Introduction
Intracerebral hemorrhage (ICH) was the cause of 10%
of strokes in the US in 2014, leading to disability, morbidity and
mortality (1). ICH remains the most
severe form of stroke, with limited options to improve survival
(2). In China, ICH accounts for a
larger, more variable proportion of strokes in community-based
Chinese compared with white populations (3). At present, China faces an increasingly
heavy burden of stroke across a variety of health care settings
(4).
Following the acute phase during ICH, injury to the
blood-brain barrier (BBB) is a key pathophysiological factor and
may contribute to perihematomal cell injury (5). BBB disruption increases the
permeability of the brain microvasculature and may result in the
development of vasogenic brain edema (6). Morbidity and mortality after ICH are
largely determined with edema surrounding the hematoma (7). Vasogenic brain edema formation is
believed to be a strong predictor of unfavorable functional outcome
(8). Hence, BBB protection to
ameliorate brain edema is a key point for treating ICH. Prevention
or reduction of BBB disruption represents a potential target for
therapeutic intervention.
The principal structures that serve the function of
the BBB are the tight junction (TJ) proteins (9). TJ proteins form the first defense in
the endothelial barrier disruption between blood and brain that
leads to vasogenic edema and cell death (10,11).
During ICH injury, the cascade of molecular events ends in the
final common pathway for BBB disruption, which degrades the TJ
proteins in endothelial cells (12).
Zonula occludens-1 (ZO-1) is one of the main TJ proteins, changes
in its levels are closely associated with the degree of BBB damage
and it becomes a symbol of the BBB destruction (13). ZO-1 reduces the permeability of
cerebral vessels by restricting the free molecular exchange between
blood and brain tissues (14).
Decreased expression and disarrangement of ZO-1 indicates reduced
BBB integrity (11). Therefore, the
normalization of ZO-1 expression may be able to promote BBB
function repair.
Numerous therapeutic strategies have been employed
in the prevention of ICH, including intravenous hemodiluting
agents, neuroprotectants and antihypertensives for secondary
prevention (15). However, the case
fatality and long-term mortality of patients with ICH has remained
unchanged over recent decades (16,17), and
there is no effective neuroprotective treatment option for BBB
improvement after ICH (18).
Traditional Chinese medicines (TCMs), such as rhubarb, have been
shown to exert multi-target effects (19). It has been suggested that TCMs may
improve ICH symptoms and reduce the risk of fatality, although
further outcomes-based research is required (20).
Rhubarb rhizome and root (Rhizoma rhei) is
one of the most commonly used and investigated TCMs (21), and is listed in the Chinese
Pharmacopoeia (22). Rhubarb is
thought to exert brain protection via the action of anthraquiones,
including aloe-emodin, rhein, emodin and chrysophanol (23), which are appointed as the bioactive
compounds and quality control standard of rhubarb in the Chinese
Pharmacopoeia (22). The traditional
primary usage of rhubarb has been for its neuroprotection.
Pharmacological studies demonstrated that rhubarb exhibited
multiple biological activities which may be of relevance to BBB
function, including anti-oxidation, anti-inflammation and
inhibition of aquaporin expression (24–26).
However, whether or not rhubarb is able to increase the expression
of TJ proteins such as ZO-1, and thus improve BBB function, remains
unclear.
In the present study, the effect of rhubarb was
evaluated in a rat model of ICH. Specifically, we investigated the
hypothesis that rhubarb would improve BBB function via the
upregulation of ZO-1 expression.
Materials and methods
Plant materials and chemicals
The raw plant material of dried rhubarb was
purchased from the Pharmacy of Xiangya Hospital, Central South
University (Changsha, China). The voucher specimen was deposited in
the Laboratory of Ethnopharmacology of Xiangya Hospital. The crude
plant material was also authenticated by the herbal medicine
botanist Professor Hu SY of the Department of Traditional Chinese
Medicine of Central South University. Marker compounds, including
aloe-emodin, rhein, emodin and chrysophanol (purity, >98%), were
supplied by the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China). Analytical
grade methanol was purchased from Tedia Company, Inc. (Fairfield,
OH, USA) and acetic acid from Sinopharm Chemical Reagent Co., Ltd.
(Shanghai, China). Triple-distilled water was prepared using silica
glass equipment (Colton Water Department, Colton, CA, USA), and was
used for preparation of the mobile phase. All other reagents were
of analytical grade.
Dried rhubarb was crushed into powder and twice
soaked in distilled water (1:12 w/v) at 100°C for 10 min. The
rhubarb was extracted twice by reflux in boiling water for 10 min,
according to a previous study (27).
Following centrifugation at 4,000 × g for 20 min at 4°C, the
supernatant was concentrated and lyophilized using the ZG-1 Vacuum
Freeze Dryer (Shanghai Cinyaa Group, Co., Ltd., Shanghai, China).
The lyophilized powder was resolved to the scale with distilled
water according to the standard of 1 g/ml (w/v) prior to the
experiment.
Determination of anthraquinones in
rhubarb by ultra performance liquid chromatography-tandem mass
spectrometry (UPLC-MS/MS)
As a quality control for rhubarb, the marker
compounds in rhubarb were determined using UPLC-MS/MS. UPLC-MS/MS
analysis was performed using a Waters Acquity UPLC™ system coupled
to Waters TQD triple quadrupole tandem mass spectrometer (Waters
Corporation, Milford, MA, USA). Chromatographic separation was
performed on an Acquity UPLC BEH 2.1×50 mm, 1.7-µm C18
column (Waters Corporation) using an Acquity ultra performance
liquid chromatography system equipped with an Acquity photo-diode
array detector (Waters Corporation). The mobile phase consisted of
methanol-deionized water containing 0.1% formic acid with gradient
elution (0 min, 45:55; 15 min, 75:25). The wavelength in the UV
spectrum was 254 nm. The flow rate was kept constant at 0.25 ml/min
during the analysis, the operating temperature was maintained at
35°C and the sample volume injected was 5 µl.
For operation in the MS/MS mode, the Waters Acquity™
TQD triple quadrupole tandem mass spectrometer equipped with
electrospray ionization interface was connected to the UPLC system.
Masslynx™ software, version 4.1 (Waters Corporation) was used for
data acquisition and processing. For mass spectrometry, the
electrospray ionization source was operated in negative mode with
the capillary voltage set at 2.5 KV. The desolvation temperature
was fixed at 365°C and the source temperature was set at 110°C.
Nitrogen was used as the desolvation gas flow (650 l/h) and cone
gas flow (50 l/h). For collision-induced dissociation, argon was
used as the collision gas at a flow rate of 0.2 ml/min. The
multiple reaction monitoring mode was used for quantification.
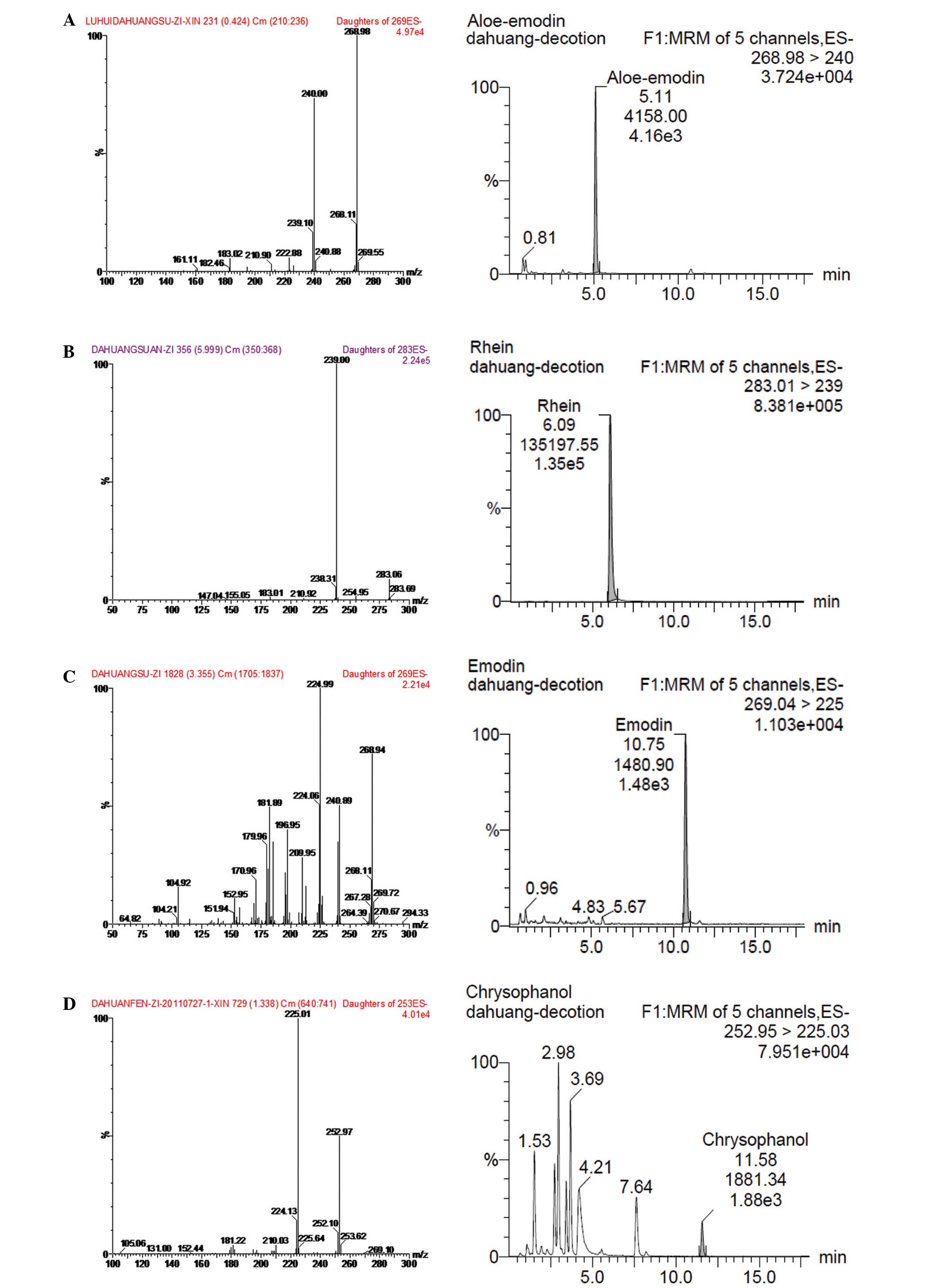
As shown in Fig. 1,
the over all intra- and inter-day variations were <5% for all
the four analytes. The method was reproducible with good precision.
The accuracy tests were conducted using a recovery test. Recovery
of all four tested compounds were >90%. The result showed that
the contents of anthraquinones in rhubarb decoction were as
follows: Aloe-emodin, 1.87±0.17 mg/g; rhein, 0.96±0.07 mg/g;
emodin, 1.55±0.12 mg/g; and chrysophanol, 0.79±0.06 mg/g.
Animal model preparation
The protocol was approved by the Medical Ethics
Committee of Xiangya Hospital of Central South University. Animal
experiments were performed according to the guidelines for the care
and use of animals, as established by the Central South University.
A total of 270 adult male Sprague-Dawley rats (age, 8–10 weeks;
weight, 180–220 g) were obtained from the Animal Center of Central
South University (SCXK2006-0002; Changsha, China). Rats were housed
at 25°C under a 12-h light/dark cycle, with ad libitum
access to food and water. The rats were randomly divided into three
groups with 90 rats per group, as follows: i) Sham-operated group;
ii) saline vehicle-treated group; and iii) rhubarb-treated group
(20 g/kg). Each group was further divided into three subgroups that
were treated with rhubarb or vehicle for 1, 3 or 5 days.
Collagenase-induced ICH was performed according to
the protocol described in a previous study (28). Briefly, the rats were anesthetized by
intraperitoneal injection with 10% chloral hydrate (400 mg/kg; from
the Pharmacy of Xiangya Hospital) and fixed in a prone position on
a stereotactic frame (Stoelting Co., Wood Dale, IL, USA). Following
a scalp incision, a small cranial burr was drilled near the right
coronal suture 3.2 mm lateral to the midline. Bacterial type VII
collagenase (0.5 U) in 2.5 µl sterile saline (0.9%; Sigma-Aldrich,
St. Louis, MO, USA) was slowly injected into the right globus
pallidus (1.4 mm posterior and 3.2 mm lateral to the bregma and 5.6
mm ventral to the cortical surface) with a 5-µl Hamilton syringe
(The Gaoge Company, Shanghai, China) over 5 min, with the needle
left in place for a further 5 min. The bone hole was sealed with
bone wax, and the wound was sutured. Each animal was placed in a
warm box to recover individually. Sham-operated rats were
administered an equal volume of saline without collagenase. The
rats with ICH were randomly divided into two groups, as follows:
The vehicle-treated and rhubarb-treated (20 g/kg) groups. Rectal
temperatures were monitored during all operations and maintained at
37.5°C with a feedback controlled heating pad (Huaibei Zhenghua
Biology Instrument Equipment Co., Ltd., Huaibei, China).
Neurobehavioral function
evaluation
Neurobehavioral function was evaluated using a
previously-described scoring system (29). The system consists of three
individual tests, each with a score range of 0–4 (0=best, 4=worst),
with a maximum total score of 12. The tests included: i)
Spontaneous ipsilateral circling behavior; ii) contra-lateral
forelimb and hindlimb retraction capability; and iii) ability to
walk a 70×32.4-cm wood beam. The evaluation was conducted by a
masked observer at days 1, 3 and 5 after the induction of ICH.
BBB permeability to Evans blue
BBB integrity was quantitatively evaluated by
assessing Evans blue (EB) dye (Sigma-Aldrich) extravasation.
Briefly, 30 rats (10/group) were intravenously infused with EB dye
solution (2% in saline, 2 ml/kg) over 1 min, which was allowed to
circulate for 2 h. Subsequently, the rats were anesthetized with
10% chloral hydrate, and then sacrificed by decapitation. The brain
was transcardially perfused with 250 ml saline until colorless
perfusion fluid was obtained. The injured hemisphere was rapidly
extracted and weighed. The samples were then placed in formamide (1
ml/100 mg; Sigma-Aldrich) for 48 h at 60°C. The absorbance of the
supernatant solution was measured using a spectrophotometer (cat.
no. 722–2000; Shandong Gaomi Caihong Analytical Instruments, Co.,
Ltd., Gaomi, China) at 620 nm. The quantitative calculation of the
dye content in the brain was quantified from a standard curve
derived from known quantities of the dye and was expressed per gram
of tissue.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cerebral cortices
obtained from the ischemic penumbra of 30 rats (10/group) using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. RNA
purity was confirmed by measuring the ratio of absorbance at 260
and 280 nm using a spectrophotometer. All RNA samples were treated
with DNase I prior to RT-qPCR to remove contaminating genomic DNA.
RNA (2 µl) was reverse transcribed into cDNA using MuLV Reverse
Transcriptase (cat. no. EP0352; Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. qPCR
was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using 2 µl cDNA, an ABsolute
Blue QPCR Mix, SYBR Green, low ROX kit (cat. no. AB4323A; Applied
Biosystems; Thermo Fisher Scientific, Inc.) and gene-specific
primers (Takara Bio, Inc., Otsu, Japan) (Table I). The PCR cycling conditions were as
follows: Initial denaturation at 95°C for 15 min, followed by 40
cycles at 95°C for 30 sec, 50°C for 30 sec and 72°C for 30 sec.
qPCR reactions were performed three times for each sample and a
negative control group without the template DNA was used to confirm
the absence of non-specific amplification. The relative mRNA
expression levels of ZO-1 were calculated using the
2−ΔΔCq method (30),
following normalization to β-actin.
 | Table I.Specific primers for ZO-1 and
β-actin. |
Table I.
Specific primers for ZO-1 and
β-actin.
| Gene | Sense primer
(5′-3′) | Antisense primer
(5′-3′) | Size (bp) |
|---|
| ZO-1 |
GGAAACCCGAAACTGATGC |
TTGGACAGAGGCGGAACT | 124 |
| β-actin |
CGTTGACATCCGTAAAGAC |
TGGAAGGTGGACAGTGAG | 201 |
Immunohistochemistry detection for
ZO-1
A total of 30 rats (10/group) were anesthetized with
10% chloral hydrate (400 mg/kg) and transcardially perfused with
0.9% saline followed by 4% paraformaldehyde (from the Pharmacy of
Xiangya Hospital). Following sacrificed by decapitation, the brains
were removed and stored in 4% paraformaldehyde until
processing.
Frozen sections (30-µm) of brain tissue were brought
to room temperature and incubated in 3% H2O2
for 15 min. After washing three times in phosphate-buffered saline
for 5 min each, nonspecific binding was blocked with 5% bovine
serum albumin (Sigma Aldrich) for 1 h at 37°C. Immunostaining was
performed using rabbit anti-ZO-1 polyclonal antibody (1:400; cat.
no. sc-5562; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
4°C overnight, followed by staining with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:200;
cat. no. BA1054; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) for 120 min. Immunoreactivity was visualized using
3,3-diaminobenzidine (Wuhan Boster Biological Technology, Ltd.)
under a light microscope (Zeiss Primo Star; Carl Zeiss AG,
Oberkochen, Germany). For the image analysis, 10 microscopic fields
were selected randomly from each group and the integrated optical
densities (IODs) were measured using Image-Pro Plus 5.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using Student's t-test and one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software, version 15.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Effect of rhubarb on neurobehavioral
functions
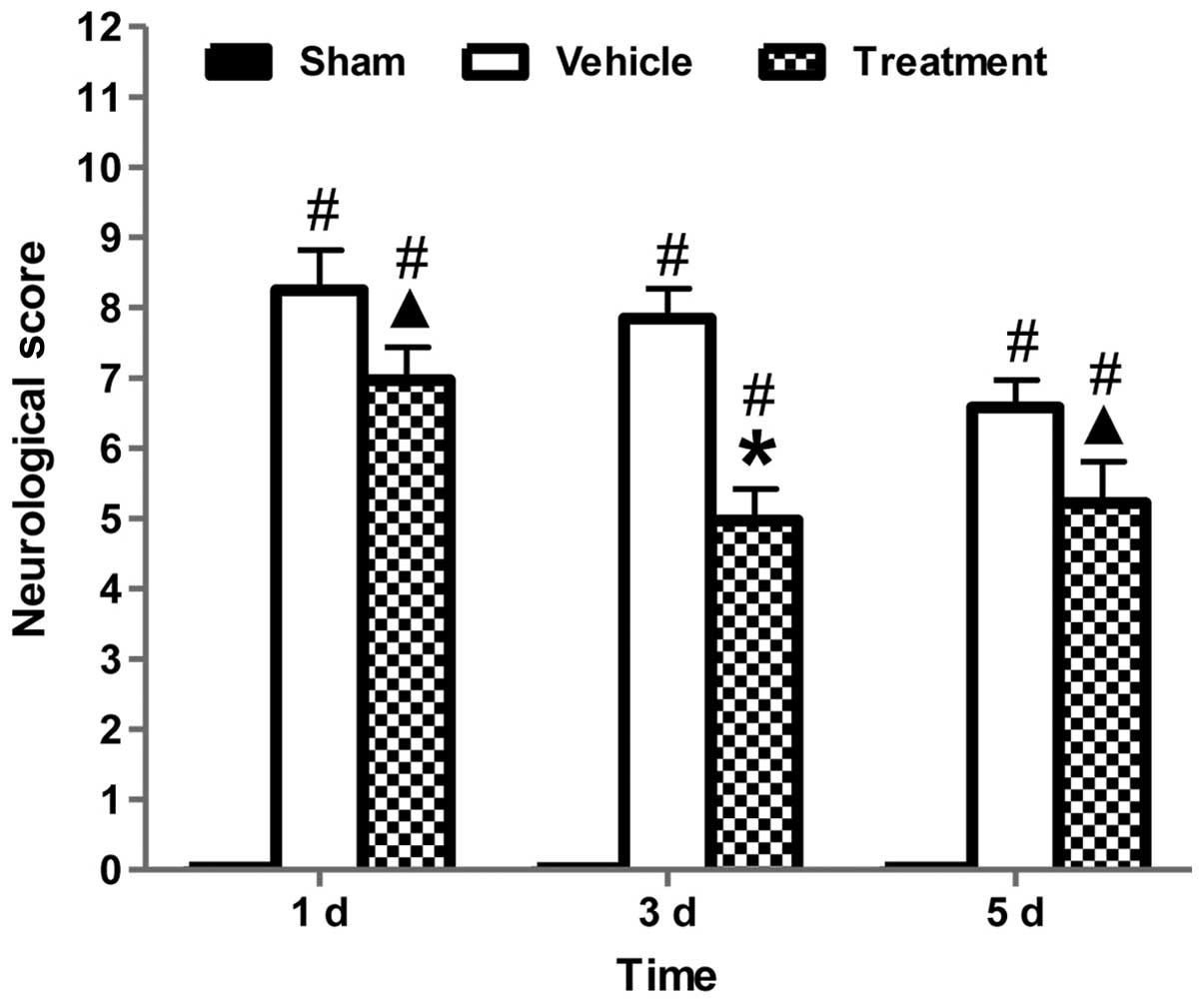
Neurobehavioral deficits were evaluated using an
established scoring system (29).
Fig. 2 indicated that after the
induction of ICH, all ICH groups showed neurobehavioral deficits as
compared with the sham-operated group. The neurobehavioral deficits
attenuated over time in each group, suggestive of self-recovery
ability of the rats. At each time point, rhubarb treatment
significantly ameliorated neurofunctional deficits compared with
the vehicle group (P<0.05).
Effect of rhubarb on BBB permeability
after ICH
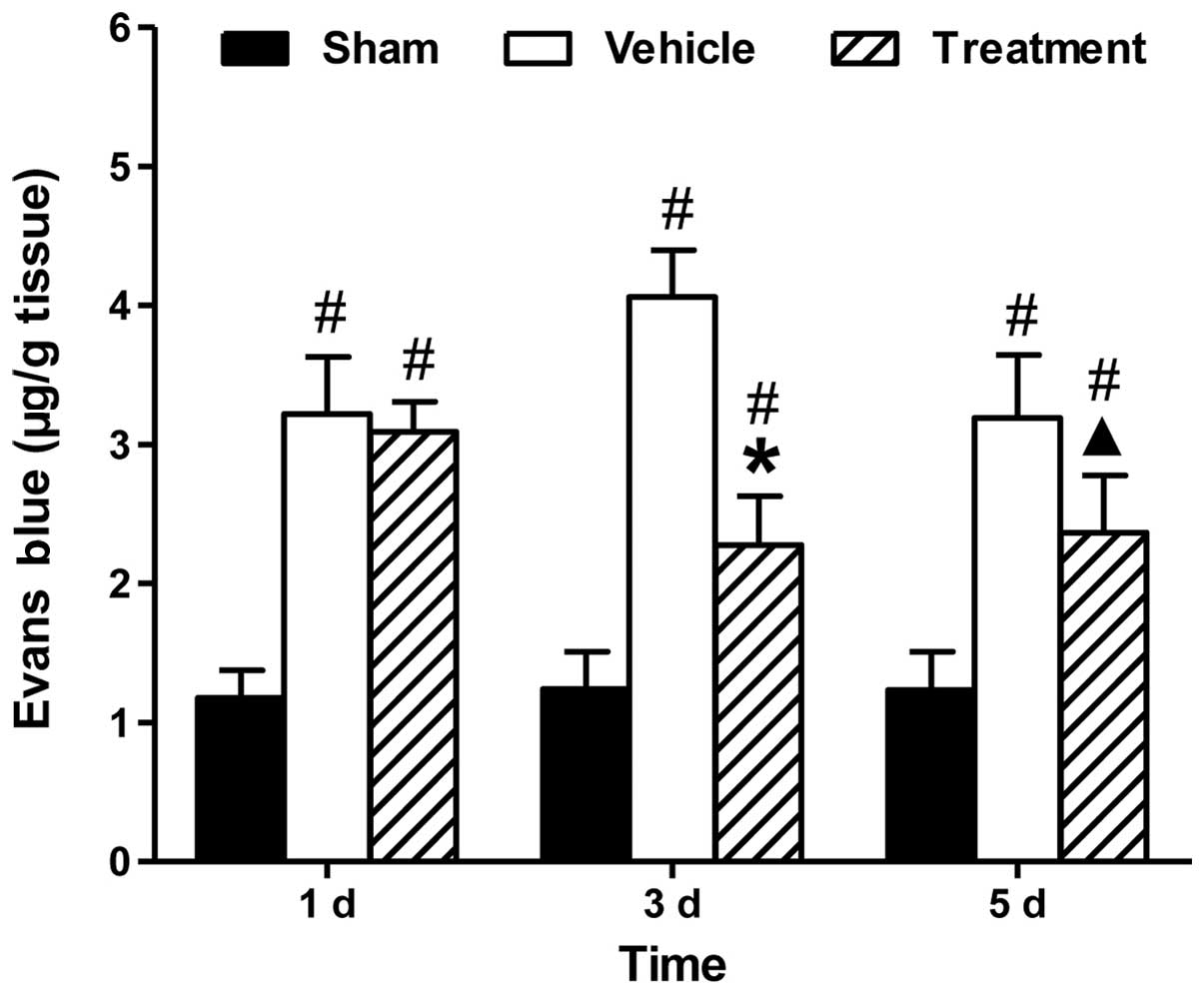
In order to assess BBB permeability, the assay of EB
dye extravasation was performed at days 1, 3 and 5 after ICH (n=10
per group). Fig. 3 showed that
significantly more extravasated dye was measured in the ICH groups,
as compared with the sham-operated group. Treatment with rhubarb
significantly reduced dye extravasation at days 3 and 5 compared
with vehicle (P<0.05).
Effect of rhubarb on ZO-1 expression in the brain.
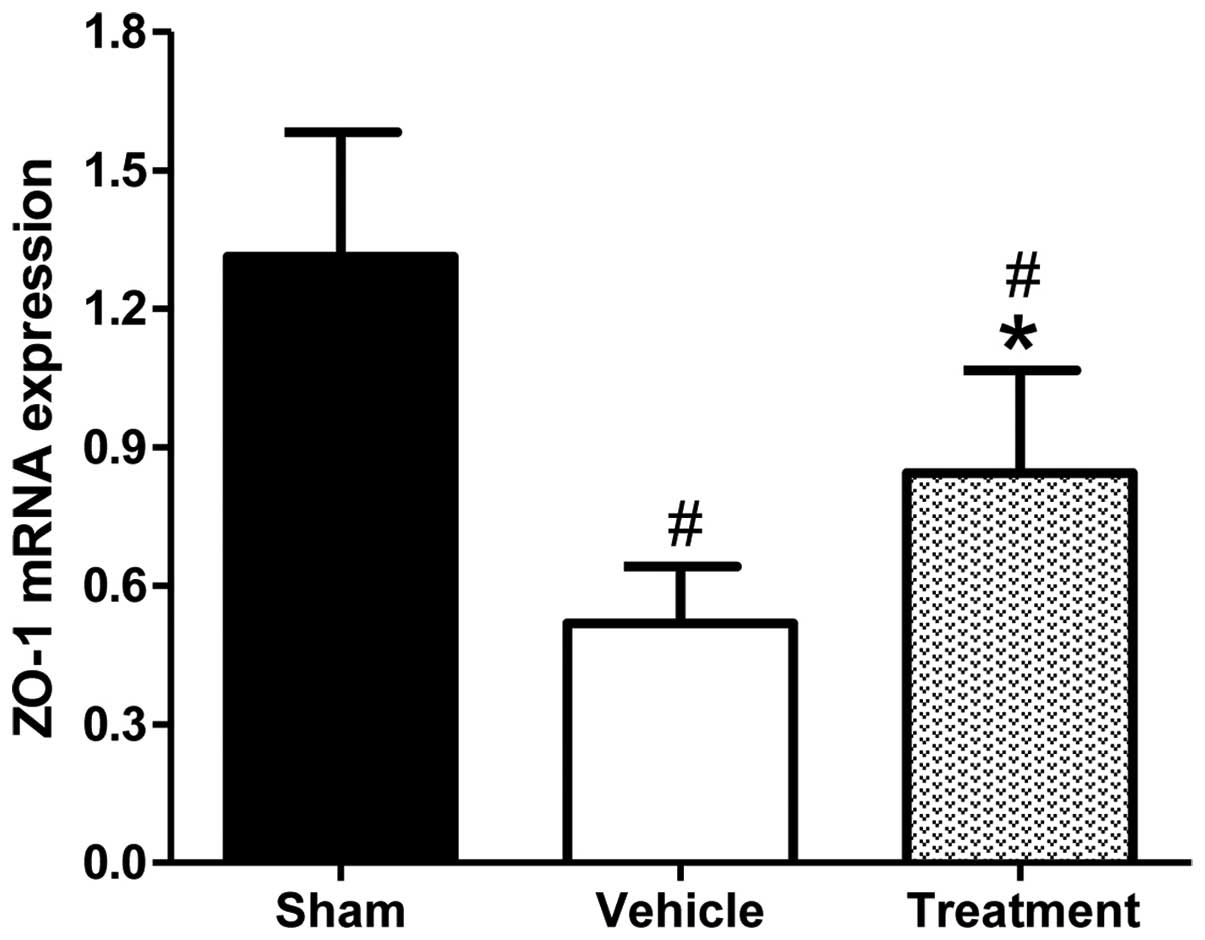
ZO-1 has been shown to have an important role in maintaining the
integrity of the BBB (31). The mRNA
expression levels of ZO-1 were analyzed by RT-qPCR at day 3, since
neurobehavioral function and BBB permeability assays (Figs. 2 and 3) had indicated that rhubarb significantly
exerted brain protective effects that peaked on day 3. As shown in
Fig. 4, ICH induced a significant
reduction in the expression levels of ZO-1 mRNA, as compared with
the sham-operated group. Conversely, rhubarb treatment resulted in
significantly upregulated ZO-1 mRNA expression levels on day 3, as
compared with the vehicle-treated group (P<0.01).
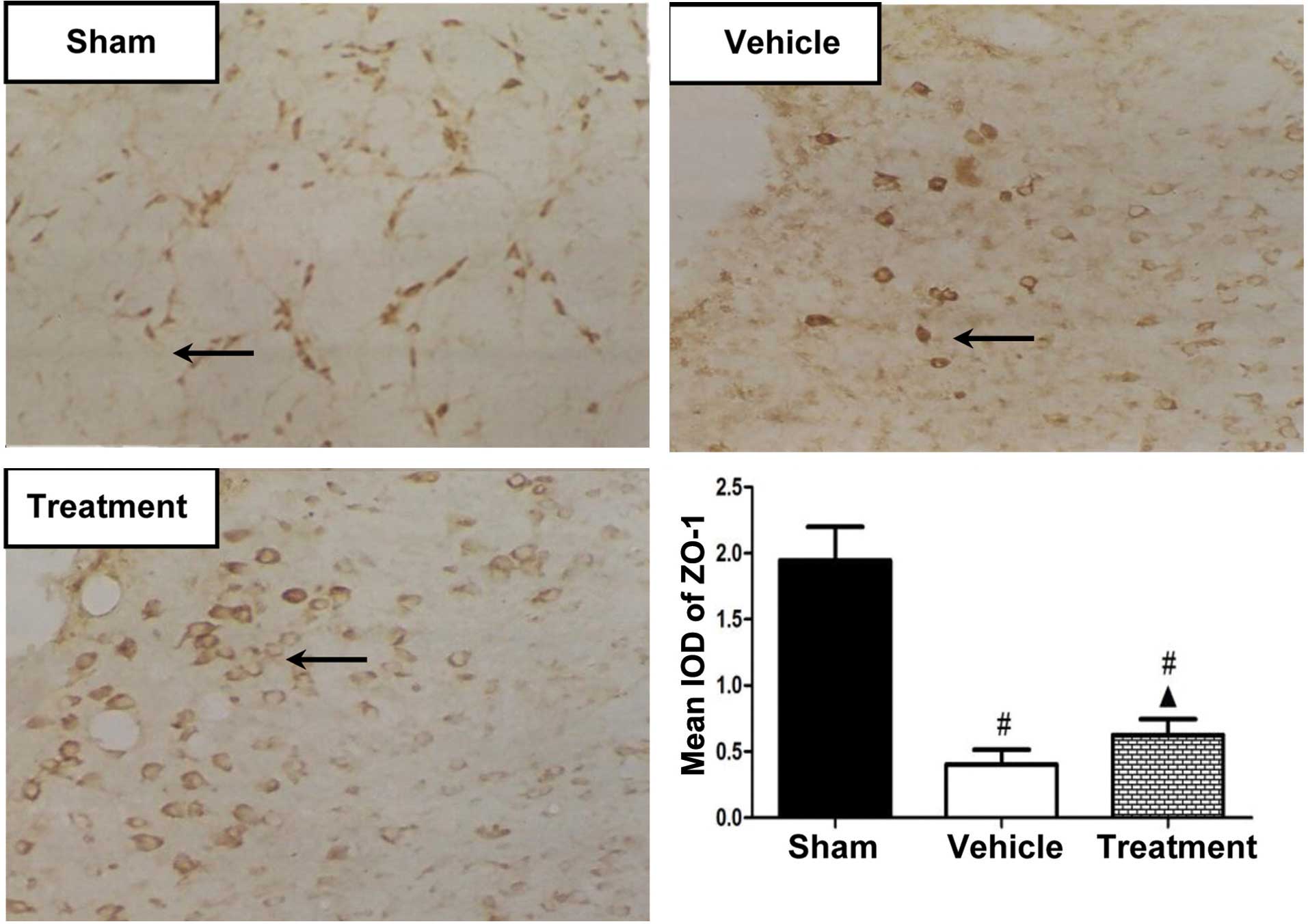
Immunohistochemical studies were performed to
investigate the protein expression of ZO-1 in the various groups,
and the IODs were calculated to quantify the protein expression
levels of ZO-1 (Fig. 5). On day 3,
the protein expression levels of ZO-1 were significantly reduced in
the rhubarb-treated group, as compared with the sham-operated group
(P<0.01), and were significantly increased in the
rhubarb-treated group, as compared with the vehicle-treated group
(P<0.05).
Discussion
BBB is a key feature of ICH and may contribute to
perihematomal cell injury (5).
Although there are a number of ongoing clinical trials, there is
currently no US Food and Drug Administration approved treatment for
BBB protection following ICH (32).
In the present study, the data showed that rhubarb (20 g/kg)
significantly ameliorated the neurological symptoms and attenuated
BBB permeability. Additionally, immunohistochemical and RT-qPCR
analyses indicated that rhubarb exerted BBB improvement effects via
the upregulation of ZO-1 expression. These results suggested that
rhubarb has the potential to be utilized as a promising
neuroprotective candidate for ICH.
The unique structure of the BBB maintains the normal
physiological state of the central nervous system by inhibiting the
passage of numerous large molecular weight, high polarity drugs and
harmful substances from the bloodstream into the interior of the
brain. Damage to the BBB is a factor in the pathogenesis of ICH,
and may occur in the initial 24 h following the onset of bleeding
and cellular damage (33). As a
result of disruption to the BBB, cells in the central nervous
system that survive the initial impact are subsequently exposed to
marked changes in their neurochemical environment, which results in
a period of secondary damage occurring in the subsequent hours and
days (34). Hence, the prevention or
reduction of BBB disruption represents a potential approach for
therapeutic intervention.
The BBB is predominantly formed by endothelial cells
with the complex TJs that serve the function of the BBB (9). TJs form the initial barrier at the
endothelial cells between the blood and brain (11). TJs are governed by intracellular
proteins, and are responsible for the restriction and control of
the paracellular flux between epithelial and endothelial cells
(35). TJ proteins form the first
defense in the endothelial barrier disruption that leads to
vasogenic edema and cell death (10). During ICH, the cascade of molecular
events ends in the final common pathway for BBB disruption which
degrades the TJ proteins in endothelial cells (12). Decreased expression and
disarrangement of the TJ proteins indicate reduced BBB integrity
and increased paracellular permeability (11).
ZO-1 is a well-characterized TJ protein and may
accurately indicate the pathological changes of BBB, making it a
valuable marker of endothelial barrier (36). It has been shown that absence of ZO-1
results in vascular leakage and the aggravated edema is likely due
to a decreased level of the TJ protein ZO-1 (37). Therefore, increasing ZO-1 protein
expression may promote repair of the BBB.
A previous study showed that rhubarb could improve
BBB function via the inhibition of the expression of the
aquaporin-4 gene (AQP4) in rats with ICH (24). However, whether or not rhubarb was
able to increase ZO-1 expression to improve BBB function was
unclear. The present results showed that rhubarb (20 g/kg)
significantly improved neurological outcome at days 1, 3 and 5
post-ICH induction compared with vehicle, which implied that
rhubarb may be able to ameliorate ICH-induced neural behavioral
defects. Collagenase induction notably increased the content of EB
extravasation after ICH, reaching a peak at day 3. Rhubarb
significantly alleviated ICH-induced BBB disruption compared with
vehicle according to the assay of EB dye extravasation. In
addition, immunochemistry and RT-qPCR analyses demonstrated that
the decreased expression and disarrangement of ZO-1 protein after
ICH were increased and rearranged by rhubarb.
Collectively, the aforementioned results suggest
that rhubarb has the potential to be utilized as a neuroprotective
drug for ICH. However, further investigation is required in order
to quantitatively evaluate the changes in ZO-1 protein expression
and to define the optimal dose of rhubarb for the treatment of
ICH.
In conclusion, the present study indicated that
rhubarb effectively attenuated BBB damage following ICH in a rat
model, raising the possibility that rhubarb or its active
components may have the potential to be utilized as a
neuroprotective drug for ICH. The protective mechanisms may involve
the preservation of the BBB integrity and elevation of ZO-1 protein
expression levels.
Acknowledgements
This study was supported by the National Natural
Science Foundation for Young Scientists of China (grant No.
81303074), Science and Technology Project of Hunan Province (grant
no. 2013SK3013), the Natural Science Foundation of Hunan Province
of China (grant no. 08JJ3077), Project of Science of Department of
Education of Hunan Province (grant no. 13C1126) and the
Administration of Traditional Chinese Medicine of Hunan Province
(grant no. 201455).
Glossary
Abbreviations
Abbreviations:
|
ICH
|
intracerebral hemorrhage
|
|
BBB
|
blood-brain barrier
|
|
TJ
|
tight junction
|
|
ZO-1
|
zonula occludens-1
|
|
TCM
|
traditional Chinese medicine
|
|
UPLC-MS/MS
|
ultra performance liquid
chromatography-tandem mass spectrometry
|
|
EB
|
Evans blue
|
References
|
1
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
Heart disease and stroke statistics-2014 update: A report from the
American heart association. Circulation. 129:e28–e292. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rincon F and Mayer SA: The epidemiology of
intracerebral hemorrhage in the United States from 1979 to 2008.
Neurocrit Care. 19:95–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsai CF, Thomas B and Sudlow CL:
Epidemiology of stroke and its subtypes in Chinese vs white
populations A systematic review. Neurology. 81:264–272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei JW, Huang Y, Wang JG, Liu M, Wong LK,
Huang Q, Wu L, Heeley EL, Arima H and Anderson CS: ChinaQUEST
Investigators: Current management of intracerebral haemorrhage in
China: A national, multi-centre, hospital register study. BMC
Neurol. 11:162011.PubMed/NCBI
|
|
5
|
Lu X, Chen-Roetling J and Regan RF:
Systemic hemin therapy attenuates blood-brain barrier disruption
after intracerebral hemorrhage. Neurobiol Dis. 70:245–251. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou QB, Jin YL, Jia Q, Zhang Y, Li LY,
Liu P and Liu YT: Baicalin attenuates brain edema in a rat model of
intracerebral hemorrhage. Inflammation. 37:107–115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zazulia AR, Diringer MN, Derdeyn CP and
Powers WJ: Progression of mass effect after intracerebral
hemorrhage. Stroke. 30:1167–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balami JS and Buchan AM: Complications of
intracerebral haemorrhage. Lancet Neurol. 11:101–118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbott NJ, Patabendige AA, Dolman DE,
Yusof SR and Begley DJ: Structure and function of the blood-brain
barrier. Neurobiol Dis. 37:13–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y and Rosenberg GA: Blood-brain
barrier breakdown in acute and chronic cerebrovascular disease.
Stroke. 42:3323–3328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krafft PR, Caner B, Klebe D, Rolland WB,
Tang J and Zhang JH: PHA-543613 preserves blood-brain barrier
integrity after intracerebral hemorrhage in mice. Stroke.
44:1743–1747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenberg GA: Neurological diseases in
relation to the blood-brain barrier. J Cereb Blood Flow Metab.
32:1139–1151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu LB, Zhou Y, Wang Q, Yang LL, Liu HQ,
Xu SL, Qi YH, Ding GR and Guo GZ: Synthetic gelatinases inhibitor
attenuates electromagnetic pulse-induced blood-brain barrier
disruption by inhibiting gelatinases-mediated ZO-1 degradation in
rats. Toxicology. 285:31–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petty MA and Wettstein JG: Elements of
cerebral microvascular ischaemia. Brain Res Brain Res Rev.
36:23–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun H, Tang Y, Guan X, Li L and Wang D:
Effects of selective hypothermia on blood-brain barrier integrity
and tight junction protein expression levels after intracerebral
hemorrhage in rats. Biol Chem. 394:1317–1324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zahuranec DB, Lisabeth LD, Sánchez BN,
Smith MA, Brown DL, Garcia NM, Skolarus LE, Meurer WJ, Burke JF,
Adelman EE and Morgenstern LB: Intracerebral hemorrhage mortality
is not changing despite declining incidence. Neurology.
82:2180–2186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Asch CJ, Luitse MJ, Rinkel GJ, van der
Tweel I, Algra A and Klijn CJ: Incidence, case fatality, and
functional outcome of intracerebral haemorrhage over time,
according to age, sex, and ethnic origin: A systematic review and
meta-analysis. Lancet Neurol. 9:167–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao L, Zhao H, Liu Q, Song J, Xu C, Liu P,
Gong W, Wang R, Liu KJ and Luo Y: Improvement of hematoma
absorption and neurological function in patients with acute
intracerebral hemorrhage treated with Xueshuantong. J Neurol Sci.
323:236–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Huang X, Liang QH, Fan R, Qin F,
Guo Y, Yan KP, Liu W, Luo JK, Li YH, et al: A strategy for
detecting absorbed bioactive compounds for quality control in the
water extract of rhubarb by ultra performance liquid chromatography
with photodiode array detector. Chin J Integr Med. 18:690–698.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu J, Zhang X, Fei Z, Wen A, Qin S, Yi S,
Chen Y and Li X: Rhubarb extracts in treating complications of
severe cerebral injury. Chin Med J (Engl). 113:529–531. 2000.(In
Chinese). PubMed/NCBI
|
|
21
|
Collins RA: A ten-year audit of
traditional Chinese medicine and other natural product research
published in the Chinese Medical Journal (2000–2009). Chin Med J
(Engl). 124:1401–1408. 2011.PubMed/NCBI
|
|
22
|
Pharmacopoeia Commission: Pharmacopoeia of
the People's Republic of China 2010 (9th). Chemical Industry Press.
Beijing: 222010.
|
|
23
|
Wei SY, Yao WX, Ji WY, Wei JQ and Peng SQ:
Qualitative and quantitative analysis of anthraquinones in rhubarbs
by high performance liquid chromatography with diode array detector
and mass spectrometry. Food Chem. 141:1710–1715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang YP, Cai DF and Liu J: Research on
acting mechanism of rhubarb on aquaporin-4 in rats with blood-brain
barrier injury after acute cerebral hemorrhage. Zhong Guo Zhong Xi
Yi Jie He Za Zhi. 26:152–156. 2006.(In Chinese).
|
|
25
|
Yong-Jun F, Yi Z, Zun-Hua K, Zhen-Guo Z,
Feng Z and Lu-Ning B: Effects of rhubarb powder on serum complement
3, complement 4, and hs-CRP in patients with intracerebral
hemorrhage. Zhong Guo Zhong Xi Yi Jie He Za Zhi. 33:168–171.
2013.(In Chinese).
|
|
26
|
Zhou X, Wang L, Wang M, Xu L, Yu L, Fang T
and Wu M: Emodin-induced microglial apoptosis is associated with
TRB3 induction. Immunopharmacol Immunotoxicol. 33:594–602. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YS and Chen FQ: The influence of ten
decoction methods on active components in rhubarb decoction. Zhong
Cheng Yao. 12:5–7. 1990.(In Chinese).
|
|
28
|
Rosenberg GA, Mun-Bryce S, Wesley M and
Kornfeld M: Collagenase induced intracerebral hemorrhage in rats.
Stroke. 21:801–807. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park HK, Lee SH, Chu K and Roh JK: Effects
of celecoxib on volumes of hematoma and edema in patients with
primary intracerebral hemorrhage. J Neurol Sci. 279:43–46. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bangsow T, Baumann E, Bangsow C, Jaeger
MH, Pelzer B, Gruhn P, Wolf S, von Melchner H and Stanimirovic DB:
The epithelial membrane protein 1 is a novel tight junction protein
of the blood-brain barrier. J Cereb Blood Flow Metab. 28:1249–1260.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Keep RF, Zhou N, Xiang J, Andjelkovic AV,
Hua Y and Xi G: Vascular disruption and blood-brain barrier
dysfunction in intracerebral hemorrhage. Fluids Barriers CNS.
11:182014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brott T, Broderick J, Kothari R, Barsan W,
Tomsick T, Sauerbeck L, Spilker J, Duldner J and Khoury J: Early
hemorrhage growth in patients with intracerebral hemorrhage.
Stroke. 28:1–5. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pardridge WM: Targeting neurotherapeutic
agents through the blood-brain barrier. Arch Neurol. 59:35–40.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liebner S, Czupalla CJ and Wolburg H:
Current concepts of blood-brain barrier development. Int J Dev
Biol. 55:467–476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nusrat A, Parkos CA, Verkade P, Foley CS,
Liang TW, Innis-Whitehouse W, Eastburn KK and Madara JL: Tight
junctions are membrane microdomains. J Cell Sci. 113:1771–1781.
2000.PubMed/NCBI
|
|
37
|
Fischer S, Wobben M, Marti HH, Renz D and
Schaper W: Hypoxia-induced hyperpermeability in brain microvessel
endothelial cells involves VEGF-mediated changes in the expression
of zonula occludens-1. Microvasc Res. 63:70–80. 2002. View Article : Google Scholar : PubMed/NCBI
|