Introduction
Cardiovascular diseases are common in the older
population, with numerous patients undergoing coronary artery
bypass grafting or cardiac valve replacement (1). Despite improvements in surgical care,
ischemia and reperfusion (IR) during cardiac surgery remains a
major cause of myocardial injury (2,3).
Reperfusion exacerbates the ischemia-induced inflammatory response
(4), thus rendering the protection
of the myocardium from IR injury extremely important.
Ischemic preconditioning (IPC) remains one of the
most effective strategies used to protect the myocardium (5). It uses repeated short periods of
ischemia to activate the myocardium in order for it to become more
resistant to subsequent ischemic insults (6). IPC consists of an early phase, which
starts a few minutes after a short ischemic stimulation and lasts
for 2–4 h, and a late phase, which is usually observed 24 h later
(7).
Cellular kinases, such as protein kinase C (PKC),
play an important role in the cardioprotective effects of IPC
(8). The upregulation of protective
genes is necessary for the development of the late phase of IPC
(9,10). Adenosine (ADO), which is released by
ischemic cells, binds to cardiac receptors (11,12),
triggering signal transductions that ultimately activate PKC and
result in cardioprotection (13,14). On
that basis, a novel concept termed adenosine-enhanced ischemic
preconditioning (APC) was proposed (5) and has been shown to enhance the
cardioprotection achieved by IPC (15); however, little has been reported
regarding the use of APC for myocardial protection in the
elderly.
A more comprehensive understanding of the IPC
mechanisms and its protective phases may enable more effective
interventions in elderly patients. A reduction in the protective
effects of IPC has been observed in the elderly, possibly due to a
decreased capacity of their body for ADO synthesis (11,12),
impaired PKC translocation in response to IPC (13), blunted sensitivity to p38
mitogen-activated protein kinase and heat shock protein 27 and/or
enhanced dephosphorylation of protective proteins (15). In addition, it has been shown that a
loss of ADO-induced cardioprotection is observed in aged hearts due
to an age-related decline in the functionality of ventricular ADO
receptors (ARs) (16). Furthermore,
a previous study suggested that the loss of ADO-induced
cardioprotection is not due to a decreased expression of ARs, but
rather to impaired downstream signaling elements (17).
The Langendorff model for investigating isolated
hearts was introduced in 1895 (18).
This model is reproducible and allows investigators to study the
heart without neurohumoral interference, under controlled
conditions and with direct access to the areas of interest
(19,20); however, this model has been shown to
have disadvantages, such as high coronary flow rate, limited supply
of high-energy phosphate, a reduced work output and oxygen
requirement, and the risk of incorrect and poor experimental
procedure (20,21). An improved version of this model is
required for its use in the modeling of IPC and APC, particularly
during the extraction of the heart, in order to avoid sudden
cardiac arrest and minimize warm ischemic time. Despite its
limitations, however, the Langendorff model has been proven useful
in the study of IR (22).
The present study was conducted with the purpose of
establishing a reliable model of aged rabbit hearts based on the
Langendorff method. Adult and elderly rabbits were subjected to
IPC. ADO levels and AR expression were detected to investigate the
factors that weaken the myocardial protective effect of IPC in
elderly hearts. Different protective approaches, including ADO
enhancement, AR agonist administration and cold crystalloid
cardioplegia, were subsequently tested alone and in combination
with one another. The results of the present study may provide
insights towards the improvement of cardioprotection in the
elderly.
Materials and methods
Animals and ethics
Elderly New Zealand white rabbits (n=64), aged 137±1
weeks and weighing 3.5±0.2 kg, and adult rabbits (n=16), aged 28±1
weeks and weighing 2.8±0.1 kg, were obtained from the SLAC
Laboratory Animal Center Co., Ltd. (Shanghai, China). All rabbits
were housed separately, fed with a standard laboratory diet and
provided with water.
All experiments were approved by the Animal Care and
Use Committee of Fujian Medical University (Fuzhou, China). The
study was performed in accordance with the Principles of Laboratory
Animal Care, proposed by the National Society for Medical Research,
and the Guide for the Care and Use of Laboratory Animals (National
Institutes of Health Publication no. 5377-3, 1996). Surgeries were
performed under anesthesia and analgesic agents were applied
immediately at the end of surgery. All surgeries and associated
tests were performed in a blinded manner.
Establishment of the isolated heart
model and sample collection
Animals were anesthetized with pentobarbital sodium
(30 mg/kg) and heparinized (200 U/Kg) via the marginal ear vein.
During the isolation, hearts were perfused at 4°C with cardioplegic
St. Thomas II (ST) solution (15 ml/kg; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) for 2 min. Following
aortic cannulation and cardiac arrest, the hearts were immersed in
ST solution at 4°C. The hearts were subjected to Langendorff
perfusion at a constant pressure of 60 mmHg (80 cm H2O)
at 37°C. Cardiac function indices, including systemic arterial
pressure (SAP), left ventricular systolic pressure (LVSP), changes
in pressure over time (+dp/dt max and -dp/dt max), heart rate (HR)
and coronary sinus flow (CSF), were monitored using an
ALCB10-MPA myocardial function analyzing system (Alcott
Biotech, Shanghai, China), and well-preserved isolated hearts were
then assigned to groups according to the IR process and the
different protective strategies. Aortic and CSF data were collected
for analyses, and left ventricular apical myocardial tissue was
extracted and frozen for further analysis.
IPC grouping
Sixteen adult and 16 elderly New Zealand white
rabbits were divided into four groups: Adult heart control, adult
heart + IPC, aged heart control and aged heart + IPC (n=8 per
group).
IPC was performed in hearts from rabbits of the IPC
groups. Hearts of the anesthetized aged rabbits were rapidly
excised, transferred to a Langendorff apparatus and perfused via
aortic cannula with an oxygenated Krebs-Henseleit buffer (Nanjing
Jiancheng Bioengineering Institute) containing 118.3 mmol/l NaCl,
2.7 mmol/l KCl, 1.0 mmol/l MgSO4, 1.4 mmol/l
KH2PO4, 29.0 mmol/l NaHCO3, 3.4
mmol/l CaCl2 and 10 mmol/l glucose, with 70 mU/l insulin
and 0.4% bovine serum albumin, at 37°C. Flow was adjusted to
achieve a retrograde perfusion pressure of 60 mmHg. All hearts were
initially perfused with the oxygenated buffer for a 15-min
stabilization period. A subset of the hearts (adult heart + IPC and
aged heart + IPC) was subjected to a preconditioning protocol
consisting of one cycle of global no-flow ischemia (5 min) followed
by normal perfusion (5 min), prior to 120 min of global ischemia,
as previously described (13). The
remaining hearts were not preconditioned but were instead perfused
with a normoxic buffer (Nanjing Jiancheng Bioengineering Institute)
for 10 min, prior to being subjected to 120 min of global ischemia.
Isolated myocardial tissue from the left ventricular apex was
immediately collected for analysis.
ADO production
Myocardial tissue of the left ventricular apex was
extracted from all hearts and immediately preserved in liquid
nitrogen. ADO production in the myocardium was detected using a
high-performance liquid chromatography system (Beckman System Gold;
Beckman Coulter, Beijing, China). ADO standard product was
purchased from Sigma-Aldrich (St. Louis, MO, USA) (23).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the myocardium using
the RNAiso Plus reagent (Takara Bio Inc., Shiga, Japan), and cDNA
was prepared using the Primescript RT Reagent (Takara). An ABI
StepOnePlus™ Real-Time-PCR System (Applied Biosystems; Life
Technologies, Carlsbad, CA, USA) was used with the SYBR® Green
Master Mix (Applied Biosystems), and primers were obtained from the
Beijing Genomics Institute (Shenzhen, China) for the measurement of
the expression of ARs (A1AR, A2AAR and
A3AR), B-cell lymphoma-2 (Bcl-2) and intercellular
adhesion molecule-1 (ICAM-1) (Table
I). PCR cycling conditions were as follows: 2 min
pre-denaturation at 95°C, 40 cycles of 10 sec denaturation at 95°C,
10 sec annealing at 61°C and a 40-sec extension step at 72°C. GAPDH
was used as a reference to obtain the relative fold change for
targets using the comparative Ct method.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Genes | Sequences | Length (bp) |
|---|
| GADPH | Forward:
5′-ACCACAGTCCATGCCATCAC-3′ | 440 |
|
| Reverse:
5′-TCCACCACCCTGTTGCTGTA-3′ |
|
|
A1AR | Forward:
5′-GCTACCACCCCTTGGACATAAC-3′ | 190 |
|
| Reverse:
5′-TGGGCACATCAGCAGACAGG-3′ |
|
|
A2AAR | Forward:
5′-TTCGCCATCACCATCAGCAC-3′ | 177 |
|
| Reverse:
5′-CCTCATACCCGTCACCAAGC-3′ |
|
|
A3AR | Reverse:
5′-AGAACGGTTACCACTCAAAGAAG-3′ | 166 |
|
| Reverse:
5′-AACTGACCACGGAACGGAAG-3′ |
|
| Bcl-2 | Forward:
5′-AGTGGGATACTGGAGATGAAGAC-3′ | 234 |
|
| Reverse:
5′-GACGGTAGCGACGAGAGAAG-3′ |
|
| ICAM-l | Forward:
5′-TGAGAAATTGGCTCCGTGGTC-3′ | 103 |
|
| Reverse:
5′-CCGTGGGAATGAGACACTGAG-3′ |
|
Cardioprotection treatments
Cardioprotection strategies are summarized in
Fig. 1. Forty-eight elderly New
Zealand white rabbits were divided into six groups (n=8 per group).
Following exposure to different interventions, the isolated hearts
were perfused for 15 min to establish stable hemodynamics. The
following six different interventions were undertaken prior to
cardiac arrest for 120 min at 15°C: i) ST group, hearts were
perfused with Langendorff solution for 10 min at 37°C, followed by
protection with ST solution (20 ml/kg) at 4°C. The hearts were
stored in the ST solution at 4°C for 120 min; ii) ADO + ST group,
hearts were subjected to a 10-ml bolus injection of ADO/KOH (1
mmol/l), followed by Langendorff perfusion for 10 min at 37°C and
protection with ST solution (20 ml/kg) at 4°C. The hearts were
stored in the ST solution at 4°C for 120 min; iii) APC + ST group,
hearts were subjected to a 10-ml bolus injection of ADO/KOH (1
mmol/l), followed by 5 min of ischemia and 5 min of reperfusion at
37°C, then protection with ST solution (20 ml/kg) at 4°C. The
hearts were stored in the ST solution at 4°C for 120 min; iv) A1AR
agonist 2-chloro-N(6)-cyclopentyladenosine (CCPA) + ST group,
rabbits received ear vein injections of CCPA (100 µg/kg) at 48 and
24 h prior to surgery. The hearts were subjected to Langendorff
perfusion for 10 min at 37°C followed by protection with ST
solution (20 ml/kg) at 4°C. The hearts were stored in ST solution
at 4°C for 120 min; v) CCPA + ADO + ST group, rabbits received ear
vein injections of CCPA (100 µg/kg) at 48 and 24 h prior to
surgery. Hearts were subjected to a 10-ml bolus injection of
ADO/KOH (1 mmol/l), followed by Langendorff perfusion for 10 min at
37°C and protection with ST solution (20 ml/kg) at 4°C. The hearts
were stored in ST solution at 4°C for 120 min; vi) CCPA + APC + ST
group, rabbits received ear vein injection of CCPA (100 µg/kg) at
48 and 24 h prior to surgery. The hearts were subjected to a 10-ml
bolus injection of ADO/KOH (1 mmol/l), followed by 5 min ischemia
and 5 min reperfusion at 37°C, followed by protection with ST
solution (20 ml/kg) at 4°C. The hearts were stored in ST solution
at 4°C for 120 min.
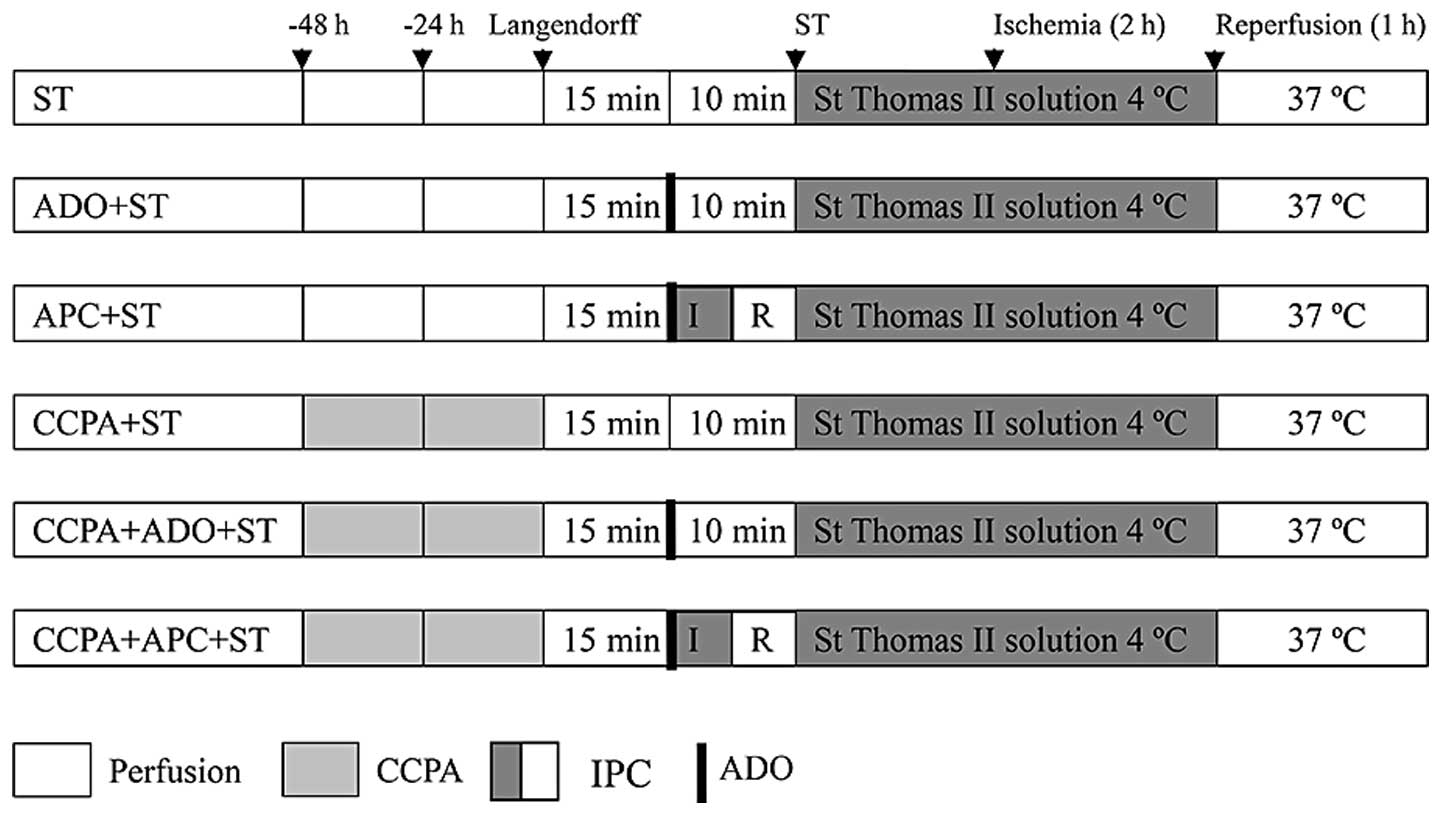 | Figure 1.Different cardioprotection strategies:
ST, ADO + ST, APC + ST, CCPA + ST, CCPA + ADO + ST and CCPA + APC +
ST. CCPA treatment comprised ear vein injections of CCPA (100
µg/kg) twice, at 48 and 24 h before surgery. IPC comprised 5 min of
ischemia and 5 min of reperfusion at 37°C, followed by preservation
with ST solution at 4°C. ADO treatment comprised 10 ml ADO/KOH (1
mmol/l). In the ST group, Langendorff perfusion was performed for
10 min at 37°C, followed by preservation with ST solution at 4°C.
ST, St. Thomas II; ADO, adenosine; CCPA,
2-chloro-N(6)-cyclopentyladenosine; APC, adenosine-enhanced
ischemic preconditioning; IPC, ischemic preconditioning; IR,
ischemia/reperfusion. |
Cardiac function indices
Cardiac function indices, including SAP, LVSP,
+dp/dt, -dp/dt, HR and CSF, were checked before treatment and 30/60
min post-treatment, using the ALCB10-MPA Cardiac
Function Analysis System (Alcott Biotech), according to the
manufacturer's instructions. Post-treatment hemodynamic parameters
were measured by catheterization (SRP-320/PVAN 3.2, Millar
Instruments, Inc., Houston, TX, USA; Chart 5 software,
ADInstruments, Dunedin, New Zealand), as previously described
(24).
Myocardial enzyme leakage
Coronary venous outflow (2 ml) was collected prior
to cardiac arrest and 20 min after reperfusion. The levels of
myocardial enzyme leakage in the preserved liquid were measured in
duplicate using a creatine kinase (CK) and lactate dehydrogenase
(LDH) ELISA testing kit (Nanjing Jiancheng Bioengineering
Institute), according to the manufacturer's instructions.
Endothelial function testing
Coronary venous outflow (2 ml) was collected prior
to cardiac arrest and 30 min after reperfusion. Levels of NO and
endothelin (ET) were detected using NO and ET testing kits (Nanjing
Jiancheng Bioengineering Institute), according to the
manufacturer's instructions.
Malondialdehyde (MDA) and superoxide
dismutase (SOD) detection
Coronary venous outflow (2 ml) was collected prior
to cardiac arrest and 60 min after reperfusion. MDA and SOD levels
were measured using MDA and SOD testing kits (Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
instructions.
Adenosine triphosphate (ATP) in
myocardial tissue
Myocardial tissues of the left ventricular apex were
collected 60 min after reperfusion. ATP levels were detected using
an ATP testing kit (Beyotime Institute of Biotechnology, Shanghai,
China).
Histopathology
Myocardial tissues of the left ventricular apex were
collected and fixed in 4% formalin, dehydrated and embedded in
paraffin. The samples were then sectioned and stained using
standard methods. Samples were observed by microscopy (Olympus
Corp., Tokyo, Japan) at x100 and x400 magnifications.
Electron microscopy
Myocardial tissues of the left ventricular apex were
collected and prepared for electron microscopy, as previously
described (25).
Apoptosis analysis
The apoptotic index of the myocardium was assessed
by the terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling (TUNEL) method using a TUNEL staining kit (Fuzhou Maxim
Biotech, Inc., Fuzhou China), according to the manufacturer's
instructions. Samples were examined under microscopy and stained
cells were counted in six random visual fields.
Statistical analysis
Statistical analysis was performed using SPSS 17.1
software (SPSS Inc., Chicago, IL, USA). Data are presented as the
mean ± standard error. Two-way repeated-measures analysis of
variance was performed to analyze the differences between the
groups at different time-points. P<0.05 was considered to
indicate a statistically significant difference.
Results
Establishment of the improved
Langendorff-perfused isolated heart and myocardial IR injury
models
Isolated heart models were successfully established
with almost no warm ischemic time in New Zealand white rabbits. All
animals had a strong heartbeat during surgery and no cardiac arrest
occurred. The duration between surgery and Langendorff perfusion
ranged from 9 to 10 min, including 4 min of cold ischemia. Cardiac
function was preserved in all isolated hearts, as determined by the
following indices: SAP, 83.98±2.18 mmHg; LVSP, 90.01±2.34 mmHg;
+dp/dt, 1,840.15±52.74 mmHg/sec; -dp/dt, 1,438.75±62.17 mmHg/sec;
HR, 193.94±6.97 bpm and CSF, 60.96±3.09 ml/min. These models were
suitable to fulfill the demands of the following experiments.
Endogenous ADO levels and AR
expression are involved in the protective effect of IPC
The baseline levels of endogenous ADO were found to
be significantly lower in the aged groups than those in the adult
groups (P<0.05, Table II). The
increase in ADO in the aged + IPC group was significantly lower
than that in the adult + IPC group (P<0.05). These findings
indicated that endogenous ADO was associated with the protective
effects of IPC in aged rabbit hearts.
 | Table II.ADO levels and relative expression of
A1AR, A2AR and A3AR mRNA. |
Table II.
ADO levels and relative expression of
A1AR, A2AR and A3AR mRNA.
| Groups | ADO (mg/g) | Fold change after
IPC |
A1AR/GADPH | Fold change after
IPC |
A2AAR/GADPH | Fold change after
IPC |
A3AR/GADPH | Fold change after
IPC |
|---|
| Adult | 0.29±0.03 |
| 1.00 |
| 1.00 |
| 1.00 |
|
| Adult + IPC | 1.23±0.18 | 4.28 |
1.51±0.25a | 1.51 |
0.51±0.06a | 0.51 |
1.68±0.20a | 1.68 |
| Aged |
0.19±0.03a |
|
0.55±0.04a |
|
0.62±0.11a |
|
0.39±0.07a |
|
| Aged + IPC |
0.63±0.04a | 3.33 |
2.40±0.34a–c | 4.36 |
0.23±0.03a–c | 0.37 |
0.73±0.21a–c | 1.87 |
As shown in Table
II, the baseline levels of A1AR, A2AAR
and A3AR were decreased in the aged control group
compared with those in the adult control group (P<0.05);
however, following IPC, the AR expression was regulated in
different manners. The mRNA levels of A1AR and
A3AR increased more significantly in the aged + IPC
group than those in the adult + IPC group (P<0.05). Following
IPC, a 4.36-fold increase was observed in the A1AR and a
1.87-fold increase in the A3AR mRNA levels in the aged
heart tissue, compared with the 1.51-fold increase in the
A1AR mRNA levels and the 1.68-fold increase in the
A3AR mRNA levels in the adult heart tissue. The mRNA
levels of A2AAR were significantly lower in the aged +
IPC group than those in the adult + IPC group (P<0.05).
Cardiac function is preserved using a
combination of CCPA, APC and cold crystalloid cardioplegic solution
in aged rabbit hearts
The results in Table
III show that the baseline values of SAP, LVSP, +dp/dt and
-dp/dt were similar in the CCPA + ST, CCPA + ADO + ST and CCPA +
APC + ST groups (P>0.05). Sixty minutes after IR, the values of
SAP, LVSP, +dp/dt and -dp/dt were improved in the combination
groups, with the most marked change observed in the CCPA + APC + ST
group (P<0.05, Fig. 2A). It was
also found that, 60 min after IR, the HR and CSF were improved in
the combination groups, particularly in the CCPA + APC + ST group,
where the improvement was more marked than that in the other groups
(P<0.05, Fig. 2B and Table III).
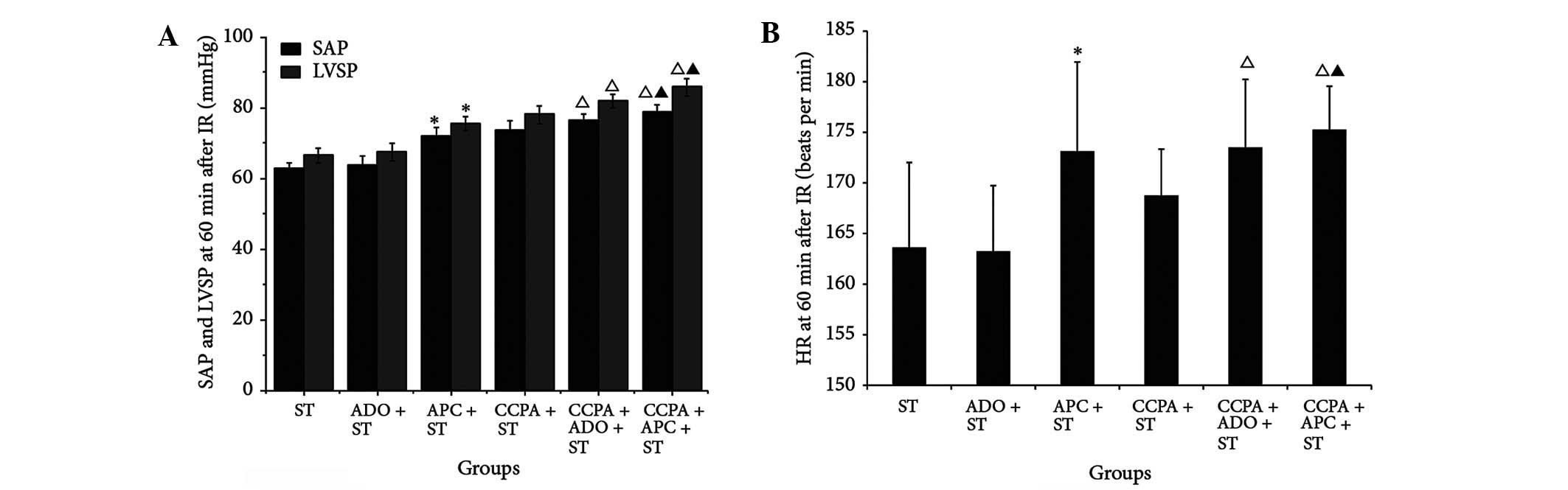 | Figure 2.Cardiac and endothelial functions and
oxidation of elderly rabbit hearts in the ST, ADO + ST, APC + ST,
CCPA + ST, CCPA + ADO + ST and CCPA + APC + ST groups. (A) SAP and
LVSP and (B) HR were detected 60 min after IR. *P<0.05 vs. ST
group; ΔP<0.05 vs. APC + ST group;
▲P<0.05 vs. CCPA + ST group. ST, St. Thomas II
solution; ADO, adenosine; APC, adenosine-enhanced ischemic
preconditioning; CCPA, 2-chloro-N(6)-cyclopentyladenosine; SAP,
systemic arterial pressure; LVSP, left ventricular systolic
pressure; IR, ischemia/reperfusion; ATP, adenosine triphosphate;
HR, heart rate. |
 | Table III.Values of indicators of cardiac and
endothelial function, myocardial enzyme leakage and levels of
reactive oxygen species, SOD and ATP. |
Table III.
Values of indicators of cardiac and
endothelial function, myocardial enzyme leakage and levels of
reactive oxygen species, SOD and ATP.
| Indicators | Time-points | ST | ADO + ST | APC + ST | CCPA + ST | CCPA + ADO +
ST | CCPA + APC +
ST |
|---|
| SAP (mmHg) | Prior to
ischemia | 80.61±1.79 | 81.68±3.14 | 81.60±1.96 |
86.53±2.19a |
86.70±2.12a |
86.77±1.88a |
|
| 30 min after
IR | 68.32±1.97 | 69.08±3.93 |
75.49±2.92a | 76.84±2.01 |
79.92±2.22b |
82.22±1.83b,c |
|
| 60 min after
IR | 63.00±1.33 | 63.53±2.97 |
71.91±2.54a | 73.76±2.50 |
76.37±2.06b |
78.91±1.94b,c |
| LVSP (mmHg) | Prior to
ischemia | 85.01±2.13 | 85.42±2.70 | 86.40±2.44 |
94.45±2.91a |
93.77±1.68a |
95.01±2.15a |
|
| 30 min after
IR | 71.06±2.16 | 71.62±2.09 |
78.31±2.49a | 82.33±1.94 |
84.95±2.21b |
89.83±1.88b,c |
|
| 60 min after
IR | 66.54±2.02 | 67.48±2.40 |
75.56±1.85a | 78.05±2.54 |
81.91±1.95b |
85.74±2.47b,c |
| +dp/dt
(mmHg/sec) | Prior to
ischemia | 1,728.26±58.24 | 1,698.35±65.59 | 1,716.42±38.61 |
1,955.75±62.37a |
1,966.48±40.21a |
1,975.63±51.42a |
|
| 30 min after
IR | 1,341.48±87.34 | 1,356.65±76.28 |
1,537.23±96.54a | 1,662.65±92.28 |
1,747.61±83.61b |
1,826.76±72.78b,c |
|
| 60 min after
IR |
1,292.22±112.31 |
1,285.46±110.21 |
1,502.21±82.25a | 1,577.12±72.62 |
1,706.71±90.27b |
1,782.81±88.35b |
| −dp/dt
(mmHg/sec) | Prior to
ischemia | 1,321.28±65.23 | 1,287.86±80.16 | 1,305.39±58.24 |
1,562.54±73.26a |
1,573.61±52.38a |
1,581.82±43.72a |
|
| 30 min after
IR | 1,030.73±85.38 | 1,004.56±69.52 |
1,156.84±79.63a | 1,324.88±82.35 |
1,385.25±72.19b |
1,457.01±65.84b,c |
|
| 60 min after
IR | 972.73±92.46 | 963.24±88.47 |
1,130.08±75.48a | 1,252.53±69.38 |
1,350.47±57.63b |
1,426.01±80.86b,c |
| HR (beats/min) | Prior to
ischemia | 191.88±10.01 | 194.25±8.33 | 193.00±9.06 | 195.25±4.95 | 194.50±5.68 | 194.75±3.81 |
|
| 30 min after
IR | 179.00±8.96 | 180.25±6.61 | 182.75±8.38 | 183.75±4.74 | 182.25±6.92 | 183.13±4.91 |
|
| 60 min after
IR | 163.63±8.38 | 163.25±6.48 |
173.13±8.82a | 168.75±4.59 |
173.50±6.72a |
175.25±4.30a |
| CSF (ml/min) | Prior to
ischemia | 54.91±3.08 | 55.40±2.28 | 55.15±2.61 |
66.13±3.12a |
66.66±3.62a |
67.48±3.80a |
|
| 30 min after
IR | 41.49±2.05 |
45.81±2.41a |
48.84±2.73a | 54.21±2.80 |
58.70±3.68b |
61.80±3.37b,c |
|
| 60 min after
IR | 32.53±1.89 |
39.36±1.94a |
42.62±2.22a | 47.17±2.67 |
50.95±3.06b |
55.82±3.21b,c |
| CK (U/l) | Prior to
ischemia | 64.88±6.87 | 72.07±8.10 | 67.91±4.60 | 66.11±3.58 | 66.68±4.12 | 67.54±4.17 |
|
| 20 min after
IR | 428.88±24.54 | 411.29±19.69 |
293.20±20.77a |
327.54±8.98a,b |
289.46±11.47a,c |
223.69±10.10a–d |
| LDH (U/l) | Prior to
ischemia | 22.19±3.16 | 22.78±3.58 | 22.82±3.24 | 23.05±3.28 | 23.21±2.97 | 22.47±3.36 |
|
| 20 min after
IR | 90.53±4.65 | 89.57±3.77 |
74.74±4.13a |
80.21±4.95a,b |
75.74±3.99a,c |
60.20±4.04a–d |
| NO (µmol/ml) | Prior to
ischemia | 76.91±3.35 | 80.69±4.40 | 78.44±3.57 |
92.37±2.04a |
94.34±2.50a |
95.06±1.77a |
|
| 30 min after
IR | 29.90±1.13 |
36.04±1.97a |
38.24±2.21a | 41.32±2.79 |
45.54±3.21b |
54.27±3.67b,c |
| ET (ng/ml) | Prior to
ischemia | 31.13±2.76 | 30.04±2.07 | 30.16±2.74 |
24.81±4.28a |
23.93±4.62a |
25.23±5.26a |
|
| 30 min after
IR | 76.56±6.95 |
53.99±3.13a |
48.42±4.63a | 52.09±10.8 |
40.80±9.07b |
32.83±5.77b,c |
| MDA (nmol/ml) | Prior to
ischemia | 8.57±1.90 | 10.03±1.56 | 8.34±2.26 |
5.19±1.43a |
4.81±1.02a |
4.49±1.20a |
|
| 60 min after
IR | 22.48±4.83 | 21.08±2.21 |
16.62±1.97a | 11.57±3.14 | 10.13±2.36 |
6.98±1.61b,c |
| SOD (U/ml) | Prior to
ischemia | 17.58±2.03 | 16.46±2.79 | 17.35±1.93 |
27.46±1.92a |
27.99±2.34a |
28.52±2.32a |
|
| 60 min after
IR | 6.65±1.25 | 6.65±1.13 |
13.72±1.29a | 15.46±2.60 |
21.70±2.92b |
24.98±2.24b |
| ATP (µmol/g) | 60 min after
IR | 1.34±0.21 | 1.44±0.20 |
3.55±0.40a |
2.33±0.26a,b |
3.62±0.36a,c |
4.77±0.47a |
Myocardial samples from the CCPA groups showed an
indefinable distribution of hematoxylin and eosin (H&E) stain.
Myocardial fibers were well preserved, although moderate regional
swelling was observed in the intercellular matrix. In the CCPA +
APC + ST group, the fibers were arranged in neat rows, the cell
size was uniform and the H&E staining was well distributed.
There was no obvious cellular swelling (Fig. 3A).
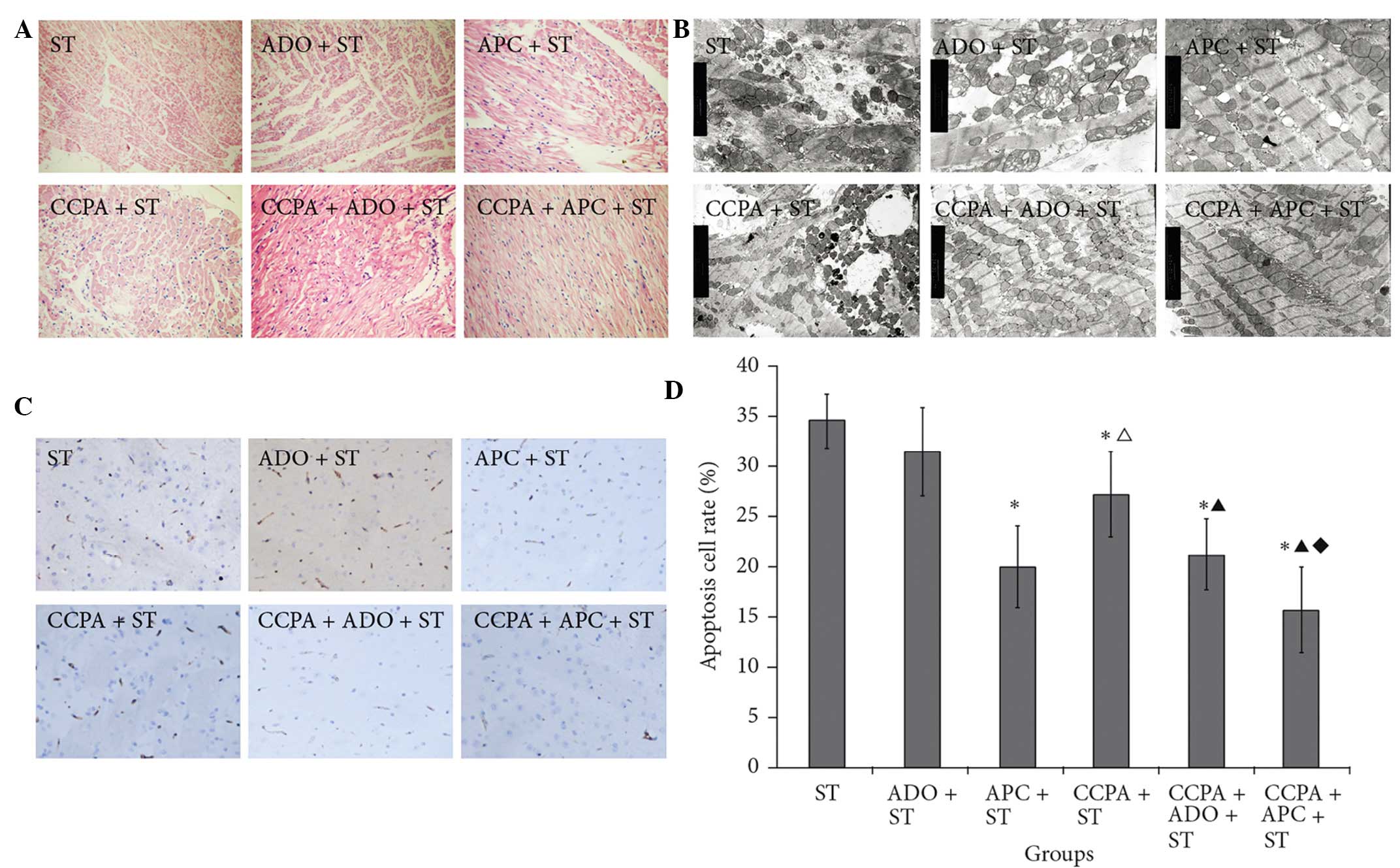 | Figure 3.(A) Hematoxylin and eosin staining
(magnification, x400), (B) ultrastructure (magnification, x8,000)
and (C) terminal deoxynucleotidyl transferase-mediated dUTP nick
end labeling staining (magnification, x400) of elderly rabbit
myocardial fibers in the ST, ADO + ST, APC + ST, CCPA + ST, CCPA +
ADO + ST and CCPA + APC + ST groups. (D) Quantitative data of (C).
*P<0.05 vs. ST group; ΔP<0.05 vs. APC + ST group;
▲P<0.05 vs. CCPA + ST group; ♦P<0.05
vs. CCPA + ADO + ST group. ST, St. Thomas II; ADO, adenosine; APC,
adenosine-enhanced ischemic preconditioning; CCPA,
2-chloro-N(6)-cyclopentyladenosine. |
Transmission electron microscopy revealed that,
following IR, myocardial fibers from the different groups varied in
size and shape and some exhibited mitochondrial hyperplasia. Both
prior to and following IR, the myocardial fibers in the ST group
were abnormal, with clear swelling observed in the cytoplasm and
mitochondria. By contrast, following IR, myocardial fibers from the
CCPA + APC + ST group showed a clear, normal structure and
contained rows of mitochondria without evidence of swelling
(Fig. 3B).
CCPA + APC+ ST treatment prevents
myocardial apoptosis by reducing enzyme leakage, increasing levels
of ATP, Bcl-2 and ICAM-1, improving endothelial function and
protecting the mitochondrial function of the myocardium
As shown in Fig. 3C,
significantly fewer apoptotic cells were found in the treatment
groups, with the exception of the ADO + ST group, compared with the
ST group (P<0.05). No significant difference in the apoptotic
index was observed between the APC + ST and CCPA + ADO + ST groups;
however, the apoptotic index was considerably lower in the CCPA +
APC + ST group compared with the other groups (P<0.05),
suggesting that both CCPA and APC protected myocardial cells from
IR-induced injury, and therefore supporting the combined use of
these two agents.
The levels of Bcl-2, ICAM-1, CK and LDH, indicators
of cardiac injury or inflammation, were subsequently investigated.
As shown in Table IV, the
expression of Bcl-2, which is a factor that protects cells from
apoptosis, was increased in all treatment groups, with the
exception of the ADO + ST group, compared with that in the ST group
(P<0.05). The levels of ICAM-1, a factor associated with the
induction of inflammation, were also decreased in the treatment
groups, with the exception of the ADO + ST group, compared with the
ST group (P<0.05). The expression of Bcl-2 in the CCPA + APC +
ST group was considerably higher than that in any of the other
groups (P<0.05) and the ICAM-1 levels were significantly lower
than those in any of the other groups (P<0.05).
 | Table IV.Relative mRNA expression of Bcl-2 and
ICAM-1. |
Table IV.
Relative mRNA expression of Bcl-2 and
ICAM-1.
| Groups | Bcl-2/GADPH | ICAM-1/GADPH |
|---|
| ST | 1.00 | 1.00 |
| ADO + ST | 1.01±0.01 | 1.03±0.04 |
| APC + ST |
1.42±0.07a |
0.70±0.03a |
| CCPA + ST |
1.16±0.02a,b |
0.85±0.02a,b |
| CCPA + ADO +
ST |
1.40±0.05a,c |
0.72±0.05a,c |
| CCPA + APC +
ST |
2.59±0.07a–d |
0.47±0.07a–d |
CK and LDH are important indicators of myocardial
injury. In order to assess the extent of IR injury, coronary venous
outflow was collected prior to and 20 min after IR. As shown in
Table III, no differences were
identified among the groups in the levels of CK and LDH prior to IR
(P>0.05); however, 20 min after IR, the CK and LDH leakage was
significantly increased in each of the studied groups (P<0.05),
but the lowest value was observed in the CCPA + APC + ST group
(P<0.05, Fig. 4A and Table III).
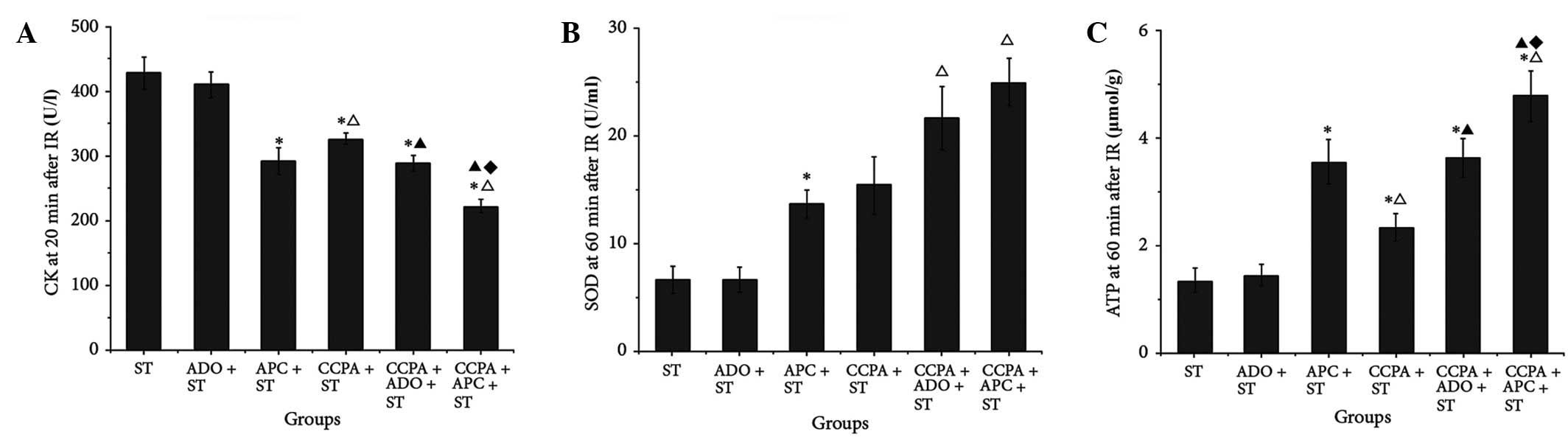 | Figure 4.Cardiac and endothelial functions and
oxidation of elderly rabbit hearts in the ST, ADO + ST, APC + ST,
CCPA + ST, CCPA + ADO + ST and CCPA + APC + ST groups. (A) CK was
detected 20 min after IR; (B) SOD and (C) ATP were detected 60 min
after IR. *P<0.05 vs. ST group; ΔP<0.05 vs. APC +
ST group; ▲P<0.05 vs. CCPA + ST group;
♦P<0.05 vs. CCPA + ADO + ST group. ST, St. Thomas II
solution; ADO, adenosine; APC, adenosine-enhanced ischemic
preconditioning; CCPA, 2-chloro-N(6)-cyclopentyladenosine; IR,
ischemia/reperfusion; CK, creatine kinase; SOD, superoxide
dismitase; ATP, adenosine triphosphate. |
NO and ET levels are indicators of endothelial
function. As shown in Table III,
prior to ischemia, NO levels were higher in the CCPA-treated groups
than those in the other groups. Following IR, NO production was
significantly reduced, but the best recovery was observed in the
CCPA + APC + ST group (P<0.05). Changes in ET levels showed an
inverse association; CCPA + APC + ST inhibited the abnormal
increase in ET production following IR.
MDA, SOD and ATP, as indicators of oxidative status,
were measured prior to and following IR. The results shown in
Table III revealed that the
mitochondrial function of the myocardium was the most effectively
protected, and the levels of reactive oxygen species (ROS) were the
lowest, in the CCPA + APC + ST group (P<0.05, Fig. 4B and C).
Discussion
It has been previously shown that IPC renders the
myocardium resistant to subsequent ischemic insult (14). Although IPC has a beneficial effect
on IR (15), the most suitable
approaches for specific populations remain controversial. In the
present study, a modified model of aged and adult rabbit hearts was
established based on the Langendorff model, in order to explore the
myocardial protective role of ADO and ARs. The protective effect of
APC in combination with pharmacological preconditioning and cold
crystalloid cardioplegia solution was also studied. The results
showed that a combination approach was more effective in preserving
cardiac function, preventing myocardial apoptosis and reducing ROS
levels in aged hearts compared with single intervention
approaches.
The isolated retrograde-perfused Langendorff heart
has previously been shown to be invaluable in studying the
pharmacological effects of different agents on myocardial function
and disease states such as IR injury (26). To successfully establish an isolated
heart model, it is necessary to avoid sudden cardiopulmonary
arrest, provide adequate time for myocardial preservation and cold
ischemia and ensure that an accurate surgical technique is being
used. These aims were achieved in the present study and an improved
isolated rabbit heart model with almost no warm ischemic time and
an optimal protection of cardiac function during cold ischemia was
established. This improved model could provide better insight into
the study of IR injury and its prevention. Traditional isolated
heart models have several limitations, such as a high incidence of
pneumothorax, damage to the left atrium and absence of a protective
strategy during myocardial preservation and cold ischemia, which
render the isolated heart models relatively unstable with high
failure rates. In the present study, several improvements were
applied to the preparation of the isolated heart model. First,
pentobarbital sodium was administered following the administration
of heparin in an attempt to avoid sudden cardiopulmonary arrest. A
medical ventilator was used to limit the impact of pneumothorax on
respiratory and circulatory function. In order to ensure zero warm
ischemic time, exogenous protection using ST solution maintained at
4°C was applied during the surgical and isolation processes. In
addition, the KOH and ST solutions were maintained within standard
ranges and at moderate constant pressure to avoid heart failure.
Other improvements included the full drainage of the left and right
ventricles prior to perfusion, in order to prevent heart injury due
to filling, and the non-conventional placement path of the
perfusion and piezometric tubes. These improvements to the standard
technique provided a stable, practical and easily reproducible
model, which allowed the measurement of cardiac function indices,
such as SAP, LVSP, +dp/dt, -dp/dt, HR and CSF. These parameters
were well preserved in all hearts included in the present study and
allowed a comprehensive assessment of the subsequent IR, IPC and
APC processes.
The AR family includes four subtypes, among which
A1AR, A2AAR and A3AR are closely
associated with cardiac function (27,28).
Studies using IR models have shown that A1AR activation
significantly decreases myocardial infarct size and enhances
post-ischemic functional recovery (5,15). In
the present study, differential regulation of A1AR,
A2AAR and A3AR expression was observed in
adult and elderly isolated rabbit heart models prior to and
following IPC. These data revealed that factors other than the
expression of ARs, such as downstream effectors of ARs, may have
important effects on heart protection; these require further
study.
CCPA is a highly selective A1AR agonist
that has been shown to induce the late-phase protective effect of
IPC (29). APC extends the
cardioprotection of IPC by interacting with ARs, primarily during
IR (30). Other studies advocate the
use of pharmacological preconditioning to reduce IR injury and
increase the time available for effective reperfusion (7,9,31,32). In
the present study, following the administration of a high dose of
CCPA in combination with APC, fully activated A1AR
significantly improved the protective effect of APC in aged
myocardial tissue. ST solution is a classical exogenous method used
to protect the myocardium. A further combination of APC with CCPA
and ST solution had the highest performance in cardioprotection
compared with all other tested approaches, indicating that the
early- and late-phase endogenous protection combined with exogenous
protection could be used simultaneously and result in an additive
effect in aged hearts.
The combination treatment also enabled endothelial
cells to produce more NO, thereby reducing harmful ET, preserving
the function of the myocardium and stabilizing HR. In addition, the
apoptotic index, the function and number of mitochondria, the ATP
levels and the expression of Bcl-2, ICAM-1, CK and LDH were all
maintained in a more favorable range in the CCPA + APC + ST group
compared with the ranges observed in the other groups.
The present study was limited, as it was mostly
observational, and further studies are required to assess the exact
molecular mechanisms involved in IPC and APC, as well as the exact
differences between adult and aged hearts.
In conclusion, the findings of the present study
suggest that the simultaneous combination of endogenous and
exogenous protective methods could prove beneficial to the
protection of aged myocardium from IR injury; therefore, the
present study could provide a basis for clinical application in
elderly patients during surgery.
References
|
1
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al:
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics − 2013
update: A report from the American Heart Association. Circulation.
127:e6–e245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu M, Zhang P, Chen M, et al: Aging might
increase myocardial ischemia/reperfusion-induced apoptosis in
humans and rats. Age (Dordr). 34:621–632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marczak J, Nowicki R, Kulbacka J and
Saczko J: Is remote ischaemic preconditioning of benefit to
patients undergoing cardiac surgery? Interact Cardiovasc Thorac
Surg. 14:634–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han S, Huang W, Liu Y, Pan S, Feng Z and
Li S: Does leukocyte-depleted blood cardioplegia reduce myocardial
reperfusion injury in cardiac surgery? A systematic review and
meta-analysis. Perfusion. 28:474–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yellon DM and Downey JM: Preconditioning
the myocardium: From cellular physiology to clinical cardiology.
Physiol Rev. 83:1113–1151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu Y, Tang XL, Park SW, Sun JZ, Kalya A
and Bolli R: The early and late phases of ischemic preconditioning:
A comparative analysis of their effects on infarct size, myocardial
stunning and arrhythmias in conscious pigs undergoing a 40-minute
coronary occlusion. Circ Res. 80:730–742. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Xiao Z, Yao J, Zhao G, Fa X and
Niu J: Participation of protein kinase C in the activation of Nrf2
signaling by ischemic preconditioning in the isolated rabbit heart.
Mol Cell Biochem. 372:169–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z and
Bolli R: Demonstration of an early and a late phase of ischemic
preconditioning in mice. Am J Physiol. 275:H1375–H1387.
1998.PubMed/NCBI
|
|
10
|
Bolli R: Cardioprotective function of
inducible nitric oxide synthase and role of nitric oxide in
myocardial ischemia and preconditioning: An overview of a decade of
research. J Mol Cell Cardiol. 33:1897–1918. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abete P, Cacciatore F, Testa G, DellaMorte
D, Galizia G, Ferrara N and Rengo F: Clinical application of
ischemic preconditioning in the elderly. Dose Response. 8:34–40.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dawson R Jr and Meldrum MJ: Norepinephrine
content in cardiovascular tissues from the aged Fischer 344 rat.
Gerontology. 38:185–191. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tani M, Honma Y, Hasegawa H and Tamaki K:
Direct activation of mitochondrial K (ATP) channels mimics
preconditioning but protein kinase C activation is less effective
in middle-aged rat hearts. Cardiovasc Res. 49:56–68. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sadigh B, Quintana M, Sylvén C, Berglund M
and Brodin LA: The ischemic preconditioning effect of adenosine in
patients with ischemic heart disease. Cardiovasc Ultrasound.
7:522009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCully JD, Toyoda Y, Uematsu M, Stewart
RD and Levitsky S: Adenosine-enhanced ischemic preconditioning:
Adenosine receptor involvement during ischemia and reperfusion. Am
J Physiol Heart Circ Physiol. 280:H591–H602. 2001.PubMed/NCBI
|
|
16
|
Schulman D, Latchman DS and Yellon DM:
Effect of aging on the ability of preconditioning to protect rat
hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ
Physiol. 281:H1630–H1636. 2001.PubMed/NCBI
|
|
17
|
Boengler K, Schulz R and Heusch G: Loss of
cardioprotection with ageing. Cardiovasc Res. 83:247–261. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langendorff O: Untersuchungen am
uberlebenden Saugethierherzen. Pflugers Archives fur die Gesamte
Physiologie des Menschen and der Tiere. 61:291–332. 1895.(In
German). View Article : Google Scholar
|
|
19
|
Suzuki Y, Yeung AC and Ikeno F: The
pre-clinical animal model in the translational research of
interventional cardiology. JACC Cardiovasc Interv. 2:373–383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
SkrzypiecSpring M, Grotthus B, Szelag A
and Schulz R: Isolated heart perfusion according to
Langendorff-still viable in the new millennium. J Pharmacol Toxicol
Methods. 55:113–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chorro FJ, SuchBelenguer L and
López-Merino V: Animal models of cardiovascular disease. Rev Esp
Cardiol. 62:69–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bell RM, Mocanu MM and Yellon DM:
Retrograde heart perfusion: The Langendorff technique of isolated
heart perfusion. J Mol Cell Cardiol. 50:940–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hancu G, Simon B, Kelemen H, Rusu A,
Mircia E and Gyeresi A: Thin layer chromatographic analysis of
Beta-lactam antibiotics. Adv Pharm Bull. 3:367–371. 2013.PubMed/NCBI
|
|
24
|
Kaneko M, Shintani Y, Narita T, et al:
Extracellular high mobility group box 1 plays a role in the effect
of bone marrow mononuclear cell transplantation for heart failure.
PLoS One. 8:e769082013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bertazzo S, Gentleman E, Cloyd KL, Chester
AH, Yacoub MH and Stevens MM: Nano-analytical electron microscopy
reveals fundamental insights into human cardiovascular tissue
calcification. Nat Mater. 12:576–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao R, Podesser BK and Lim CC: The
continuing evolution of the Langendorff and ejecting murine heart:
New advances in cardiac phenotyping. Am J Physiol Heart Circ
Physiol. 303:H156–H167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fredholm BB, Abbracchio MP, Burnstock G,
et al: Nomenclature and classification of purinoceptors. Pharmacol
Rev. 46:143–156. 1994.PubMed/NCBI
|
|
28
|
VintenJohansen J, Thourani VH, Ronson RS,
et al: Broad-spectrum cardioprotection with adenosine. Ann Thorac
Surg. 68:1942–1948. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lasley RD, Keith BJ, Kristo G, Yoshimura Y
and Mentzer RM Jr: Delayed adenosine A1 receptor preconditioning in
rat myocardium is MAPK dependent but iNOS independent. Am J Physiol
Heart Circ Physiol. 289:H785–791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomai F, Crea F, Chiariello L and Gioffrè
PA: Ischemic preconditioning in humans: Models, mediators and
clinical relevance. Circulation. 100:559–563. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yun N, Kim SH and Lee SM: Differential
consequences of protein kinase C activation during early and late
hepatic ischemic preconditioning. J Physiol Sci. 62:199–209. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naderi R, Imani A, Faghihi M and Moghimian
M: Phenylephrine induces early and late cardioprotection through
mitochondrial permeability transition pore in the isolated rat
heart. J Surg Res. 164:e37–e42. 2010. View Article : Google Scholar : PubMed/NCBI
|


















