Introduction
Atrial fibrillation (AF) is a common complication
following cardiac operations that typically occurs within the first
week following open-heart surgery, such as coronary artery bypass
grafting (CABG) or heart valve surgery (1–3).
Postoperative AF (POAF) is frequently considered to be a temporary
problem; however, this complication results in significant adverse
effects. POAF increases the risk of a cerebrovascular accident,
extending the duration of hospital stay and the requirement for
intensive care (4–6).
According to various studies, the risk factors
associated with the development of POAF include right atrial
manipulation, prolonged preoperative atrial conduction duration,
atrial myocardial ischemia, prolonged aortic cross-clamping time
and advanced age (7–9). Although nearly all patients present at
least one of the aforementioned risk factors, ~60% of patients
undergoing cardiac surgery do not develop AF; thus, only certain
patients seem to possess an inherent or acquired preoperative risk
(6,10). Therefore, these factors suggest that
the major determinants of the risk for the development of POAF
include the patient's preoperative status and condition of the
atria, as well as the ischemia and stress induced by the surgical
procedure.
The aim of the present study was to identify the
markers of increased vulnerability in developing AF subsequent to
CABG or heart valve surgery. The aim of the present study was to
investigate the association among clinical (systemic hypertension),
echocardiographic features (ejection fraction, left atrial dilation
and pulmonary hypertension) and histological atrial lesions (cell
atrophy, cell hypertrophy, interstitial fibrosis, myocytolysis,
pericardial adiposity and inflammation) and the occurrence of
POAF.
Materials and methods
Ethical approval
Ethical approval for the experiments conducted in
the present study was obtained from the Institutional Board of
‘Prof. Dr. George I.M. Georgescu’, Institute of Cardiovascular
Diseases (Iasi, Romania). All patients provided written informed
consent.
Patient population
Between January 2010 and December 2011 a total of
103 selected patients underwent cardiac surgical procedures
requiring cardiopulmonary bypass (CPB) at the ‘Prof. Dr. George
I.M. Georgescu’ Institute of Cardiovascular Diseases. The patient
population included 76 men and 27 women, with an age range of 42–77
years and a mean age of 61.8 years.
Cardiac assessment
All patients had undergone preoperative
electrocardiography (SE 12; Edan Instruments, Inc., Nanshan, China)
for selecting known cardiac patients in sinus rhythm and
transthoracic echocardiography (Vivid E9 Electrocardiograph; GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA) was used to measure
left atrial size along the anteroposterior diameter, left
ventricular function and right atrial pressure
Sample collection
Right atrial appendage (RAA) tissue samples were
collected from 103 patients in normal sinus rhythm, who were
subjected to CABG (82 patients; 79.61%) or heart valve surgery (21
patients; 20.38%), prior to CPB. Right atrial appendage specimens
were collected after opening of the pericardium, prior to
cannulation of the right atrium. The age of the patients ranged
between 42 and 77 years (mean age, 61.8 years), and 76 patients
were females (73.8%).
Histological and morphological
examinations
Formalin-fixed atrial tissue was processed for
paraffin embedding and the samples were cut into 4–6 µm sections.
Next, histological sections were stained with hematoxylin and eosin
and collagen-specific sirius red (Bio Optica Milano SpA, Milan,
Italy). To highlight the cardiomyocyte (CM) nuclei, the sections
stained with sirius red were re-stained with hematoxylin.
The specimens were examined histologically by a
trained pathologist who was blinded to the patient characteristics
and AF occurrence. Since atrial tissue analysis is not a routine
procedure for pathologists, a previously-developed standard
protocol was used for the examination of the RAA, as described by
Ad et al (9) (Table I). The standard protocol was modified
by adding the endocardial analysis step.
 | Table I.Modified protocol for routine
histopathological examination of right atrial appendage
specimens. |
Table I.
Modified protocol for routine
histopathological examination of right atrial appendage
specimens.
| A, Endocardium |
|
|---|
|
|---|
| Features
investigated | Possible
observations |
|---|
| Endocardial
fibrosis | Absent/Present |
| Mononuclear
exudates | Absent/Present |
|
| B,
Myocardium/myocytes |
|
|
| Features
investigated | Possible
observations |
|
| Myolytic
vacuolation | Absent/Present |
| Size of
vacuole | Mild/Severe |
|
Frequency | 25% or >25% |
| Cell hypertrophy | Absent/Present |
| Cell atrophy | Absent/Present |
| Lipofuscin | Absent/Present |
| Abnormal nuclei | Apoptotic figure |
|
| C, Interstitial
myocardium |
|
|
| Features
investigated | Possible
observations |
|
| Interstitial
fibrosis | Absent/Present |
|
Amount | Mild/Severe |
|
Frequency | 25% or >25% |
| Perivascular
fibrosis | Absent/Present |
|
| D, Pericardium |
|
|
| Features
investigated | Possible
observations |
|
| Mononuclear
exudate | Absent/Present |
| Pericardial
adiposity | Absent/Present |
| Pericardial
fibrosis | Absent/Present |
An Olympus BX40 light microscope (Olympus
Corporation, Tokyo, Japan) was used for morphological evaluation.
Histologically, the CM examination included analysis of the degree
of myocytolysis, the existence of atrophy and other degenerative
cell lesions. In the atrial interstitium, the degree of fibrosis
was assessed. Furthermore, in the pericardium, the presence of
mononuclear or fibrinous exudates, adiposity and fibrosis was
investigated. Fibrosis was also evaluated at the endocardium.
Semi-quantitative scales were used to evaluate the pathology of
connective tissue components and atrial myocytes, as previously
described by Ad et al (8,9). Various
degrees of lesions were identified and compared between the POAF
and POSR groups.
Morphometric assessment
Atrial myocytes included the degree of vacuolization
from loss of myofibrils. Vacuolization was scored as 0 or 1 (0,
absent; 1, observed at any rate). The existence of hypertrophy and
atrophy were rated between 0 and 1 as percentage of the number of
hypertrophic or atrophic cells reported to total nucleated cell
number, by assessing 10 high power field (HPF) sections from each
group. Myocyte nuclear derangement encountered an evaluation of
apoptotic pyknotic figures rated between 0 and 1 as percentage of
the number of myolytic cells reported to total nucleated cell
number, by assessing 10 HPF sections from each group. The analysis
of connective tissue components focused on fibrosis, rated between
0 and 1 as percentage of the fibrous interstitial area observed in
the studied histological section area by assessing 10 HPF sections
per group, as described by Ad et al (9).
POAF and risk factors
POAF was identified on electrocardiogram in 37/103
patients (35.9%), and it occurred between 12 and 144 h after
surgery (mean value, 45.1 h). Following drug therapy, all patients
affected by AF subsequently regained sinus rhythm prior to patient
hospital discharge.
Monitoring
For post-operative AF detection, all patients were
monitored daily until hospital discharge with standard 12-lead
electrocardiography. Only AF episodes of >15 min duration were
considered. Patients were diagnosed with POAF if interventional
therapy (drug administration or electrical cardioversion) was
required in order to restore the sinus rhythm. The majority of
patients with POAF responded well to drug therapy, which included
disopyramide, amiodarone and sotalol. In rare cases, including
elderly or heart failure patients, electrical cardioversion was
required to achieve sinus rhythm. Hospitalization durations of
>14 days were required for all patients (mean, 19.93 days;
range, 14–31 days).
Statistical analysis
The data were analyzed using the Excel software
(Microsoft Corporation, Redmond, WA, USA). Pearson's χ2
test was used for categorical variables. A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
POAF and risk factors
POAF was identified using an electrocardiogram in
37/103 patients (35.92%), and it occurred between 12 and 144 h
after surgery (mean, 45.1 h). Following drug therapy, all patients
affected by AF subsequently regained sinus rhythm prior to patient
hospital discharge. The mean age of the patients with POAF (61.7
years) was higher compared with that of the patients remaining in
sinus rhythm (SR) subsequent to cardiac surgery (58.7 years). In
addition, only 42.5% of the patients with a postoperative sinus
rhythm (POSR) were >60-years-old compared with 75.6% of patients
with POAF. Thus, the results indicate that the risk of POAF is
higher in patients with an age of >60 years (P<0.001).
Several risk factors associated with the occurrence
of POAF in cardiac surgery were analyzed, as shown in Table II. In the univariate analysis, the
only independent clinical predictors of POAF risk were as follows:
Age, >60 years; gender, male; ejection fraction, <50%;
increased pulmonary hypertension; interstitial fibrosis;
myocytolysis; cell hypertrophy; and pericardial adiposity
(P<0.001 for all factors) (Table
II).
 | Table II.Risk factors associated with normal
POSR and POAF. |
Table II.
Risk factors associated with normal
POSR and POAF.
| Variable | POSR (%) | POAF (%) | P-value |
|---|
| Age of >60
years | 42.5 | 75.6 | P<0.001 |
| Male gender | 13.63 | 51.35 | P<0.001 |
| Ejection fraction
of <50% | 1.51 | 75.67 | P<0.001 |
| Left atrial
dilatation of >44 mm | 50 | 56.75 | NS |
| Pulmonary
hypertension | 1.51 | 18.91 | P<0.001 |
| Systemic
hypertension | 77.27 | 72.97 | NS |
| Fibrinogen | 25.75 | 64.86 | NS |
| Hospital stay of
>14 days | 39.39 | 29.72 | NS |
| Cell
hypertrophy | 31.81 | 98.9 | P<0.001 |
| Cell atrophy | 56.06 | 40.54 | NS |
| Interstitial
fibrosis | 31.81 | 97.75 | P<0.001 |
| Myocytolysis | 38.84 | 91.89 | P<0.001 |
| Abnormal
nuclei | 30.30 | 91.89 | P<0.01 |
| Pericardial
adiposity | 27.27 | 75.67 | P<0.001 |
| Pericardial
inflammatory infiltrate | 27.27 | 33.33 | NS |
| Endocardial
fibrosis | 15.2 | 20.3 | NS |
Left atrial size, measured by echocardiography,
exceeded the upper limit (range, 25–44 mm) in 56.75% of patients
with POAF and 50% of patients with POSR. However, no correlation
was identified between POAF and atrial dimensions (P>0.05).
Histopathological examination
results
Upon histopathological examination, mild to severe
myocytolysis was detected in the majority of the specimens,
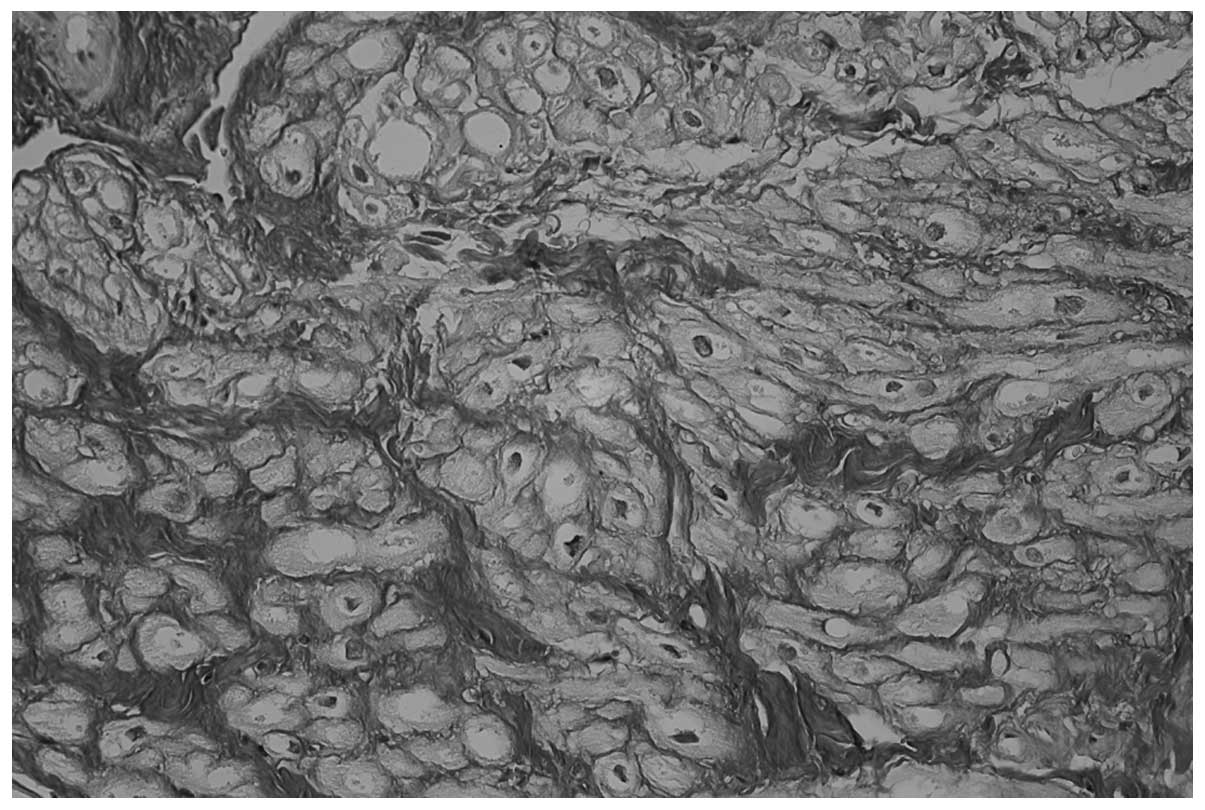
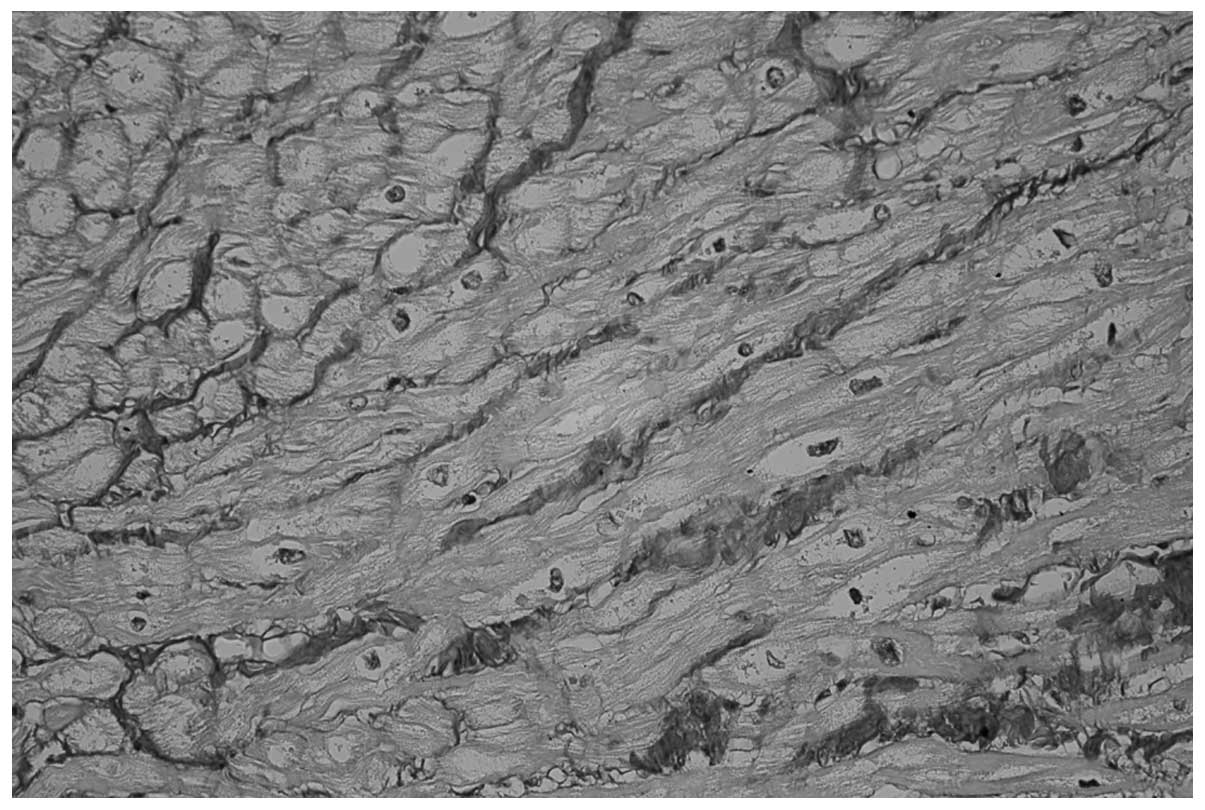
including 34/37 patients in the POAF group (91.8%; Fig. 1) and 25/66 patients in the POSR group
(38.8%; P<0.0001; Fig. 2). In
addition, CM hypertrophy was identified in 98.9 and 31.81% of
patients in the POAF and POSR groups, respectively (P<0.0001).
CM atrophy was detected in 40.5% of patients in the POAF group and
56.0% in the POSR group. In the POAF group, the percentage of
abnormal nuclei in each specimen was higher compared with that in
the POSR group (91.8 vs. 30.3%, respectively; P<0.01). CMs with
contraction band necrosis were rare findings in both groups, and
were associated with ongoing IF only in the POAF patients.
Several histopathological abnormalities were
encountered in the atrial interstitium of the two groups, but only
IF exhibited a statistically significant difference. IF was
detected in 97.7% of patients in the POAF group and 31.8% of
patients in the POSR group (P<0.0001; Figs. 1 and 2, respectively). Furthermore, no
interstitial inflammatory infiltrate was observed, but a
statistically significant difference in pericardial adiposity was
detected between the two groups (76.7% in POAF patients and 27.2%
in POSR patients; P<0.0001). A limited number of pericardial
inflammatory foci, associated with localized pericardial fibrosis
were observed in the POAF (33.3%) and POSR (27.2%) groups
(P>0.05). The presence of pericardial inflammatory foci in both
groups may indicate an ongoing healing process, associated with
previous pericardial lesions. Endocardial fibrosis exhibited a
focal and mild extension in the POAF (20.3%) and POSR (15.2%)
groups (P>0.05), which may be associated by the connective
organization of small parietal thrombi.
Discussion
POAF is one of the most common causes of morbidity
following cardiac surgery (10,11).
Although AF is a common postoperative complication, the incidence
of POAF in patients undergoing cardiac surgery is unclear.
Previously-reported incidence rates are between 10 and 65%. In
1996, Mathew et al (10)
reported an overall postoperative AFIB incidence of 27%, while in
2001 Maisel et al (11)
estimated that POAF occurs in 10–65% of patients following cardiac
surgery. This is a wide range since the definition of AF, detection
methods, baseline patient characteristics and surgery type differ
in previous studies (10). Maisel
et al (11) estimated that
POAF is ~30% following standard CABG surgery and 40% subsequent to
valve repairs or replacements, increasing to ~50% after combined
procedures.
Identifying the patients at a risk of developing
POAF subsequent to cardiac surgery would result in the reduction of
the incidence of POAF, as well as the prevention of undesired
clinical consequences associated with this complication (4). Several studies have reported various
risk factors for AF development following open-heart surgery
(3,4). Therefore, in addition to old age,
numerous other risk factors have been identified by Almassi et
al (3), such as chronic
obstructive pulmonary disease, use of digoxin within 2 weeks prior
to surgery, low resting pulse rate (<80 bpm), high resting
systolic blood pressure (>120 bpm) and use of inotropic agents
for >30 min following the termination of CPB. In addition,
Banach et al (12) identified
further risk factors, including history of supraventricular
arrhythmias, preoperative heart failure, operation with standard
CABG technique and repeated revascularization. The common risk
factors associated with POAF investigated in the present study,
which were consisted with Aranki et al (5), were increasing age, male gender and
hypertension.
The patient's age is the most common risk factor
identified by previous studies. For instance, Hogue and Hyder
(1) observed that in addition to
age, valvular heart operation is the most consistently identified
risk factor for cardiac arrhythmia. Similar to the present results
(age, ≥60 years; P<0.001), Zaman et al (6) found that advanced age is markedly
associated with postoperative AF (65.9 vs. 61.7 years;
P<0.0005). The frequency of this arrhythmia is increasing,
possibly due to the rising proportions of elderly patients
undergoing cardiac surgery. In the present study, the low incidence
of POAF (35.92%) may be associated with the relatively young mean
age of the patients included in the present study (61.8 years).
Kitzman and Edwards (13) reported that fibrosis and atrophy in
the atria, which are typical in older patients, as well as left
atrial dilation, contribute to the susceptibility to develop POAF.
However, the association between these features and AF occurring
subsequent to cardiac surgery has not received considerable
attention. Similar to other authors (14,15), in
the present study, we hypothesized that in the atrial myocardium of
aged patients, atrophy and fibrosis may decrease the conductive
tissue.
Data in the literature suggest that the left atrial
size is an important factor in AF development (16–18). For
instance, Henry et al (17)
observed that AF is rare (3%) when the left atrial size is <44
mm, but common (54%) when the size is >44 mm. In addition, Ausma
et al (18) noted that the AF
risk increases by 1.4 times per 5-mm increase in left atrial size.
According to Li et al (19),
atrial enlargement due to structural remodeling is a particularly
important determinant of the occurrence of multiple-circuit
reentry.
In the current study, histological lesions were
observed in the majority of specimens, with generally higher values
in POAF patients (hypertrophy, 98.9%; interstitial fibrosis, 97.8%;
myocytolysis, 91.8%; abnormal nuclei, 91.8%; pericardial adiposity,
75,6%) compared with those in POSR patients (hypertrophy, 31.8%;
interstitial fibrosis, 31.8%; myocytolysis, 38.8%; abnormal nuclei,
30.3%; pericardial adiposity, 27.2%). Consistent with the results
of Li et al (19), the
present results indicate that interstitial fibrosis is a
significant risk factor for POAF development, having values ~3
times higher in POAF patients (97.7%) compared with POSR patients
(31.8%). Usually, interstitial fibrosis is an expression of cardiac
remodeling associated with various causes, such as chronic
ischemia. In agreement with Boldt et al (20), we propose that fibrosis may explain
the tissue anisotropy that results in inhomogeneous conduction, and
may be responsible for the slow conduction and reentry that
stabilizes AF.
A notable finding of the present study was the
increased CM vacuolation observed in patients with POAF (91.8%).
Myocytolysis is a reversible, vacuolar degeneration of myocytes.
Myocytolytic CMs are viable cells with a reduced function, due to
loss of myofibrils. In addition, lesions may be associated with
chronic ischemia. According to the results of Kitzman and Edwards
(13) and Pirolo et al
(21), CM vacuolation occurs as a
consequence of the normal aging process or in response to the
exposure to hypoxic stimuli in cardiac cells. Two previous studies
by Ad et al (8,9) identified myocytolysis as a key
preoperative histopathological predictor for the development of
POAF (65%). In the present study, it was observed that the two
patient groups (POAF, 91.8%; POSR, 38.8%) presented increased CM
vacuolation as a possible arrhythmogenic substrate for the
development of POAF; however, ultimately only certain patients
developed POAF. Taking in consideration myolysis, Ausma et
al (18) observed that the
degree of myolysis and glycogen accumulation could increase with AF
persistence. The enhanced accumulation of glycogen in the
structurally altered atrial myocytes may imply an alteration of
cellular metabolic status.
The majority of histologic changes, including
atrophy and fibrosis, are characteristic of an ischemic myocardium.
The presence of CM atrophy was not dominant in the current study,
and CM hypertrophy was a compensatory lesion. Thus, atrophy and
hypertrophy appear to induce background abnormality independently.
Another two histological variables considered to be POAF predictors
were pericardial adiposity and inflammation.
Although no interstitial atrial inflammation was
identified in the current study, Ishii et al (22) noted that inflammation plays an
important role in the pathogenesis of POAF, by altering atrial
conduction, facilitating re-entry and predisposing to the
development of POAF. The current results revealed only a small
number of pericardial inflammatory foci, as possible AF trigger.
Issac et al (23) considers
that extracorporeal circulation contains enough systemic
inflammatory mediators that may be, in part, responsible for the
occurrence of POAF.
In the current analysis, a strong correlation was
observed between extensive pericardial adiposity and POAF. Al
Chekakie et al (24) and
Batal et al (25), referring
to the association between pericardial fat volume and AF, suggested
that the local effects of proinflammatory cytokines released from
the pericardial adipose tissue may be a potential mechanism for the
development of AF.
In conclusion, in the present study, the
preoperative status of atrial morphology was examined in
correlation with various clinical risk factors. The results
suggested that preoperative morphologic alterations, such as CM
vacuolation and increased IF, may constitute a pathologic substrate
and predictive factors for POAF development. However, the study had
certain evident limitations. First, the number of patients in the
study was small. Increasing the number of patients included in
future studies would lead to a more accurate data analysis.
Secondly, a limitation of the study was participation of a single
pathologist. In addition, only the right atrial appendage was
sampled, while the left atrial tissue was not examined. Pulmonary
veins and left atrial tissue are known to be critical regions in
the initiation and maintenance of AF. However, as the left atrium
is more difficult to access during surgery due to its posterior
position, the investigation of this area may require the use of
necropsy samples or an experimental animal study.
References
|
1
|
Hogue CW Jr and Hyder ML: Atrial
fibrillation after cardiac operation: Risks, mechanisms and
treatment. Ann Thorac Surg. 69:300–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Creswell LL, Schuessler RB, Rosenbloom M
and Cox JL: Hazards of postoperative atrial arrhythmias. Ann Thorac
Surg. 56:539–549. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Almassi GH, Schowalter T, Nicolosi AC,
Aggarwal A, Moritz TE, Henderson WG, Tarazi R, Shroyer AL, Sethi
GK, Grover FL and Hammermeister KE: Atrial fibrillation after
cardiac surgery: A major morbid event? Ann Surg. 226:501–511. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cox JL: A perspective of postoperative
atrial fibrillation in cardiac operations. Ann Thorac Surg.
56:405–409. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aranki SF, Shaw DP, Adams DH, Rizzo RJ,
Couper GS, VanderVliet M, Collins JJ Jr, Cohn LH and Burstin HR:
Predictors of atrial fibrillation after coronary artery surgery.
Current trends and impact on hospital resources. Circulation.
94:390–397. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaman AG, Archbold RA, Helft G, Paul EA,
Curzen NP and Mills PG: Atrial fibrillation after coronary artery
bypass surgery: A model for preoperative risk stratification.
Circulation. 101:1403–1408. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groves PH and Hall RJ: Atrial
tachyarrhythmias after cardiac surgery. Eur Heart J. 12:458–463.
1991.PubMed/NCBI
|
|
8
|
Ad N, Snir E, Vidne BA and Golomb E:
Potential preoperative markers for the risk of developing atrial
fibrillation after cardiac surgery. Semin Thorac Cardiovasc Surg.
11:308–313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ad N, Snir E, Vidne BA and Golomb E:
Histologic atrial myolysis is associated with atrial fibrillation
after cardiac operation. Ann Thorac Surg. 72:688–693. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathew JP, Parks R, Savino JS, Friedman
AS, Koch C, Mangano DT and Browner WS: Atrial fibrillation
following coronary artery bypass graft surgery: Predictors,
outcomes and resource utilization. Multi Center Study of
Perioperative Ischemia Research Group. JAMA. 276:300–306. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maisel WH, Rawn JD and Stevenson WG:
Atrial fibrillation after cardiac surgery. Ann Intern Med.
135:1061–1073. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banach M, Rysz J, Drozdz JA, Okonski P,
Misztal M, Barylski M, Irzmanski R and Zaslonka J: Risk factors of
atrial fibrillation following coronary artery bypass grafting: A
preliminary report. Circ J. 70:438–441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitzman DW and Edwards WD: Age-related
changes in the anatomy of the normal human heart. J Gerontol.
45:M33–M39. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lie JT and Hammond PI: Pathology of the
senescent heart: Anatomic observations on 237 autopsy studies of
patients 90 to 105 years old. Mayo Clin Proc. 63:552–564. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goette A, Juenemann G, Peters B, Klein HU,
Roessner A, Huth C and Röcken C: Determinants and consequences of
atrial fibrosis in patients undergoing open heart surgery.
Cardiovasc Res. 54:390–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaziri SM, Larson MG, Benjamin EJ and Levy
D: Echocardiographic predictors of nonrheumatic atrial
fibrillation. The framingham heart study. Circulation. 89:724–730.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henry WL, Morganroth J, Pearlman AS, Clark
CE, Redwood DR, Itscoitz SB and Epstein SE: Relation between
echocardiographically determined left atrial size and atrial
fibrillation. Circulation. 53:273–279. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ausma J, Litjens N, Lenders MH, Duimel H,
Mast F, Wouters L, Ramaekers F, Allessie M and Borgers M: Time
course of atrial fibrillation-induced cellular structural
remodeling in atria of the goat. J Mol Cell Cardiol. 33:2083–2094.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li D, Fareh S, Leung TK and Nattel S:
Promotion of atrial fibrillation by heart failure in dogs: Atrial
remodeling of a different sort. Circulation. 100:87–95. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boldt A, Wetzel U, Lauschke J, Weigl J,
Gummert J, Hindricks G, Kottkamp H and Dhein S: Fibrosis in left
atrial tissue of patients with atrial fibrillation with and without
underlying mitral valve disease. Heart. 90:400–405. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pirolo JS, Hutchins GM and Moore GW:
Myocyte vacuolization in infarct border zones is reversible. Am J
Pathol. 121:444–450. 1985.PubMed/NCBI
|
|
22
|
Ishii Y, Schuessler RB, Gaynor SL, Yamada
K, Fu AS, Boineau JP and Damiano RJ Jr: Inflammation of atrium
after cardiac surgery is associated with inhomogeneity of atrial
conduction and atrial fibrillation. Circulation. 111:2881–2888.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Issac TT, Dokainish H and Lakkis NM: Role
of inflammation in initiation and perpetuation of atrial
fibrillation: A systematic review of the published data. J Am Coll
Cardiol. 50:2021–2028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al Chekakie MO, Welles CC, Metoyer R,
Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ and Akar JG:
Pericardial fat is independently associated with human atrial
fibrillation. J Am Coll Cardiol. 56:784–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Batal O, Schoenhagen P, Shao M, Ayyad AE,
Van Wagoner DR, Halliburton SS, Tchou PJ and Chung MK: Left atrial
epicardial adiposity and atrial fibrillation. Circ Arrhythm
Electrophysiol. 3:230–236. 2010. View Article : Google Scholar : PubMed/NCBI
|