Introduction
The prolapse of a lumbar intervertebral disc is a
common ailment that places pressure on the surrounding nerves and
causes pain in the waist and leg. Intervertebral disc degeneration
(IDD) has been demonstrated to be the main cause of lumbar
intervertebral disc prolapse. Approximately 20% of teenage
indiciduals have discs with mild signs of degeneration, while ~10%
of 50-year-old individuals and ~60% of 70-year-old individuals
posses discs that exhibit severe degeneration (1). However, the pathogenesis of IDD is
associated with various cellular and biochemical alterations
(2). IDD is associated with numerous
risk factors, including lifestyle, co-morbidities, genetic
predisposition and age (3).
Previous studies have detected high expression
levels of cytokines in degenerated intervertebral disc tissues,
including interleukin (IL)-1, interferon (IFN)-γ and tumor necrosis
factor (TNF)-α, and these cytokines are associated with the
pathogenesis of IDD (4–6).
IL-2, which is a pleiotropic cytokine primarily
produced by T lymphocytes, has been implicated in the development
and control of inflammatory disease (7,8). A
previous study demonstrated that IL-2 mRNA expression was
downregulated in the epidural compartment following intervertebral
disc extrusion (9), and IL-2
expression levels in the disc samples of patients with lumbar
degenerative disc disease were significantly increased, as compared
with the controls (10). These
findings suggested that IL-2 may be associated with intervertebral
disc disease; however, the role of IL-2 in the cellular functions
of intervertebral disc tissues remains unclear.
In the present study, the expression levels of IL-2
in the nucleus pulposus (NP) tissues, the avascular and aneural
tissues in the center of the intervertebral disc, of patients with
prolapsed lumbar intervertebral discs were investigated, and in
vitro experiments were performed in order to investigate the
role of IL-2 in the proliferation of human NP cells (HNPCs),
apoptosis and extracellular matrix (ECM) metabolism. In addition,
alterations in p38 mitogen-activated protein kinase (MAPK)
signaling were examined in the HNPCs, a potential target for
treating IDD, following IL-2 treatment.
Materials and methods
Clinical samples
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Soochow University
(Suzhou, China). Informed consent was obtained from all patients
prior to enrollment onto the present study. Human NP tissues were
obtained from 30 patients undergoing surgery for prolapsed lumbar
intervertebral discs, whereas control NP tissues were obtained from
10 patients undergoing surgery for spinal trauma. The 30 patients
diagnosed with prolapsed lumbar intervertebral discs included 15
males and 15 females, and the mean age was 51 years. The 10
patients with spinal trauma included 4 males and 6 females, with a
mean age of 49 years.
Cell culture and treatment
HNPCs were purchased from ScienCell Research
Laboratories (Carlsbad, CA, USA) and cultured in NP Cell Medium
(ScienCell Research Laboratories) in an atmosphere containing 5%
CO2 and 95% air. The NP Cell culture medium was changed
every three days. IL-2 (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in sterile distilled water, and the HNPCs were treated
with 1, 5, 10 or 20 ng/ml IL-2.
Enzyme-linked immunosorbent assay
(ELISA)
IL-2 expression levels in the NP tissues were
determined using an IL-2 ELISA kit (R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's protocol.
Briefly, the NP tissues were homogenized using a Dounce tissue
grinder (Sigma-Aldrich) and centrifuged at 1,000 × g for 10 min at
4°C, and 100 µl of either standard, control (phosphate-buffered
saline; PBS) or sample was subsequently pipetted into each well,
and incubated with 100 µl assay diluent for 2 h at room
temperature. Following washing, 200 µl conjugate was added and
incubated for 2 h at room temperature prior to the addition of 200
µl substrate solution to develop the color. Reactions were
terminated by adding 50 µl Stop solution and the absorbance was
measured at 450 nm using a Multiskan Spectrum spectrophotometer
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell viability
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability was measured using an MTT assay. The
cells were seeded into 96-well plates and incubated with 1, 5, 10
or 20 ng/ml IL-2 for 12, 24, 48 and 72 h, respectively. Following
this, 0.5 mg/ml MTT solution (Sigma-Aldrich, St. Louis, MO, USA)
was added to each well, and the plates were incubated for 4 h at
37°C. Subsequently, the medium was removed and 200 µl dimethyl
sulfoxide (Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA,
USA) was subsequently added into each well. The absorbance was
measured at 570 nm using a Multiskan Spectrum spectrophotometer
(Thermo Fisher Scientific, Inc.).
Analysis of caspase-3 and caspase-8
activities
Caspase-3 and caspase-8 activities in HNPCs were
measured using a Caspase Activity kit (Beyotime Institute of
Biotechnology, Shanghai, China), according to the manufacturer's
protocol. Briefly, the cells were washed and lysed prior to
incubation of the supernatant with acetyl-Asp-Glu-Val-Asp
p-nitroanilide and N-Acetyl-Ile-Glu-Thr-Asp-p-nitroanilide,
respectively. The release of p-nitroaniline was determined by
measuring the absorbance at 405 nm using a SpectraMax 190
microplate reading (Molecular Devices, LLC, Sunnyvale, CA, USA).
The caspase activities were expressed as a percentage, relative to
the control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Tissues were homogenized using the Dounce tissue
grinder. Total RNA was harvested from the NP tissues or the HNPCs
using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific Inc.),
and subsequently reverse transcribed into cDNA using a First Strand
cDNA Synthesis kit (Fermentas; Thermo Fisher Scientific Inc.),
according to the manufacturer's instructions. The primers for
RT-qPCR are outlined in Table I. PCR
amplification was performed with 2 ng cDNA using a 7300 Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with a SYBR® Green PCR kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The cycling conditions were as follows: 5 min at
95°C, followed by 42 cycles of 5 min at 95°C, 15 sec at 58°C and 10
sec at 72°C, finally 10 min at 95°C. Relative mRNA expression
levels were calculated using the 2−ΔΔCq method (11). Data are presented as fold changes,
normalized to the control.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Name | Primer sequences
(5′-3′) |
|---|
| IL-2 | F:
ACCTCAACTCCTGCCACAAT |
|
| R:
TCCTGGTGAGTTTGGGATTC |
| Aggrecan | F:
CTGCTTCCGAGGCATTTCAG |
|
| R:
CTTGGGTCACGATCCACTCC |
| Type I collagen | F:
GTCGAGGGCCAAGACGAAG |
|
| R:
CAGATCACGTCATCGCACAAC |
| Type II collagen | F:
GGTCTTGGTGGAAACTTTGCT |
|
| R:
GGTCCTTGCATTACTCCCAAC |
| ADAMTS-4 | F:
ACTGGTGGTGGCAGATGACA |
|
| R:
TCACTGTTAGCAGGTAGCGCTTT |
| ADAMTS-5 | F:
GGACCTACCACGAAAGCAGATC |
|
| R:
GCCGGGACACACGGAGTAC |
| MMP-3 | F:
TGAAGAGTCTTCCAATCCTACTGT TG |
|
| R:
CTAGATATTTCTGAACAAGGTTCATGCA |
| MMP-13 | F:
GGACAAGTAGTTCCAAAGGCTACA A |
|
| R:
CTTTTGCCGGTGTAGGTGTAGATAG |
| GAPDH | F:
CAATGACCCCTTCATTGACC |
|
| R:
GACAAGCTTCCCGTTCTCAG |
Western blot analysis
Proteins were isolated using a Total Protein
Extraction kit (Nanjing KeyGEN Biotech Inc., Nanjing, China).
Protein concentrations were determined using a BCA Protein Assay
Kit (C503021; Sangon Biotech Co., Ltd., Shanghai, China). Total
proteins (25 µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred via
electroblotting onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
bovine serum albumin at 4°C overnight and subsequently incubated at
37°C for 1 h with the following primary antibodies: Mouse
monoclonal antibodies to p38 MAPK (#9217; 1:400),
phosphorylated-p38 MAPK (#9216; 1:200) and Fas cell surface death
receptor (Fas; #8023; 1:500; Cell Signaling Technology, Inc.,
Beverly, MA, USA); rabbit polyclonal antibody to matrix
metalloproteinase (MMP)-13 (ab39012; 1:400); mouse monoclonal
antibodies to collagen I (ab90395; 1:500), collagen II (ab185430;
1:500) and MMP-3 (ab17790; 1:400) Abcam, Cambridge, UK); rabbit
polyclonal antibodies to a disintegrin and metalloproteinase with
thrombospondin motifs (ADAMTS)-4 (sc-25582; 1:800) and ADAMTS-5
(sc-83186; 1:400); and mouse monoclonal antibodies to aggrecan and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sc-365062;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). GAPDH
was used as a housekeeping gene. Following this, the membranes were
washed with PBS and incubated with a horseradish peroxidase-labeled
goat anti-rabbit IgG/HRP (sc-2004; 1:5,000) and goat anti-mouse
IgG/HRP (sc-2031; 1:5,000; Santa Cruz Biotechnology, Inc.)
secondary antibody at 37°C for 1 h. Immunolabeling was detected
using an enhanced chemiluminescence reagent and a Western Blotting
kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The blots were
analyzed using Image-Pro Plus software (Media Cybernetics, Inc.,
Rockville, MD, USA)
Statistical analysis
Data were analyzed using SPSS 19.0 statistical
software (IBM SPSS, Armonk, NY, USA) and presented as the mean ±
standard deviation. Statistical differences between the two groups
were analyzed using a Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
IL-2 expression levels are increased
in patients with a prolapsed lumbar intervertebral disc
In order to determine the expression levels of IL-2
in the NP tissues, NP tissues were collected from the patients and
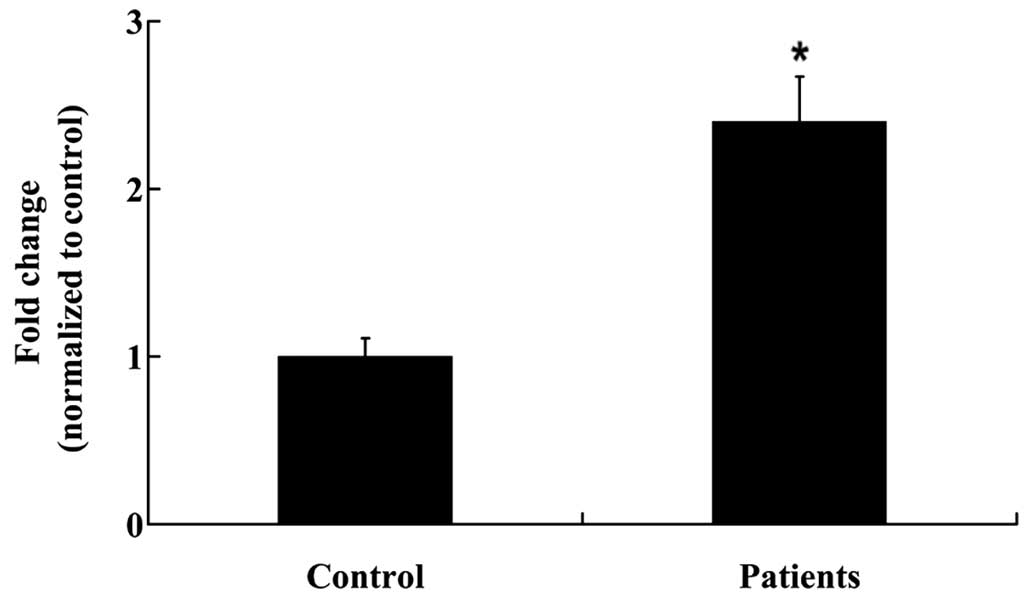
controls as outlined, prior to RT-qPCR and ELISA analyses. IL-2
mRNA expression levels were significantly increased in the NP
tissues of patients with a prolapsed lumbar intervertebral disc, as
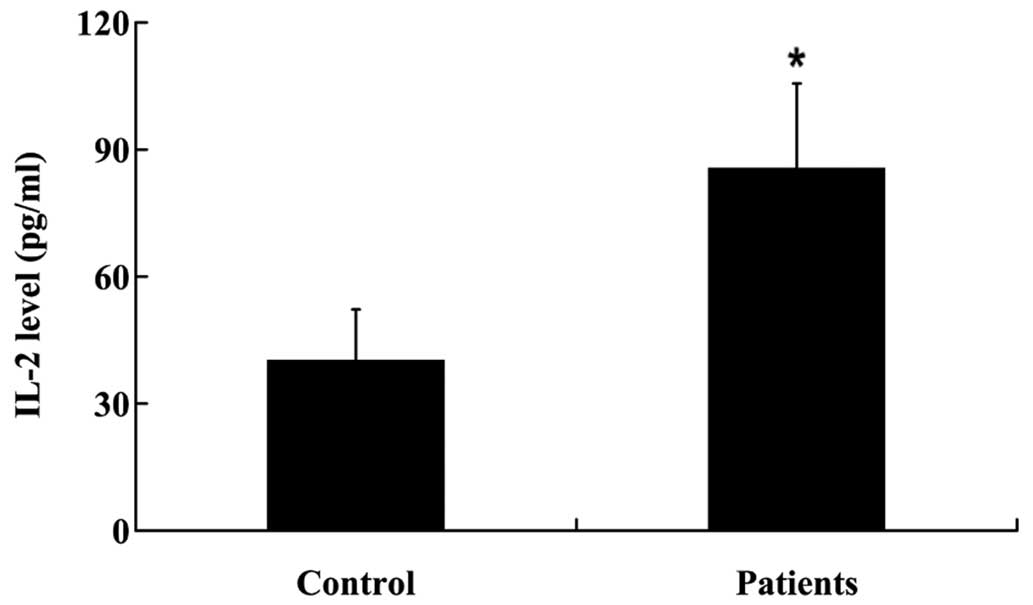
compared with the controls (P<0.01; Fig. 1). Furthermore, IL-2 protein
expression levels were significantly increased in the NP tissues of
patients with a prolapsed lumbar intervertebral disc (P<0.05;
Fig. 2).
IL-2 inhibits the proliferation of
HNPCs in a dose-dependent manner
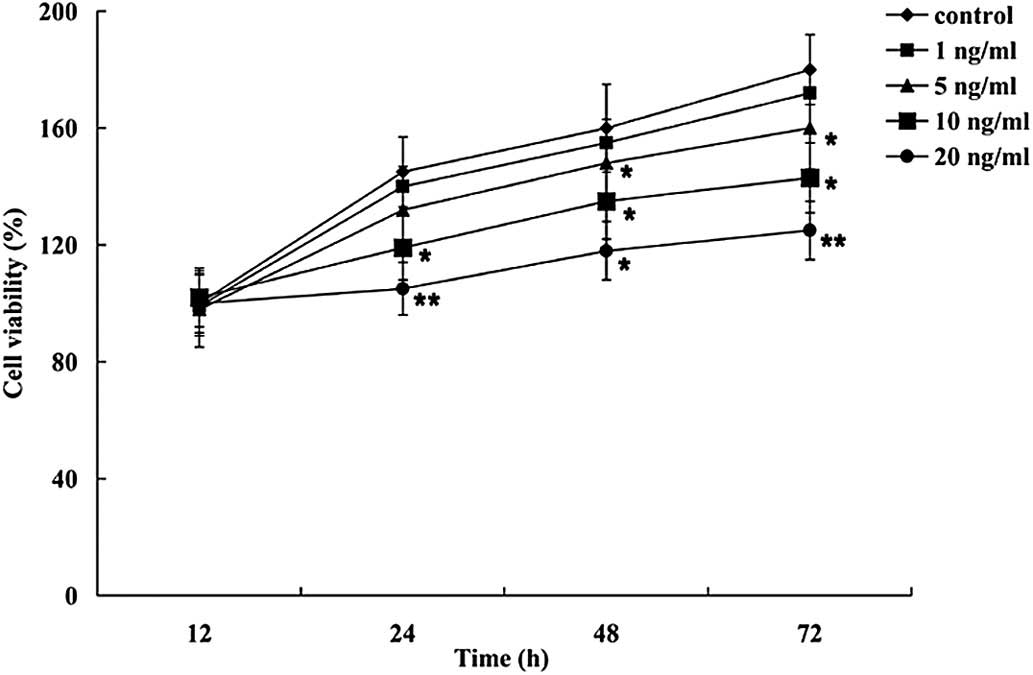
To investigate the effects of IL-2 on the
proliferation of HNPCs, the cells were treated with various
concentration of IL-2 (1, 5, 10 and 20 ng/ml), and an MTT assay was
performed. As demonstrated in Fig.
3, IL-2 inhibited the proliferation of the HNPCs in a
dose-dependent manner. No significant effects on the viability of
the HNPCs were observed at the lowest IL-2 concentration (1 ng/ml);
however, 5, 10 and 20 ng/ml IL-2 significantly decreased the
viability of the HNPCs.
IL-2 significantly increases the
expression levels of apoptosis-related proteins in HNPCs
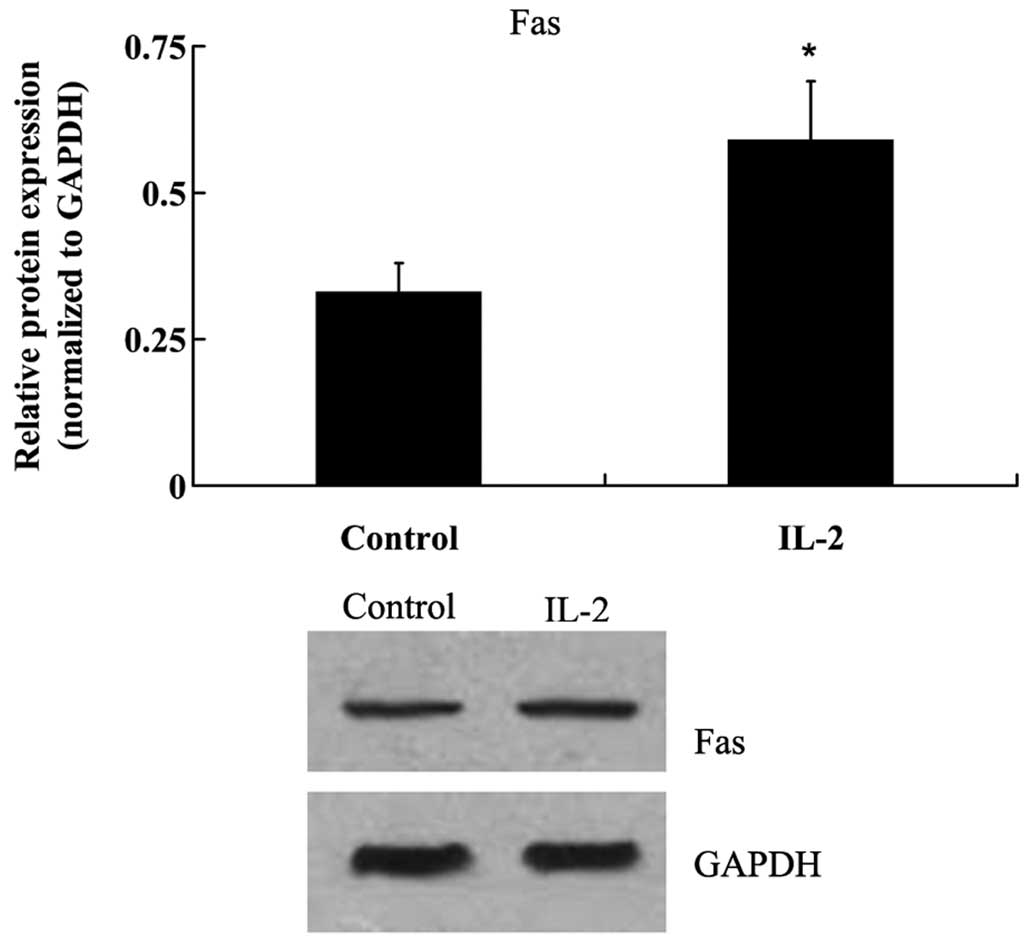
To investigate the effects of IL-2 on the apoptosis
of HNPCs, the cells were treated with 20 ng/ml IL-2 for 24 h. Fas
protein expression levels were analyzed by western blot analysis,
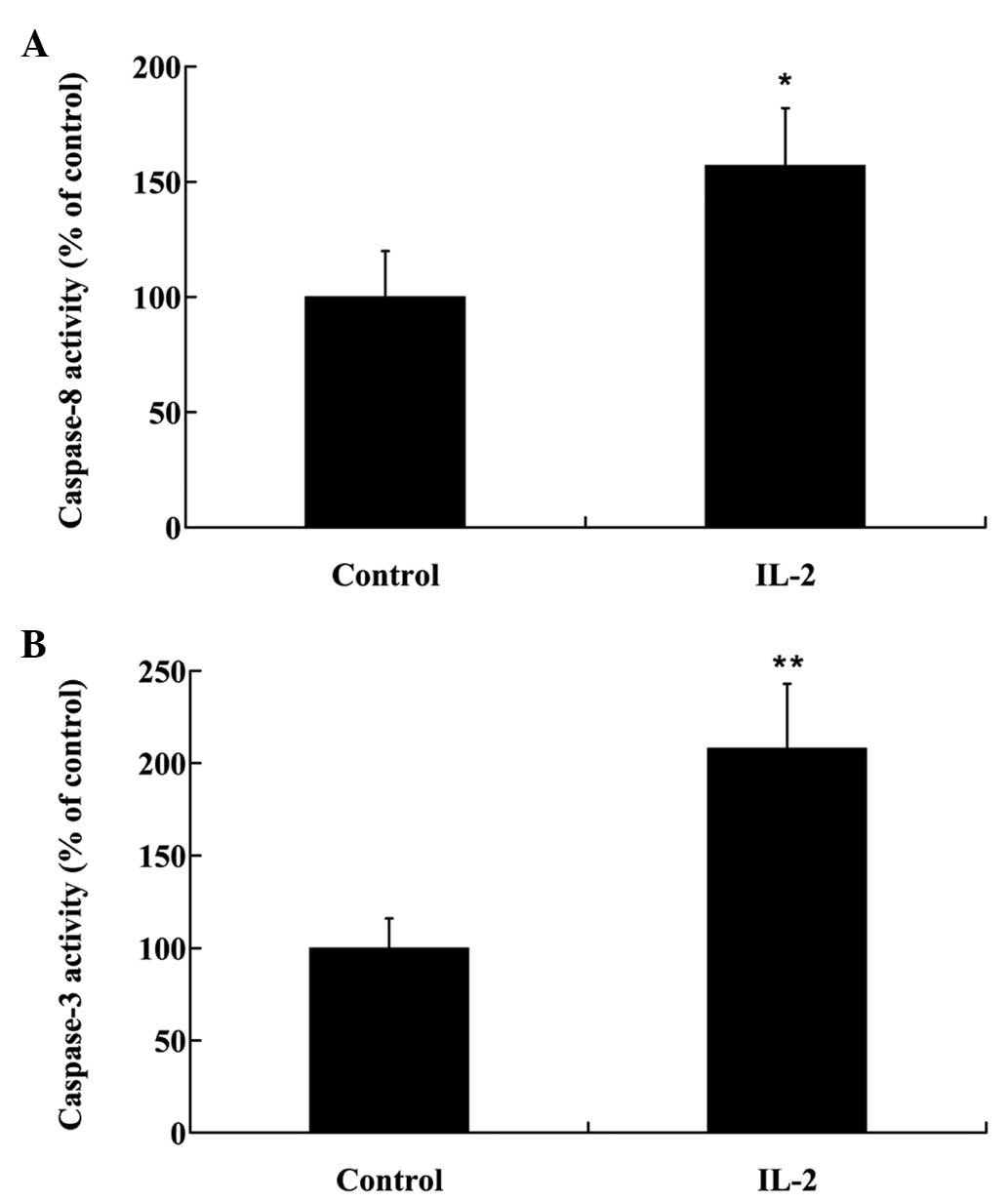
and the activities of caspase-3 and −8 were analyzed in the HNPCs,
using commercial kits. As demonstrated in Fig. 4, IL-2 significantly increased
(P<0.05) the protein expression levels of Fas in the HNPCs; and
the activities of caspase-3 and −8 were also increased in the HPNCs
following treatment with IL-2 (Fig. 5A
and B)
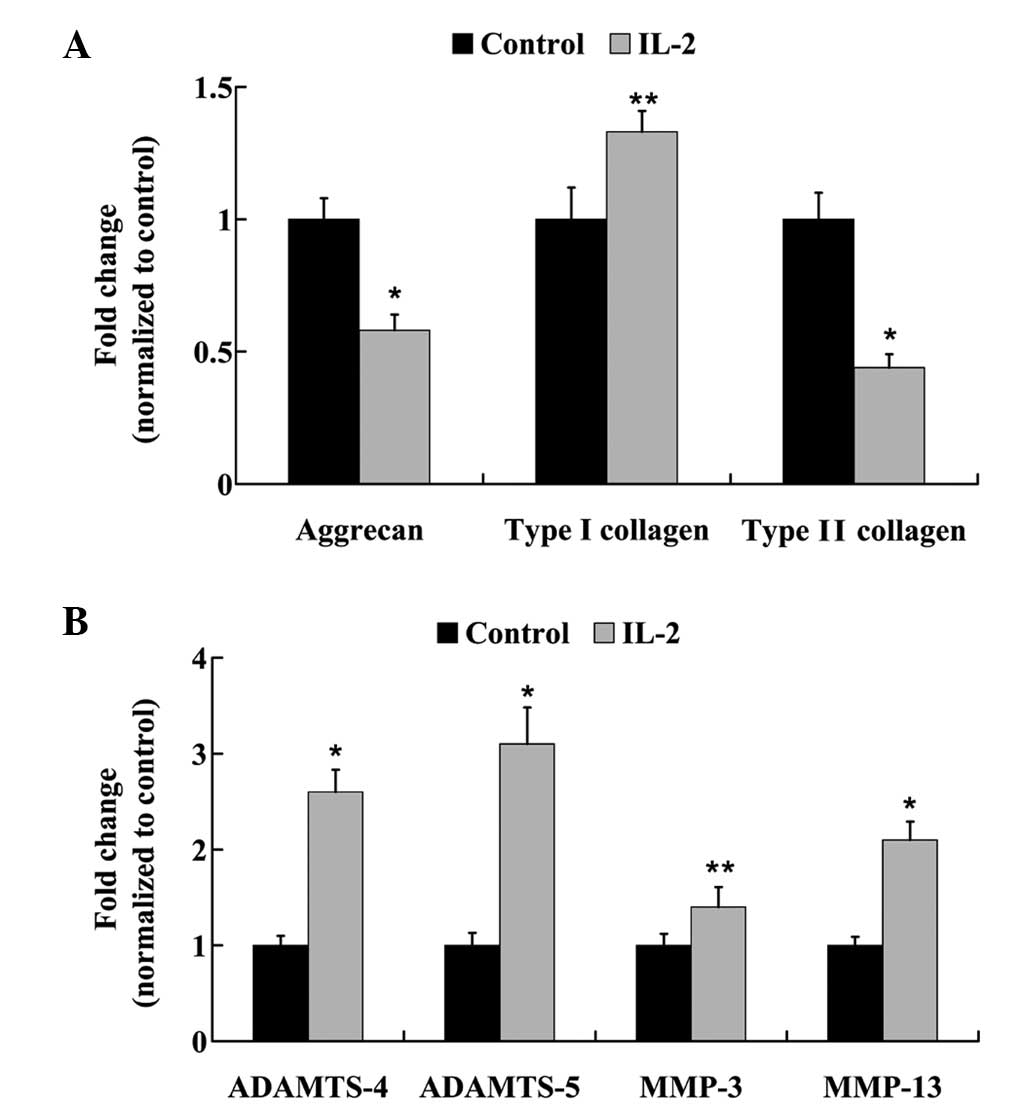
IL-2 affects ECM metabolism in
HNPCs
HNPCs were treated with 20 ng/ml IL-2 for 24 h, and
the mRNA expression levels of: Aggrecan; type I and type II
collagen; ADAMTS-4 and −5; and MMP-3 and −13 were analyzed using
RT-qPCR. As outlined in Fig. 6A, the
mRNA expression levels of aggrecan were reduced by 58% following
treatment with IL-2 in the HNPCs. Furthermore, following IL-2
administration, a 1.33-fold increase in the mRNA expression levels
of type I collagen was detected; however, type II collagen mRNA
expression levels were significantly decreased (P<0.05).
Furthermore, IL-2 treatment significantly increased (P<0.05) the
mRNA expression levels of ADAMTS-4 and −5, and MMP-3 and −13
(Fig. 6B).
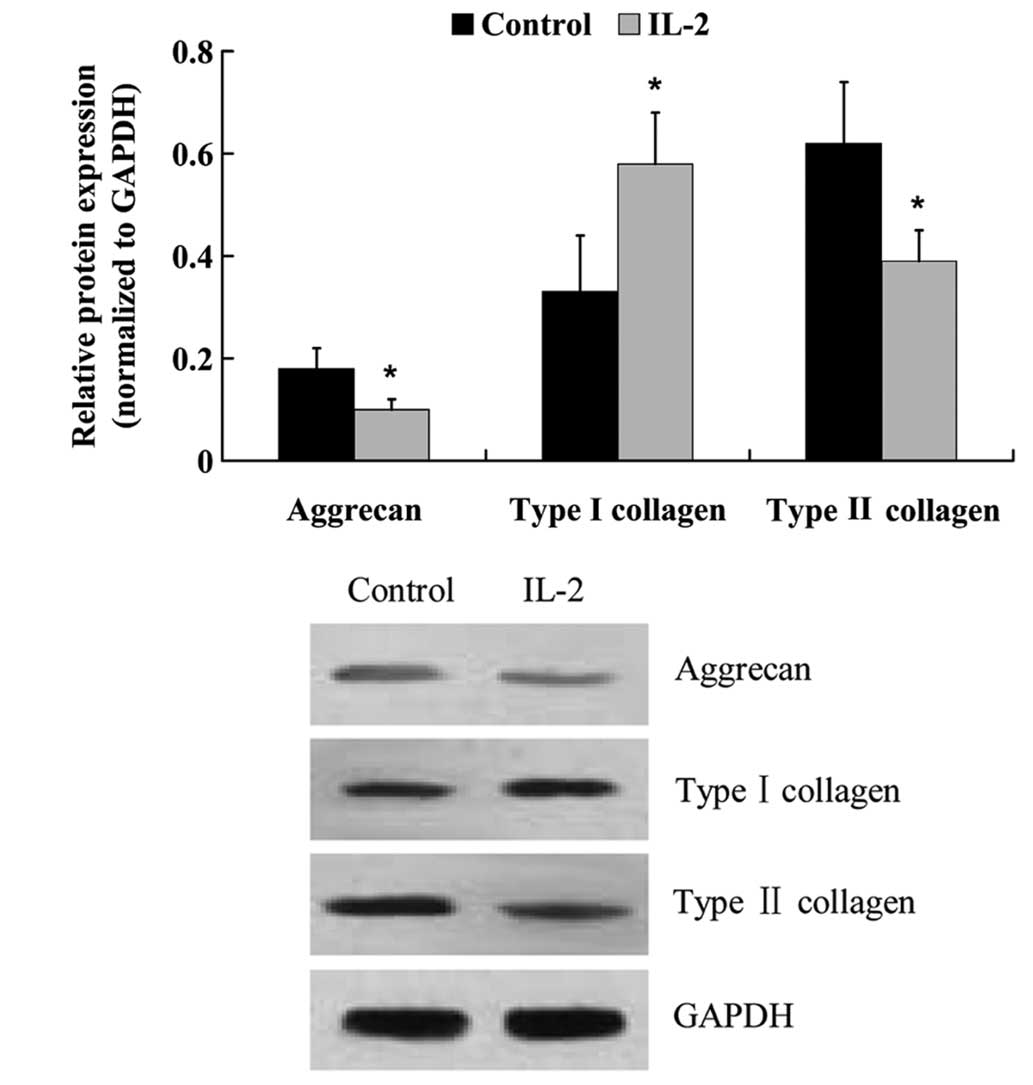
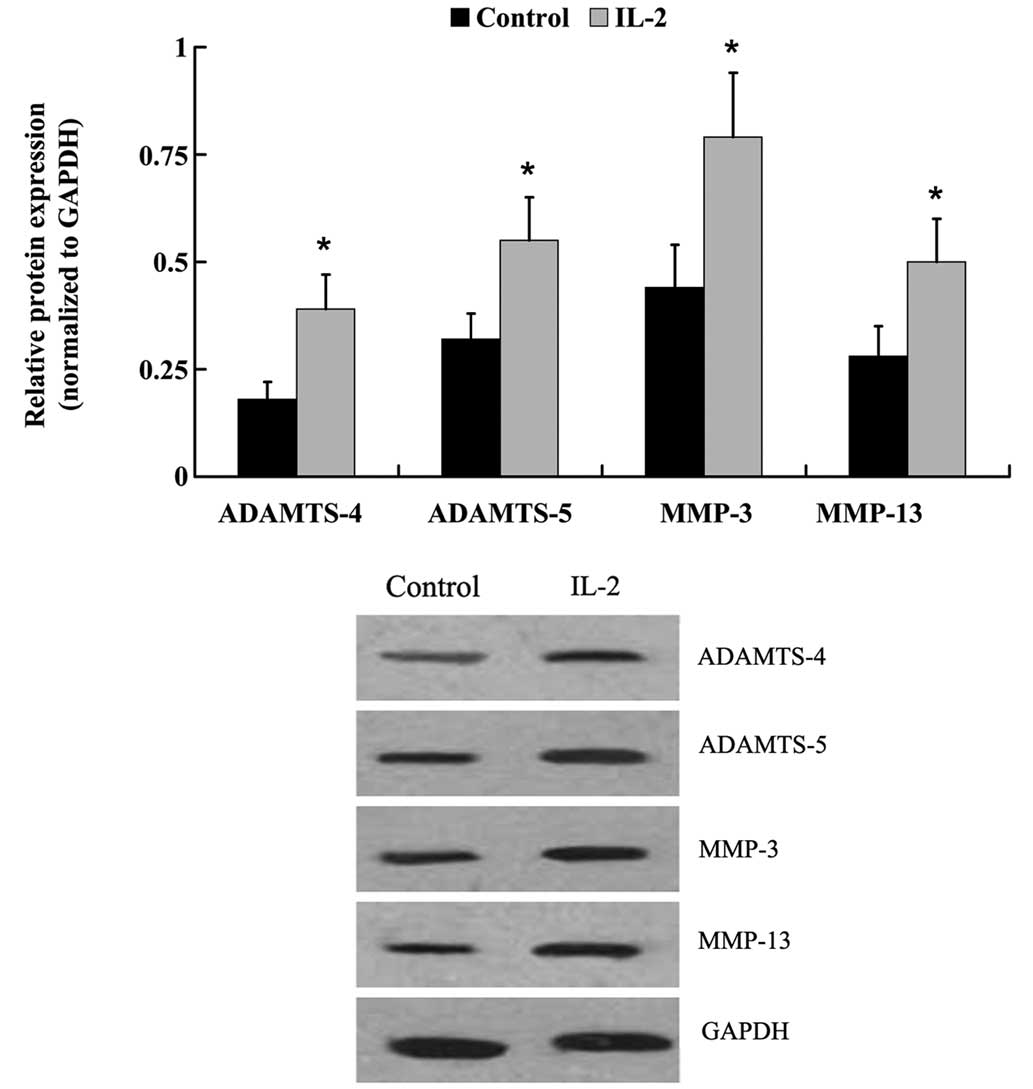
The protein expression levels of: Aggrecan; type I
and type II collagen; ADAMTS-4 and −5; and MMP-3 and −13 were
examined by western blot analysis. Similar to the mRNA expression
level results, IL-2 significantly increased (P<0.05) the protein
expression levels of type I collagen, ADAMTS-4 and −5, MMP-3 and
−13, whereas the protein expression levels of aggrecan and type II
collagen were decreased (Fig. 7 and
8).
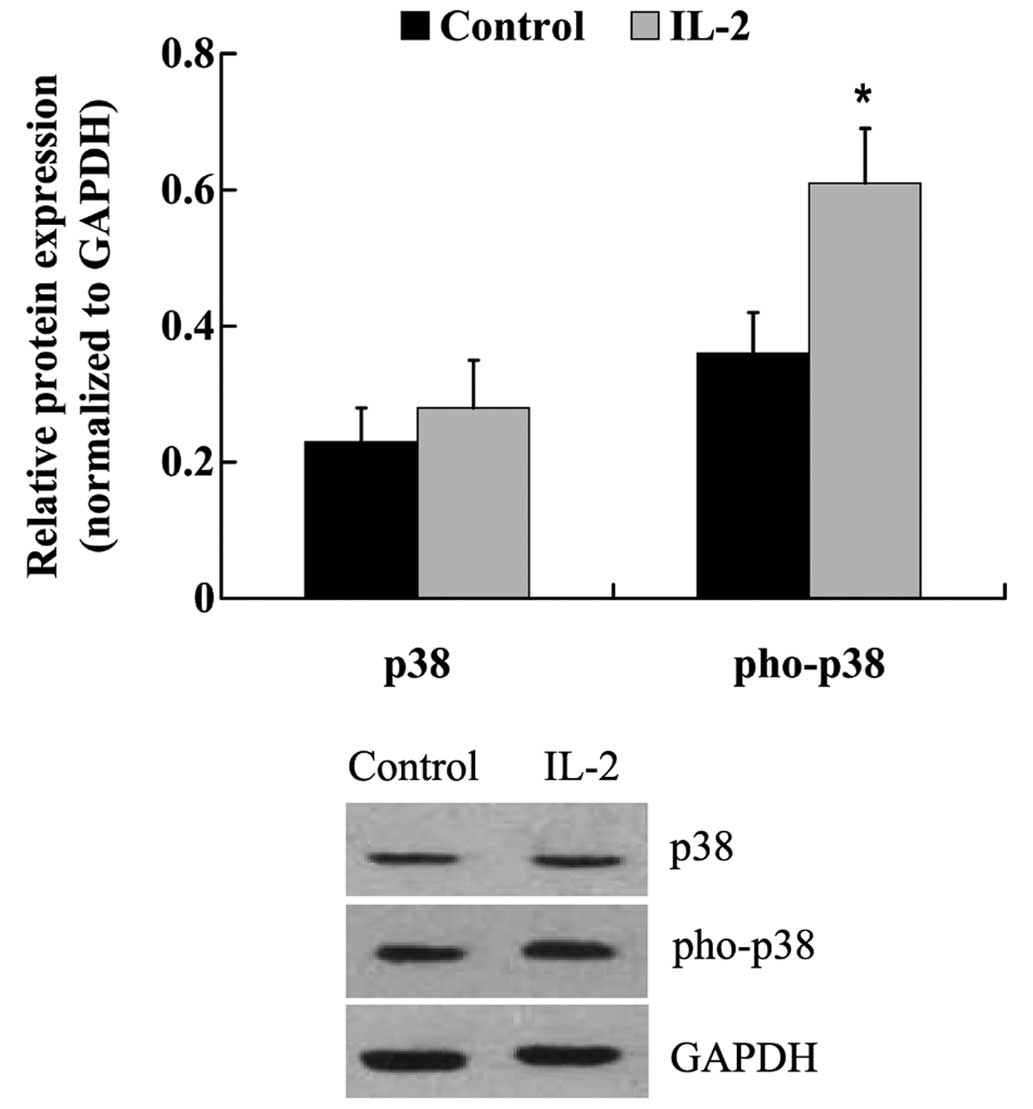
IL-2 induces the phosphorylation of
p38 in HNPCs
The expression levels of p38 and its phosphorylated
form were examined in the HNPCs following treatment with IL-2. As
outlined in Fig. 9, the protein
expression levels of p38 were not altered in response to IL-2
treatment; however, IL-2 successfully induced the phosphorylation
of p38 in the HNPCs. The expression of phosphorylated-p38 protein
was significantly upregulated in IL-2-treated HNPCs, as compared
with the untreated control cells (P<0.05).
Discussion
The intervertebral disc contains a central NP
encapsulated by endplates and the annulus fibrosus (12). The NP is an avascular and aneural
tissue capable of resisting compressive loads, which acts as a pump
in order to regulate the flow of liquids and gases in the disc.
Therefore, the NP has a significant influence on the overall
function and homeostasis of the intervertebral disc (13). In the present study, human NP tissues
from patients presenting with a prolapsed lumbar intervertebral
disc were harvested and the mRNA and protein expression levels of
IL-2 were analyzed. The results demonstrated that IL-2 was highly
upregulated in the NP tissues of patients with a prolapsed lumbar
intervertebral disc, as compared with the control patients. These
results suggested that IL-2 may have a pathogenic role in the
prolapse of lumbar intervertebral discs.
Previous studies have demonstrated that the
expression levels of cytokines, including IL-1, IL-17 and TNF-α,
are elevated in pathological disc tissue, and they increase in line
with the grade of degeneration (6,14). These
cytokines interact with a large genetic program that is
instrumental in maintaining the appropriate homeostasis of cells
within the intervertebral disc. A previous in vivo study
demonstrated that IL-2 is upregulated in patients with a prolapsed
lumbar intervertebral disc (4);
however, the mechanisms underlying the association between IL-2 and
IDD have yet to be elucidated. In the present study, in order to
further understand the role of IL-2 in the pathophysiological
mechanisms associated with disc degeneration, in vitro
experiments were performed to determine the effects of IL-2 on
apoptosis, ECM metabolism and the proliferation of HNPCs.
In the present study, the HNPCs were cultured and
treated with various concentrations of IL-2. An MTT assay
demonstrated that IL-2 inhibits the proliferation of HNPCs in a
dose-dependent manner, and 20 ng/ml IL-2 was determined to be the
optimal concentration for the inhibition of HNPCs. Furthermore, 20
ng/ml IL-2 was applied to investigate the effect of IL-2 on the
apoptosis and ECM metabolism of HNPCs.
The NP of the intervertebral disc contains a
proteoglycan-rich ECM, which normally functions to retain water
(15,16); however, degenerated intervertebral
discs lose their normal architecture, leading to alterations in
cell shape and biochemical characteristics. The role of apoptosis
and apoptosis-related genes in the development of degenerative
diseases has garnered much attention in recent years. The excessive
apoptosis of NP cells, which are capable of producing ECM
components, is one of the most discernable cellular and biochemical
changes credited to degeneration (17–22). A
previous study demonstrated that NP cells predominantly undergo
apoptosis via the death receptor pathway, which contributes to
degeneration of the intervertebral disc (23). The death receptor pathway is
initiated by the binding of cell surface receptors, such as Fas,
which activates initiator caspases, primarily caspase-8, which in
turn activate effector caspases, predominantly caspase-3 (24). In the present study, IL-2 induced the
expression of Fas protein and increased the activities of caspase-3
and −8 in the HNPCs, thus suggesting that the death receptor
pathway may be activated by IL-2 in HNPCs in order to promote cell
apoptosis.
NP cells secrete a complex ECM comprised mainly of
type II collagen and proteoglycans (25). Disc degeneration results from an
imbalance between the degradation and synthesis of ECM components.
Alterations in collagen type, and a decrease in proteoglycan
content may cause dehydration and desiccation within the NP, and
the intervertebral disc may become a more fibrotic and less
cartilaginous structure (26–29) as a
result. Aggrecan is a large proteoglycan that forms aggregates, the
loss of which is considered to be an early biochemical abnormality
of IDD (30). Previous studies have
demonstrated that destructive enzymes, such as MMPs or ADAMTS, are
upregulated in NP cells, leading to the downregulation of aggrecan
and collagen type II (31–35). In order to elucidate the effects of
IL-2 on ECM metabolism in the HNPCs, the expression levels of
degrading enzymes that have been demonstrated to hydrolyze aggrecan
and collagen were measured. RT-qPCR and western blot analysis
demonstrated that the expression levels of ADAMTS-4 and −5, and
MMP-3 and −13 were significantly increased following treatment with
IL-2. In addition, aggrecan expression levels were significantly
decreased, and collagen was altered from type II to I. These
findings suggested that IL-2 may promote ECM degradation in
HNPCs.
Studer et al (36) demonstrated that the p38 MAPK
signaling pathway is a potential target for the treatment of IDD.
NP cells respond to inflammatory cytokines via p38 MAPK; therefore,
inhibition of p38 MAPK signaling may decrease the factors that
negatively affect the metabolic balance and viability of NP cells.
In the present study, phosphorylated-p38 was significantly
upregulated following IL-2 treatment, suggesting that IL-2 may
activate p38 MAPK signaling in HNPCs.
In conclusion, the expression of IL-2 was
significantly upregulated in the NP tissues of patients with a
prolapsed lumbar intervertebral disc. In addition, the present
study demonstrated that IL-2 inhibits cell proliferation, induces
cell apoptosis and ECM degradation, and activates p38 MAPK
signaling in HNPCs. These findings provide a novel insight into the
molecular pathogenesis of IDD.
References
|
1
|
Miller JA, Schmatz C and Schultz AB:
Lumbar disc degeneration: Correlation with age, sex, and spine
level in 600 autopsy specimens. Spine (Phila Pa 1976). 13:173–178.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith LJ, Nerurkar NL, Choi KS, Harfe BD
and Elliott DM: Degeneration and regeneration of the intervertebral
disc: Lessons from development. Dis Model Mech. 4:31–41. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adams MA and Roughley PJ: What is
intervertebral disc degeneration, and what causes it? Spine (Phila
Pa 1976). 31:2151–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Markova D, Anderson DG, Zheng Z,
Shapiro IM and Risbud MV: TNF-α and IL-1β promote a
disintegrin-like and metalloprotease with thrombospondin type I
motif-5-mediated aggrecan degradation through syndecan-4 in
intervertebral disc. J Biol Chem. 286:39738–39749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shamji MF, Setton LA, Jarvis W, So S, Chen
J, Jing L, Bullock R, Isaacs RE, Brown C and Richardson WJ:
Proinflammatory cytokine expression profile in degenerated and
herniated human intervertebral disc tissues. Arthritis Rheum.
62:1974–1982. 2010.PubMed/NCBI
|
|
7
|
Kołodziejska-Sawerska A, Rychlik A, Depta
A, Wdowiak M, Nowicki M and Kander M: Cytokines in canine
inflammatory bowel disease. Pol J Vet Sci. 16:165–171.
2013.PubMed/NCBI
|
|
8
|
Shachar I and Karin N: The dual roles of
inflammatory cytokines and chemokines in the regulation of
autoimmune diseases and their clinical implications. J Leukoc Biol.
93:51–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karli P, Martlé V, Bossens K, Summerfield
A, Doherr MG, Turner P, Vandevelde M, Forterre F and Henke D:
Dominance of chemokine ligand 2 and matrix metalloproteinase-2 and
−9 and suppression of pro-inflammatory cytokines in the epidural
compartment after intervertebral disc extrusion in a canine model.
Spine J. 14:2976–2984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akyol S, Eraslan BS, Etyemez H, Tanriverdi
T and Hanci M: Catabolic cytokine expressions in patients with
degenerative disc disease. Turk Neurosurg. 20:492–499.
2010.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minogue BM, Richardson SM, Zeef LA,
Freemont AJ and Hoyland JA: Characterization of the human nucleus
pulposus cell phenotype and evaluation of novel marker gene
expression to define adult stem cell differentiation. Arthritis
Rheum. 62:3695–3705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCann MR, Bacher CA and Séguin CA:
Exploiting notochord cells for stem cell-based regeneration of the
intervertebral disc. J Cell Commun Signal. 5:39–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Maitre CL, Hoyland JA and Freemont AJ:
Catabolic cytokine expression in degenerate and herniated human
intervertebral discs: IL-1beta and TNFalpha expression profile.
Arthritis Res Ther. 9:R772007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deyo RA and Tsui-Wu YJ: Descriptive
epidemiology of low-back pain and its related medical care in the
United States. Spine (Phila Pa 1976). 12:264–268. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buckwalter JA: Aging and degeneration of
the human intervertebral disc. Spine (Phila Pa 1976). 20:1307–1314.
1995.PubMed/NCBI
|
|
17
|
Chen JW, Ni BB, Li B, Yang YH, Jiang SD
and Jiang LS: The responses of autophagy and apoptosis to oxidative
stress in nucleus pulposus cells: Implications for disc
degeneration. Cell Physiol Biochem. 34:1175–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gruber HE and Hanley EN Jr: Biologic
strategies for the therapy of intervertebral disc degeneration.
Expert Opin Biol Ther. 3:1209–1214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao CQ, Jiang LS and Dai LY: Programmed
cell death in intervertebral disc degeneration. Apoptosis.
11:2079–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao CQ, Wang LM, Jiang LS and Dai LY: The
cell biology of intervertebral disc aging and degeneration. Ageing
Res Rev. 6:247–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YF, Zhang YZ, Zhang WL, Luan GN, Liu
ZH, Gao Y, Wan ZY, Sun Z, Zhu S, Samartzis D, et al: Insights into
the hallmarks of human nucleus pulposus cells with particular
reference to cell viability, phagocytic potential and long process
formation. Int J Med Sci. 10:1805–1816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu
Y, Tian N, Huang Y, Xue E, Wang X and Xu H: Apoptosis, senescence,
and autophagy in rat nucleus pulposus cells: Implications for
diabetic intervertebral disc degeneration. J Orthop Res.
31:692–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng S, Liu L and Wang J: Significance of
BNIP3 gene expression and cell apoptosis in nucleus pulposus of
degenerative intervertebral disc in rabbits. Zhongguo Xiu Fu Chong
Jian Wai Ke Za Zhi. 24:1367–1371. 2010.(In Chinese). PubMed/NCBI
|
|
24
|
Yurube T, Hirata H, Kakutani K, Maeno K,
Takada T, Zhang Z, Takayama K, Matsushita T, Kuroda R, Kurosaka M
and Nishida K: Notochordal cell disappearance and modes of
apoptotic cell death in a rat tail static compression-induced disc
degeneration model. Arthritis Res Ther. 16:R312014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erwin WM and Hood KE: The cellular and
molecular biology of the intervertebral disc: A clinician's primer.
J Can Chiropr Assoc. 58:246–257. 2014.PubMed/NCBI
|
|
26
|
Sivan SS, Hayes AJ, Wachtel E, Caterson B,
Merkher Y, Maroudas A, Brown S and Roberts S: Biochemical
composition and turnover of the extracellular matrix of the normal
and degenerate intervertebral disc. Eur Spine J. 23(Suppl 3):
S344–S353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Antoniou J, Demers CN, Beaudoin G, Goswami
T, Mwale F, Aebi M and Alini M: Apparent diffusion coefficient of
intervertebral discs related to matrix composition and integrity.
Magn Reson Imaging. 22:963–972. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Antoniou J, Steffen T, Nelson F,
Winterbottom N, Hollander AP, Poole RA, Aebi M and Alini M: The
human lumbar intervertebral disc: Evidence for changes in the
biosynthesis and denaturation of the extracellular matrix with
growth, maturation, ageing, and degeneration. J Clin Invest.
98:996–1003. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pritzker KP: Aging and degeneration in the
lumbar intervertebral disc. Orthop Clin North Am. 8:66–77.
1977.PubMed/NCBI
|
|
30
|
Pockert AJ, Richardson SM, Le Maitre CL,
Lyon M, Deakin JA, Buttle DJ, Freemont AJ and Hoyland JA: Modified
expression of the ADAMTS enzymes and tissue inhibitor of
metalloproteinases 3 during human intervertebral disc degeneration.
Arthritis Rheum. 60:482–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arana CJ, Diamandis EP and Kandel RA:
Cartilage tissue enhances proteoglycan retention by nucleus
pulposus cells in vitro. Arthritis Rheum. 62:3395–3403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hamilton DJ, Pilliar RM, Waldman S and
Kandel RA: Effect of circumferential constraint on nucleus pulposus
tissue in vitro. Spine J. 10:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le Maitre CL, Freemont AJ and Hoyland JA:
Human disc degeneration is associated with increased MMP 7
expression. Biotech Histochem. 81:125–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
MacLean JJ, Roughley PJ, Monsey RD, Alini
M and Iatridis JC: In vivo intervertebral disc remodeling: Kinetics
of mRNA expression in response to a single loading event. J Orthop
Res. 26:579–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Studer RK, Aboka AM, Gilbertson LG,
Georgescu H, Sowa G, Vo N and Kang JD: p38 MAPK inhibition in
nucleus pulposus cells: A potential target for treating
intervertebral disc degeneration. Spine (Phila Pa 1976).
32:2827–2833. 2007. View Article : Google Scholar : PubMed/NCBI
|