rhizoma on the morphology and
viability of human stem cells derived from gingiva. To the best of
our knowledge, this study is the first to elucidate the effect of
Cimicifugae Rhizoma on stem cells derived from gingiva.
Materials and methods
Preparation of materials
The dry roots of Cimicifuga heracleifolia
Komarov (500 g; Chungju Hospital of Korean Medicine, College of
Korean Medicine, Semyung University, Chungju, Republic of Korea)
were immersed in distilled water and boiled under reflux for 2 h 30
min, and the resulting extract was centrifuged at 5,000 × g for 10
min. The supernatant was concentrated to 300 ml using a rotary
evaporator under reduced pressure (Eyela NE-1001; Tokya Rikakikai
Co., Ltd, Tokyo, Japan). The concentrates were then freeze-dried
using a lyophilizer (Labconco, Kansas, MO, USA) to obtain 92.8 g
solid residue, resulting in a yield of 18.6% (w/w).
Isolation and culture of stem cells
derived from gingiva
Healthy gingival tissues were obtained from healthy
patients undergoing crown-lengthening procedures. This study was
reviewed and approved by the Institutional Review Board of Seoul
St. Mary's Hospital, College of Medicine, The Catholic University
of Korea (Seoul, Korea; KC11SISI0348), and informed consent was
obtained from all patients.
The tissues were immediately placed in sterile
phosphate-buffered saline (PBS; Welgene, Inc., Daegu, Korea) with
100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich, St.
Louis, MO, USA) at 4°C. The gingival tissue was de-epithelialized,
minced, digested with collagenase IV (Sigma-Aldrich) and incubated
at 37°C in a humidified incubator with 5% CO2 and 95%
O2. The non-adherent cells were washed with PBS after 24
h, supplied with essential minimal medium α (α-MEM; Gibco Life
Technologies, Grand Island, NY, USA) containing 15% fetal bovine
serum (Gibco), 100 U/ml penicillin, and 100 µg/ml streptomycin
(Sigma-Aldrich), 200 mM L-glutamine (Sigma-Aldrich) and 10 mM
ascorbic acid 2-phosphate (Sigma-Aldrich), and fed every 2–3 days.
These cells showed the characteristics of stem cells, including
colony-forming abilities, plastic adherence and multi-lineage
differentiation (osteogenic, adipogenic, chondrogenic) potency.
Approximately 3×105 cells were incubated with specific
PE-, APC-, BV421-, PerCP-cyTM5.5- or fluorescein
isothiocyanate-conjugated mouse monoclonal antibodies for human
CD44, CD73, CD90, CD105, CD14, CD45, CD34 and CD19 (BD Biosciences,
San Jose, CA, USA). The cells expressed CD44, CD73, CD90 and CD105,
but did not express CD14, CD45, CD34 and CD19 when examined by flow
cytometry (FACSCanto II; BD Biosciences).
Evaluation of stem cell
morphology
The stem cells were plated at a density of
2.0×103 cells/well in 96-well plates. The cells were
incubated in α-MEM (Gibco Life Technologies, Grand Island, NY, USA)
that was composed of 15% fetal bovine serum (Gibco Life
Technologies), 100 U/ml penicillin, 100 µg/ml streptomycin, 200 mM
L-glutamine (Sigma-Aldrich) and 10 mM ascorbic acid 2-phosphate
(Sigma-Aldrich) in the presence of the Cimicifugae Rhizoma extract
at final concentrations that ranged from 0.001 to 1,000 µg/ml. The
concentrations used were 0 (untreated control), 0.001, 0.01, 0.1,
1, 10, 100 and 1,000 µg/ml, respectively. The morphology of the
cells was viewed under an inverted microscope (Leica DM IRM; Leica
Microsystems, Wetzlar, Germany) on days 1, 3, 5 and 7. The images
were saved as JPEG files.
Determination of cell
proliferation
The analysis of cell proliferation was performed on
days 1, 3, 5 and 7. Viable cells were identified using a Cell
Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) assay.
The spectrophotometric absorbance was measured with a Synergy MX
microplate reader (BioTek Instruments, Inc.; Winooski, VT, USA),
and the analysis was performed in triplicate.
Statistical analysis
The results are represented as the means ± standard
deviation. A test of normality was performed, and a one-way
analysis of variance (ANOVA) with post hoc test was performed to
determine the differences between the groups using commercially
available software (SPSS version 12 for Windows; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
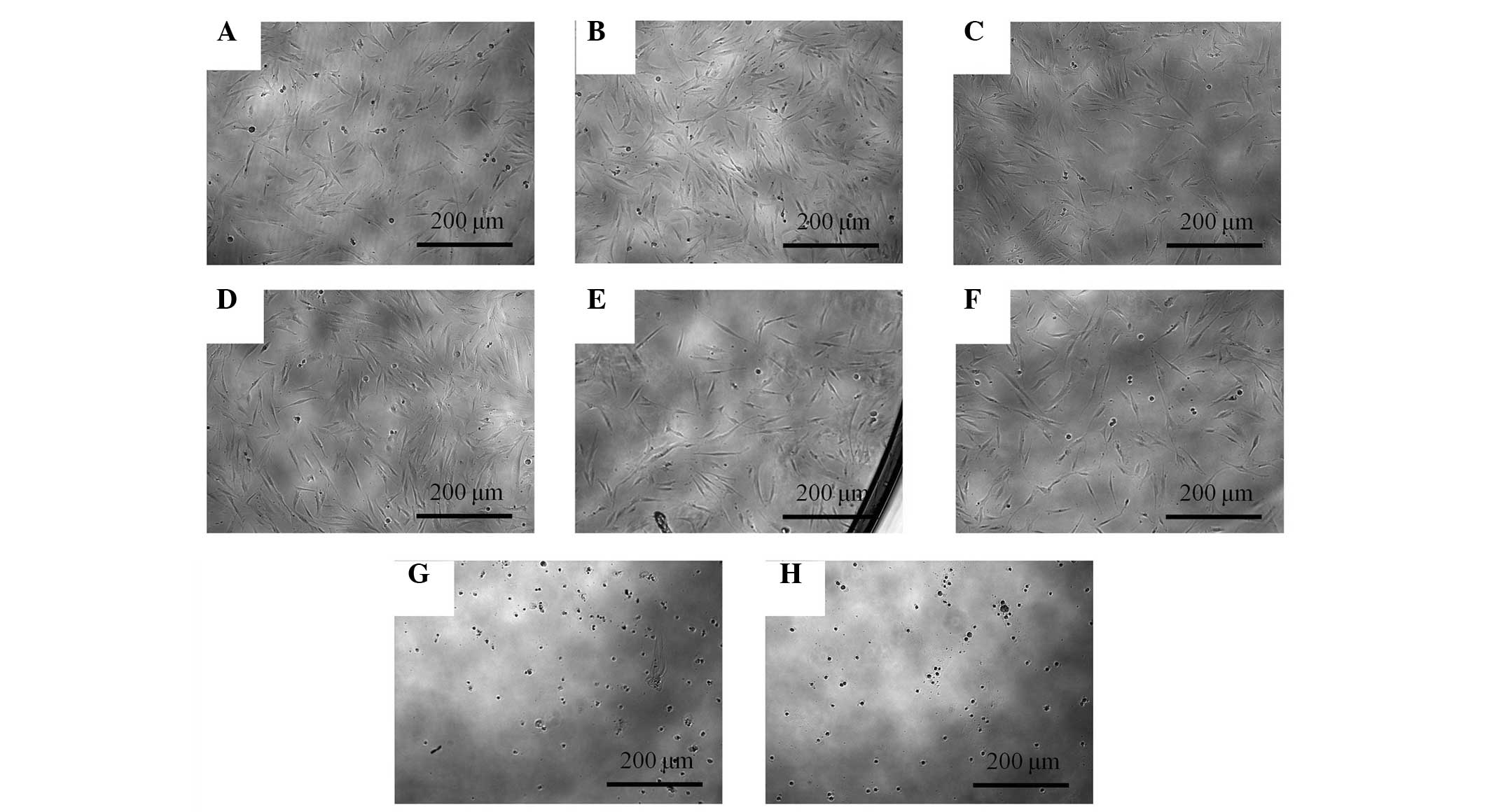
Evaluation of cell morphology
The morphology of the stem cells at day 1 is shown
in Fig. 1. Under an optical
microscope, the control group showed spindle-shaped,
fibroblast-like morphology. The shapes of the cells treated with
0.001–10 µg/ml Cimicifugae Rhizoma were similar to the shapes of
the control group. Significant alterations were noted in the 100
and 1,000 µg/ml groups when compared with the control group. The
shapes of the cells in the 100 and 1,000 µg/ml groups were rounder,
and fewer cells were present.
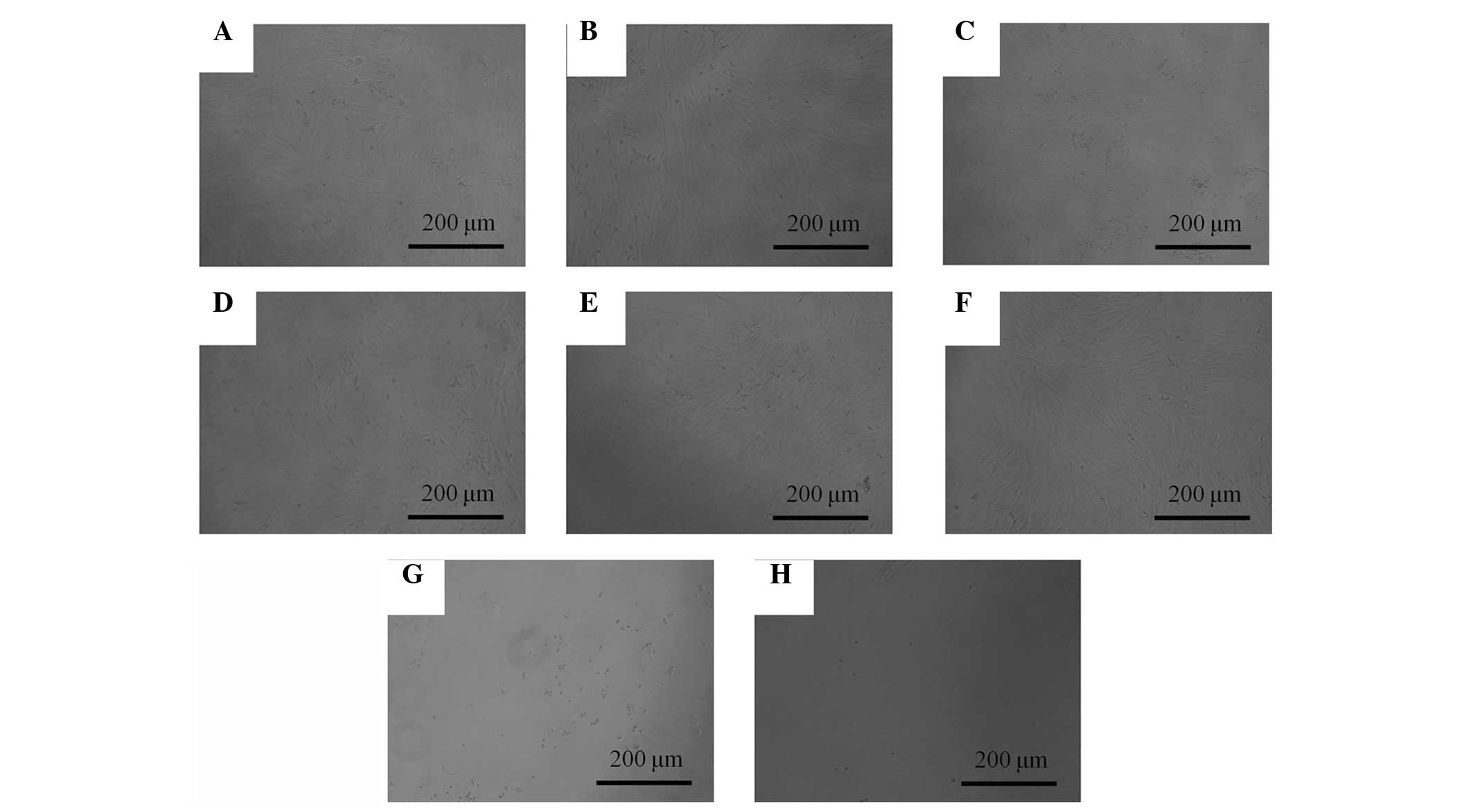
The morphology of the cells on day 3 is shown in
Fig. 2. The shapes of the cells in
treated with 0.001–10 µg/ml were similar to the shapes of the
control group. Marked alterations in cytoskeletal organization were
observed in the 100 and 1,000 µg/ml groups. The shapes of the cells
in the 100 and 1000 µg/ml groups were rounder, and fewer cells were
present, when compared with the control group.
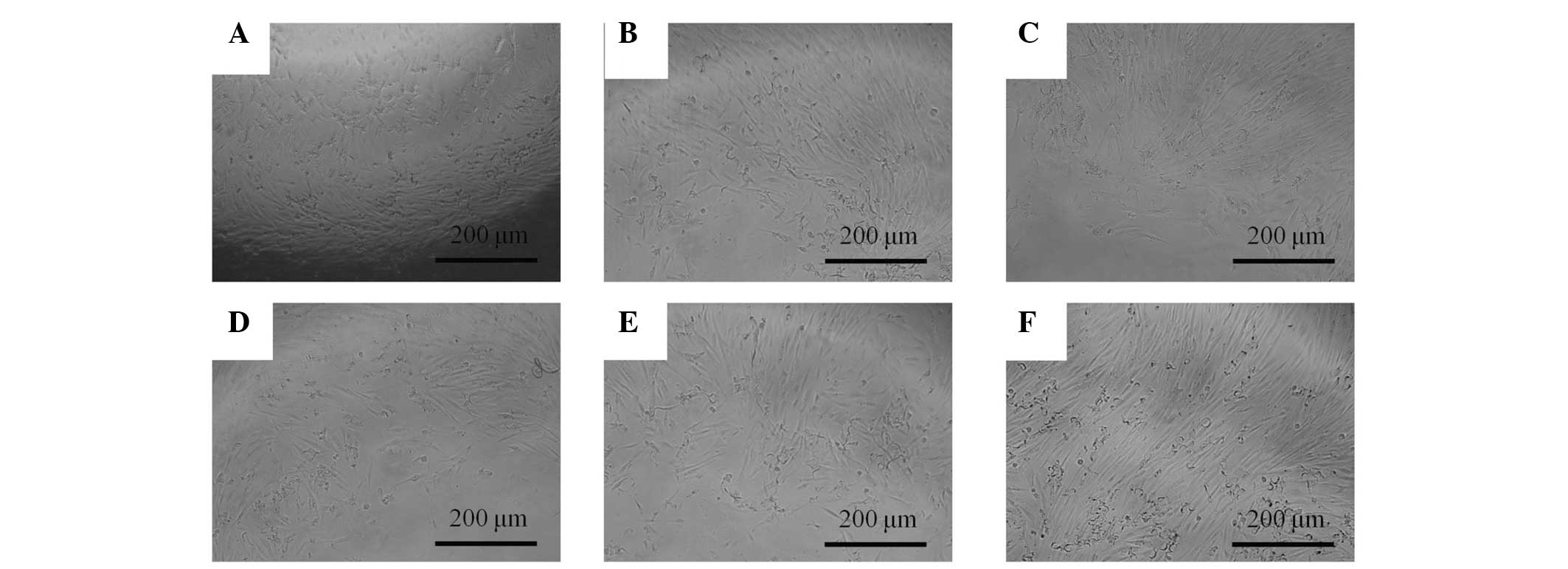
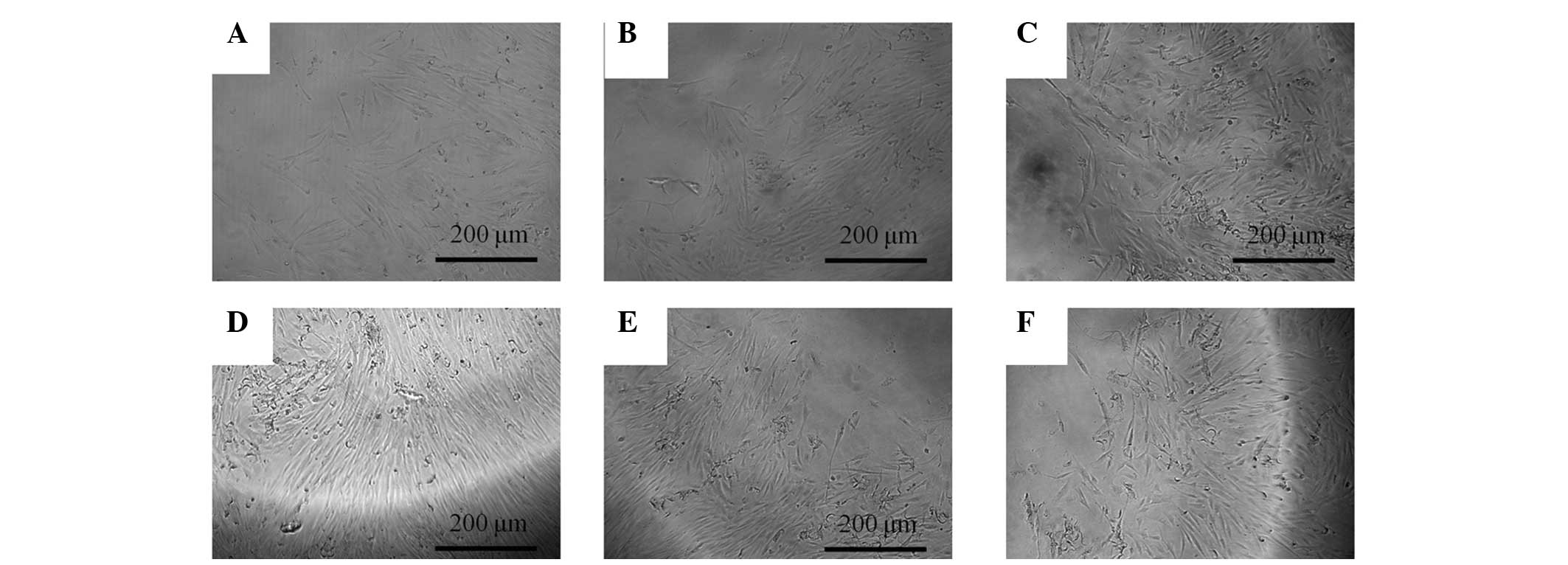
The morphology of the cells on days 5 and 7 is shown
in Figs. 3 and 4, respectively. The shapes of the cells in
the 0.001–10 µg/ml groups were similar to the shapes of the
untreated control group.
Cell proliferation
The results of the cell proliferation assay on days
1, 3, 5 and 7 are shown in Fig. 5,
6, 7
and 8, respectively. The cultures
that were grown in the presence of Cimicifugae Rhizoma on day 1
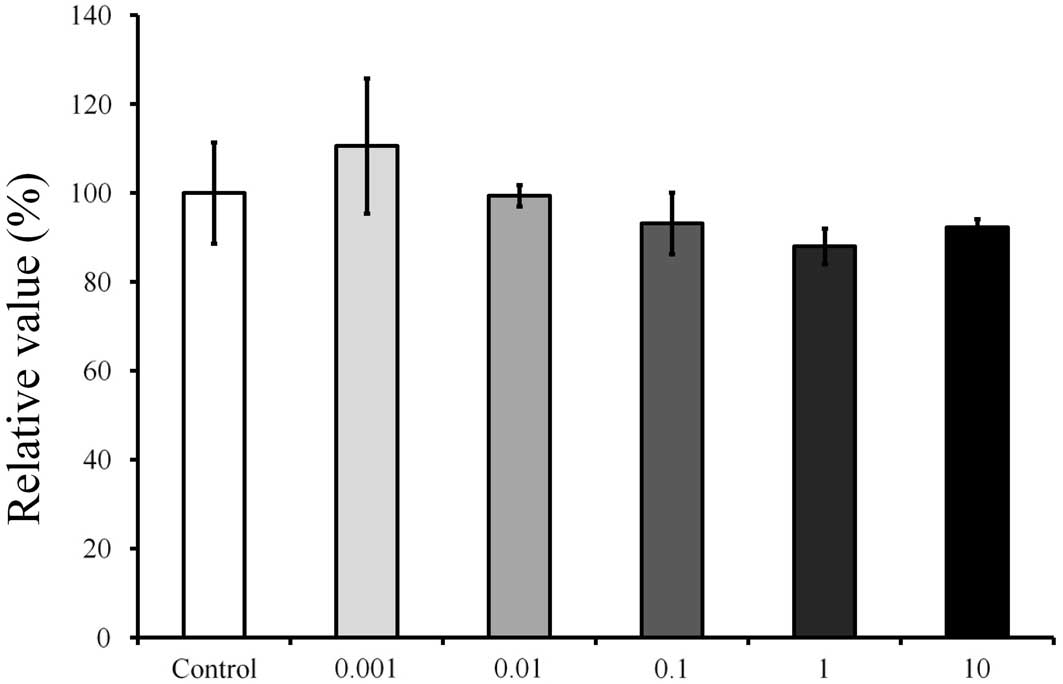
showed an increase in the CCK-8 result at 0.001 µg/ml (Fig. 5). The relative value of the CCK-8
assay result for 0.001 µg/ml Cimicifugae Rhizoma was 110.6±15.2%,
when the CCK-8 result of the untreated control group on day 1 was
considered to be 100%; however, there were no significant
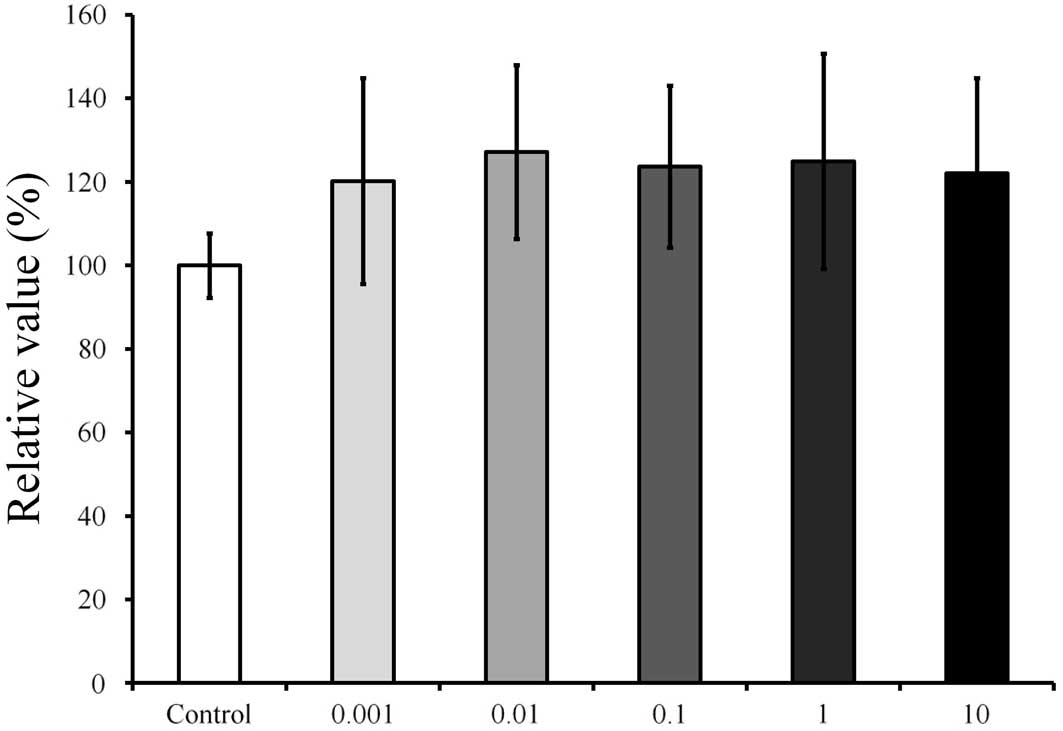
differences (P>0.05). On day 3 (Fig.
6), the relative value of the CCK-8 result for 0.001 µg/ml
Cimicifugae Rhizoma was 120.2±24.6%, when the CCK-8 result of the
untreated control group on day 3 was considered to be 100%. On day
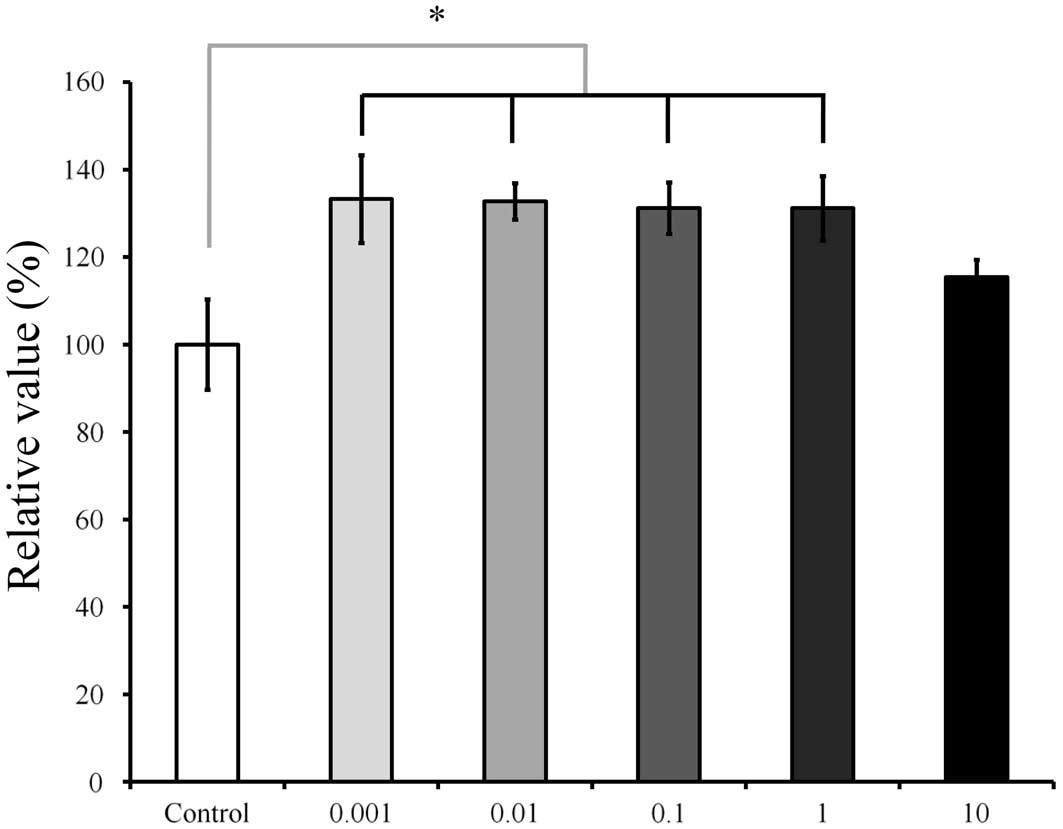
5 (Fig. 7), the cultures grown in
the presence of Cimicifugae Rhizoma at concentrations of 0.001,
0.01, 0.1 and 1 µg/ml resulted in increased CCK-8 values
(P<0.05). The relative values of the CCK-8 results for 0.001,
0.01, 0.1, 1 and 10 µg/ml Cimicifugae Rhizoma were 133.3±10.0,
132.7±4.2, 131.2±5.8, 131.2±7.3 and 115.5±4.0%, respectively, when
the CCK-8 result of the untreated control group on day 5 was
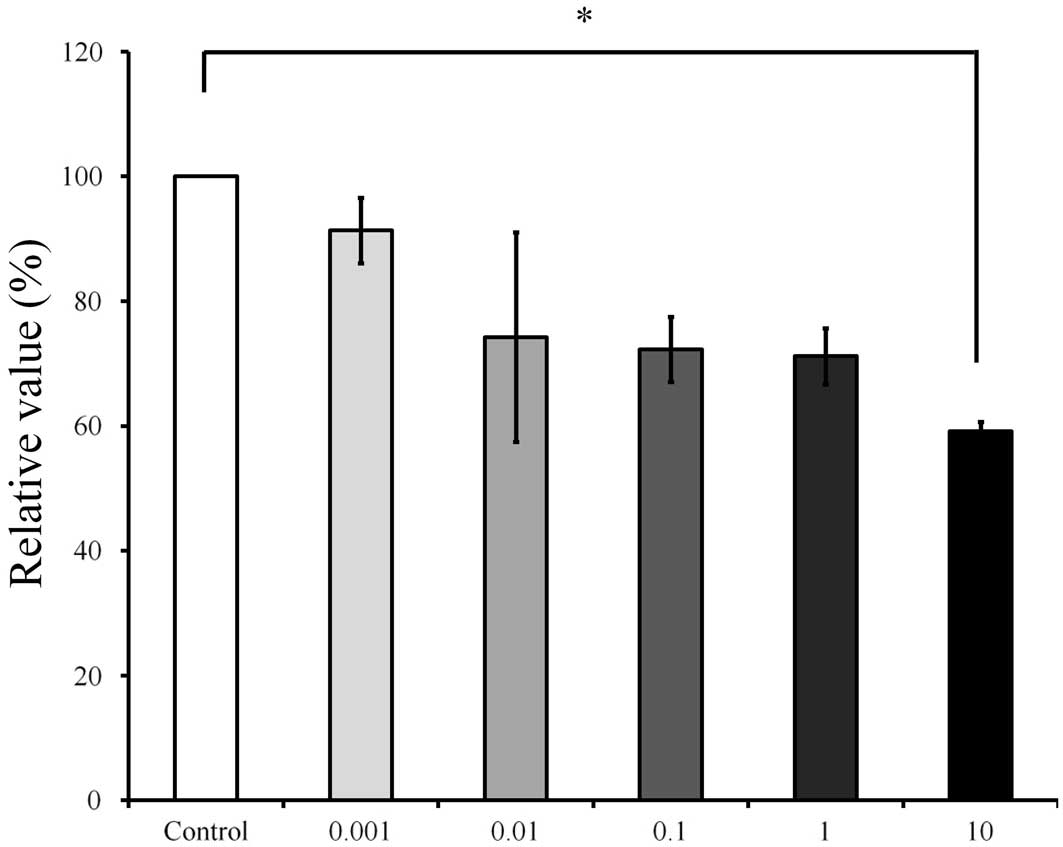
considered to be 100% (100.0±7.7%). On day 7 (Fig. 8), The relative value of the CCK-8
result for 10 µg/ml Cimicifugae Rhizoma was 59.2±1.5%, when the
CCK-8 result of the untreated control group on day 7 was considered
to be 100% (P<0.05).
Discussion
In this study, the effects of Cimicifugae Rhizoma on
the morphology and proliferation of human mesenchymal stem cells
derived from periodontal tissue were investigated. The results
clearly demonstrated that the stem cells were sensitive to
Cimicifugae Rhizoma at high concentrations and that a significant
reduction in cellular viability occurred at 100 and 1,000 µg/ml
concentrations.
The effects of Cimicifugae Rhizoma have previously
been tested in in vitro and in vivo experiments
(10–12). A Cimicifugae Rhizoma extract has
shown cytotoxicity toward human cancer cell lines, including
promyelocytic, lung carcinoma and human colon adenocarcinoma cell
lines (12). The cytotoxicity of
Cimicifugae Rhizoma has been tested and the half maximal inhibitory
concentration (IC50) values on hepatocellular carcinoma
and drug-resistant hepatocellular carcinoma cell lines and primary
cultured normal mouse hepatocytes were found to be 21, 43 and 80
µg/ml, respectively (10). In a rat
model, Cimicifugae Rhizoma extract at a dosage of 50 mg/kg showed a
slight toxicity in the liver and kidney via disturbance of the
metabolisms of energy and amino acids, which provides a reasonable
explanation for the clinical hepatotoxicity (11).
Cimicifugae Rhizoma is a traditional herbal medicine
used to treat various diseases. The anticancer properties of plants
of the genus Cimicifuga have received considerable attention
in recent years (12). Cimicifugae
Rhizoma can be used for the treatment of cardiovascular disorders
such as atherosclerosis (13).
Ovariectomized rats treated with extracts of Cimicifugae Rhizoma
exhibited a significant increase in bone mineral density compared
with that in untreated rats (2), and
it was suggested that these anti-bone resorption effects of
Cimicifugae Rhizoma may be applied therapeutically against
osteoporosis (14,15). Aqueous extracts of Cimicifugae
Rhizoma have shown central nervous system effects by binding to the
5-HT1A receptor (4). Cimicifugae
Rhizoma has also demonstrated inhibitory effects on histamine,
bradykinin and COX-2-mediated inflammatory actions (5). The analgesic and sedative effects of
Cimicifugae Rhizoma have been noted using animal model experiments
(16). As an effective antioxidant,
Cimicifugae Rhizoma can protect deoxyribonucleic acid and lipids
against oxidative damage, and its antioxidant ability may be
responsible for its various pharmacological effects (17). Cimicifugae Rhizoma has also been
reported to protect the intestines and hematopoietic organs against
radiation damage (18). A herbal
drug containing multiple medicinal plants, including Cimicifugae
Rhizoma, has demonstrated the ability to decrease bacterial counts
in urine culture (19), and
Cimicifugae Rhizoma has also shown an antiviral effect (20).
There is great interest in stem cells due to their
promising potential for the treatment of diseases and the
regeneration of tissue (21). Stem
cells may be obtained from various tissues, including bone marrow
and adipose tissue (22,23). Moreover, stem cells may be obtained
intraorally, including from dental pulp and periodontal ligaments
(24,25). However, tissue obtained intraorally
may not be easily accessible, and only a limited amount of the
tissue can be obtained in a limited number of procedures. By
contrast, the gingiva is an readily accessible tissue source
(26). Thus, stem cells derived from
gingiva may be useful for the research and treatment of
disease.
Within the limits of the present study, Cimicifugae
Rhizoma influenced the viability of stem cells derived from
gingiva, and its direct application onto oral tissues may produce
adverse effects at high doses. The concentration and application
time of Cimicifugae Rhizoma should be meticulously controlled to
obtain optimal results.
Acknowledgements
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(NRF-2014R1A1A1003106).
References
|
1
|
Kim CM, Shin MK, Lee KS and Ahn DK: The
Encyclopedia of Oriental Herbal Medicine. Jung Dam Publishing Co.;
Seoul: 1998, pp. 3362–3372. 1998
|
|
2
|
Li JX, Kadota S, Li HY, et al: Effects of
Cimicifugae rhizoma on serum calcium and phosphate levels in low
calcium dietary rats and on bone mineral density in ovariectomized
rats. Phytomedicine. 3:379–385. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakurai N and Nagai M: Chemical
constituents of original plants of Cimicifugae rhizoma in Chinese
medicine. Yakugaku Zasshi. 116:850–865. 1996.(In Japanese).
PubMed/NCBI
|
|
4
|
Liao JF, Jan YM, Huang SY, Wang HH, Yu LL
and Chen CF: Evaluation with receptor binding assay on the water
extracts of ten CNS-active Chinese herbal drugs. Proc Natl Sci
Counc Repub China B. 19:151–158. 1995.PubMed/NCBI
|
|
5
|
Kim SJ and Kim MS: Inhibitory effects of
cimicifugae rhizoma extracts on histamine, bradykinin and COX-2
mediated inflammatory actions. Phytother Res. 14:596–600. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwon IH, Kim NK, Chiang HC, Lim HG and Kim
JM: Antipyretic effect of Rhizoma Cimicifugae in a rat model of
LPS-induced fever. Daehan Hanui Hakhoeji. 23:32–44. 2002.(In
Korean).
|
|
7
|
Kim DK, Kim T, Pi SH, et al: Effects of
several natural medicines on alkaline phosphatase synthesis in
MC3T3-E1 cells. J Korean Acad Periodontol. 29:751–764. 1999.
View Article : Google Scholar
|
|
8
|
Wong RW, Hägg U, Samaranayake L, Yuen MK,
Seneviratne CJ and Kao R: Antimicrobial activity of Chinese
medicine herbs against common bacteria in oral biofilm. A pilot
study. Int J Oral Maxillofac Surg. 39:599–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hidaka S, Nishimura H, Nakajima K and Liu
SY: Effects of a rhubarb (Rhei rhizoma) solution and its fractions
on the formation of calcium phosphate precipitates. J Periodontal
Res. 31:408–413. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian Z, Pan R, Chang Q, Si J, Xiao P and
Wu E: Cimicifuga foetida extract inhibits proliferation of
hepatocellular cells via induction of cell cycle arrest and
apoptosis. J Ethnopharmacol. 114:227–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He CC, Dai YQ, Hui RR, et al: NMR-based
metabonomic approach on the toxicological effects of a Cimicifuga
triterpenoid. J Appl Toxicol. 32:88–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu L, Chen JC, Li Y, et al: Studies on the
constituents of Cimicifuga foetida collected in Guizhou Province
and their cytotoxic activities. Chem Pharm Bull (Tokyo).
60:571–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mun L, Jun MS, Kim YM, et al:
7,8-didehydrocimigenol from Cimicifugae rhizoma inhibits
TNF-α-induced VCAM-1 but not ICAM-1expression through upregulation
of PPAR-γ in human endothelial cells. Food Chem Toxicol.
49:166–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li JX and Yu ZY: Cimicifugae Rhizoma: from
origins, bioactive constituents to clinical outcomes. Curr Med
Chem. 13:2927–2951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li JX, Liu J, He CC, et al: Triterpenoids
from Cimicifugae rhizoma, a novel class of inhibitors on bone
resorption and ovariectomy-induced bone loss. Maturitas. 58:59–69.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao L, Sun H, Li Z and Pan RL: Comparison
of activities of various species of Rhizoma Cimicifugae and their
honey processed products. Zhong Yao Cai. 30:1561–1563. 2007.(In
Chinese). PubMed/NCBI
|
|
17
|
Li X, Lin J, Gao Y, Han W and Chen D:
Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem
Cent J. 6:1402012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SH, Lee SE, Oh H, et al: The
radioprotective effects of Bu-Zhong-Yi-Qi-Tang: a prescription of
traditional Chinese medicine. Am J Chin Med. 30:127–137. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishida S: Effect of Hochu-ekki-to on
asymptomatic MRSA bacteriuria. J Infect Chemother. 9:58–61. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan L, Huang YW, Ye YR and Wang YQ: A
model for screening anti-viral agents based on yeast killer system.
Wei Sheng Wu Xue Bao. 47:517–521. 2007.(In Chinese). PubMed/NCBI
|
|
21
|
Sekiya I, Larson BL, Smith JR, Pochampally
R, Cui JG and Prockop DJ: Expansion of human adult stem cells from
bone marrow stroma: conditions that maximize the yields of early
progenitors and evaluate their quality. Stem Cells. 20:530–541.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuznetsov SA, Friedenstein AJ and Robey
PG: Factors required for bone marrow stromal fibroblast colony
formation in vitro. Br J Haematol. 97:561–570. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodriguez AM, Elabd C, Amri EZ, Ailhaud G
and Dani C: The human adipose tissue is a source of multipotent
stem cells. Biochimie. 87:125–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ballini A, De Frenza G, Cantore S, Papa F,
Grano M, Mastrangelo F, Tetè S and Grassi FR: In vitro stem cell
cultures from human dental pulp and periodontal ligament: new
prospects in dentistry. Int J Immunopathol Pharmacol. 20:9–16.
2007.PubMed/NCBI
|
|
25
|
Nagatomo K, Komaki M, Sekiya I, Sakaguchi
Y, Noguchi K, Oda S, Muneta T and Ishikawa I: Stem cell properties
of human periodontal ligament cells. J Periodontal Res. 41:303–310.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y,
Shi S and Le AD: Mesenchymal stem cells derived from human gingiva
are capable of immunomodulatory functions and ameliorate
inflammation-related tissue destruction in experimental colitis. J
Immunol. 183:7787–7798. 2009. View Article : Google Scholar : PubMed/NCBI
|