Introduction
Hypersensitivity reaction is a type of B-type
adverse reaction, which is an abnormal reaction unrelated to dose
and conventional pharmacological effects and unpredictable in
preclinical experiments, with low incidence and high mortality
(1). Hypersensitivity reaction is
referred to as anaphylaxis (also known as Type I hypersensitivity)
and is a strong reaction produced by the body when the immune
system is stimulated by a certain allergen repeatedly. Type I
hypersensitivity is usually caused by an immunogenic macromolecule
substance or hapten molecules capable of being combined with a
macromolecular carrier (2).
Allergens may stimulate lymphocytes to generate antibody IgE, which
is combined with high-affinity IgE receptor (FcεRI) of mast cells
or basophils via the circulatory system. Once the allergen enters
the body, its combination with IgE leads to FcεRI coupling, thus
triggering the degranulation of mast cells or basophils, and the
release of allergic mediators causes local or systemic reactions
(3).
The majority of drug-induced allergic reactions are
Type I hypersensitivity reactions ranging from the local to the
body; local reactions include allergic dermatitis, and systemic
allergic reactions may lead to anaphylactic shock and patient
fatality (4). Artemisinin is an
antimalarial active chemical component isolated from
Artemisia extract. Artemisia apiacea (also known as
Artemisia annua L) contains >140 types of chemical
substance, of which >30 have anti-malarial activity. A recently
identified chemical substance in A. apiacea extract,
isolated by the China Traditional Chinese Medicine Academy
(http://www.catcm.ac.cn/), has shown a good
antimalarial effect that is equal to artemisinin (5–7). A.
apiacea is a herb commonly used in traditional Chinese
medicine, which exhibits anti-inflammatory and antipyresis,
antibacterial, antiparasitic and immunosuppressive effects
(8). Therefore, the aim of the
present study was to evaluate the effect of Artemisia
extract on the allergic response induced by compound 48/80 in
rats.
Materials and methods
Animals and grouping
A total of 60 male Wistar rats (weight, 230–280 g;
age, 7 weeks) were obtained from the Animal Experimental Center of
Southern Medical University (Guangzhou, China) and were maintained
at a constant temperature (24±2°C) with a relative humidity of
55±15% in a 12-h light/dark cycle. All rats were administered
standard laboratory rodent feed and water ad libitum. A
total of 50 rats were randomly assigned into 5 groups: A
spontaneous group (n =10), a control (n=10), 100 mg/kg of
Artemisia extract-treated group (n=10), 200 mg/kg of
Artemisia extract-treated group (n=10), 400 mg/kg of
Artemisia extract-treated group (n=10). This study was in
accordance with the Southern Medical University Guidelines and
Regulations on the Use and Care of Lab Animals. Ethical approval
was obtained for this study from the Southern Medical
University.
Preparation of the extract
The dried flowering tips of A. apiacea were
obtained from Kunming Institute of Medicine (Kunming, China) and
were ground into fine powder using an electric blender (Thermo
Fisher Scientific Inc., Waltham, MA, USA). Next, 20 g powder was
ground and refluxed with 80% ethanol (600 ml) using a Soxhlet
extractor (Thermo Fisher Scientific Inc.) for 8 h. The extraction
process was repeated twice. The extract was separated by passing
the mixture through filter paper. and the resulting
Artemisia extract was freshly dissolved in a 5% gum arabic
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) solution
prior to use.
Compound 48/80-induced systemic
anaphylactic reaction
All rats received an intraperitoneal (i.p.)
injection of compound 48/80 (8 mg/kg; Sigma-Aldrich, St. Louis, MO,
USA) to induce a systemic anaphylactic reaction. The systemic
anaphylactic reaction model was conducted according to the method
previously described by Shin et al (9). Artemisia extract was
administered 1 h prior to the injection of compound 48/80.
Following the induction of anaphylactic shock, mortality rate was
recorded over a 1-h period.
Compound 48/80-induced histamine
release from isolated rat peritoneal mast cells
The rats were cervically dislocated under anesthesia
with 40 mg/kg pentobarbital sodium). Peritoneal mast cells were
harvested from male Wistar strain rats using decollation under 350
mg/kg chloral hydrate. A total of 5 ml of medium was injected into
enterocoelia of every mouse and the abdomen was gently massaged for
5–10 min. The cells were purified using Percoll density
centrifugation (Thermo Fisher Scientific, Inc.) at 8,000xg at 4°C
for 10 min, as described previously (10). All cells (3×104 cells/tube) were
incubated in physiological buffer solution (Beyotime Institute of
Biotechnology, Haimen, China) for 20 min at 37°C. Artemisia
extract, 0.1 ml phosphate-buffered saline (Wuhan Procell Science
and Technology Co., Ltd., Wuhan, China) and 0.5 mg/ml compound
48/80 were blended altogether. The miscible liquids were incubated
for 20 min on ice. The histamine content was measured by means of a
fluorometric assay (ELISTA kit; E-EL-0032c, Elabscience, Wuhan,
China) by an ELx800 microplate reader (Biotek Instruments, Inc.,
Winooski, VT, USA). Briefly, the miscible liquids were incubated
for 20 min on ice and 80 µl of affinity chain enzyme-HRP at 37°C
was added for 1 h. Next, every well was washed with scrubbing
solution. The histamine content was measured by means of a
fluorometric assay (Synergy 2 Microplate Reader, Bio-Tek, USA) at
450 nm.
Scratching behavior
Scratching behavior was surveyed using a previously
described method (11). The
Artemisia extract was administered orally and scratching
behavior was observed for 1 h. Next, 10 µg/0.02 ml compound 48/80,
100 nmol/0.02 ml histamine and 100 nmol/0.02 ml serotonin (both
from Sigma-Aldrich) were injected intradermally into the rostral
part of the back of the rats. Scratching behavior of rat was
observed for a further 1 h, and evaluated using a HF-200 camera
(Canon Legria, Canon Inc., Tokyo, Japan).
Vascular permeability of the skin
Artemisia extract was administered orally and
1 h passed before subsequent experiments. Next, 0.5 mg/0.02 ml
compound 48/80 (i.p.), 10 nmol/0.02 ml histamine (i.p.) or 10
nmol/0.02 ml serotonin (i.p.) was administered into the rostral
part of the back. In addition, 2% Evans blue solution (Beyotime
Institute of Biotechnology) was intravenously injected into each
animal. After a pentobarbital sodium injection (1.5%;
Sigma-Aldrich) the rats were sacrificed using cervical dislocation
and after 30 min, then the ‘bluing’ reaction diameter was measured
at the injection site.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Statistical significance
was tested using one-way analysis of variance followed by Dunnett's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of Artemisia extract on the
systemic anaphylactic shock induced by compound 48/80
To determine the effect of artemisia extract on the
systemic anaphylactic shock induced by compound 48/80, the
mortality rate within 1 h after compound 48/80 injection was
recorded. The administration of 8 mg/kg compound 48/80 (i.p.)
increased the mortality rate in the allergic model rats (Table I). However, the Artemisia
extract pretreatment reduced the mortality rate of allergic model
rats in a concentration- and time-dependent manner (Table I).
 | Table I.Effect of Artemisia extract on
the systemic anaphylactic shock induced by compound 48/80. |
Table I.
Effect of Artemisia extract on
the systemic anaphylactic shock induced by compound 48/80.
|
|
| Mortality rate
(%) |
|---|
|
|
|
|
|---|
| Group | 10 min | 20 min | 60 min |
|---|
| Control | 10 | 60 | 100 |
| Artemisia
extract (mg/kg) |
|
|
|
| 100 | 0 | 30 | 70 |
| 200 | 0 | 20 | 50 |
| 400 | 0 | 10 | 40 |
Effect of Artemisia extract on the
histamine released from rat peritoneal mast cells induced by
compound 48/80
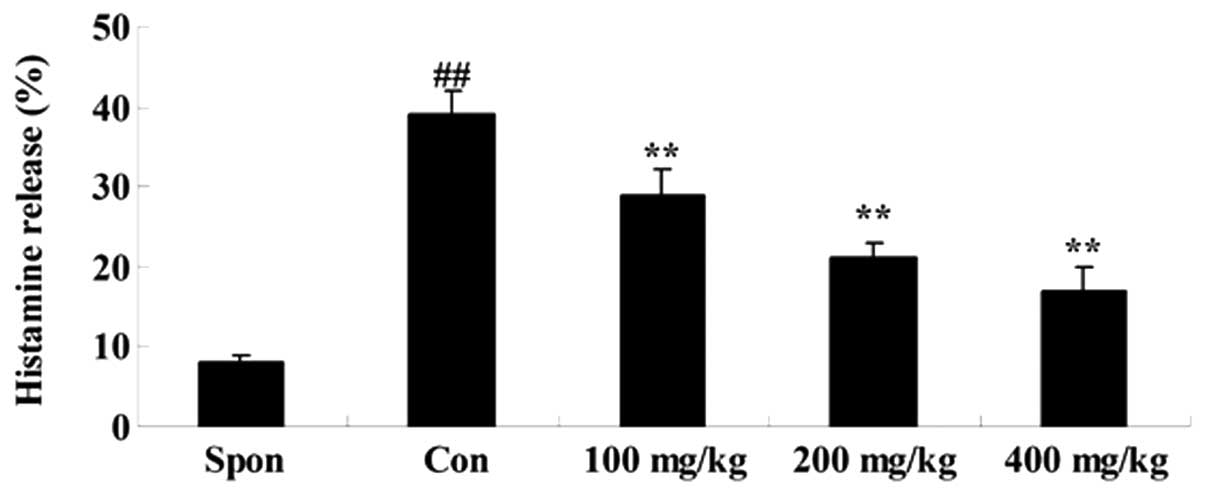
A fluorometric assay was performed to investigate
the effect of Artemisia extract on histamine release in the
allergic model rats. Treatment with 0.5 mg/ml compound 48/80
significantly increased the histamine release in the allergic model
rats (Fig. 1). Artemisia
extract pretreatment (100, 200 and 400 mg/kg) significantly reduced
the histamine release in the allergic model rats, compared with the
untreated control (Fig. 1).
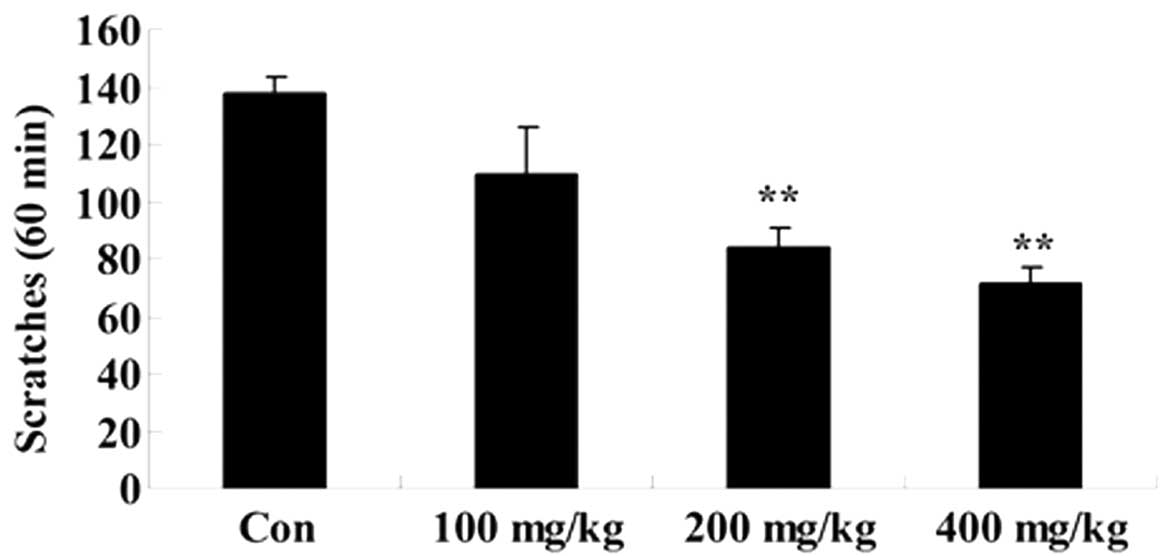
Effect of Artemisia extract on the
scratching behavior induced by compound 48/80
To elucidate the effect of Artemisia extract
on the scratching behavior of allergic model rats, the scratching
behavior was induced by treatment with compound 48/80. Furthermore,
the Artemisia extract pretreatment (200 and 400 mg/kg)
significantly reduced the scratching behavior of the allergic model
rats (Fig. 2).
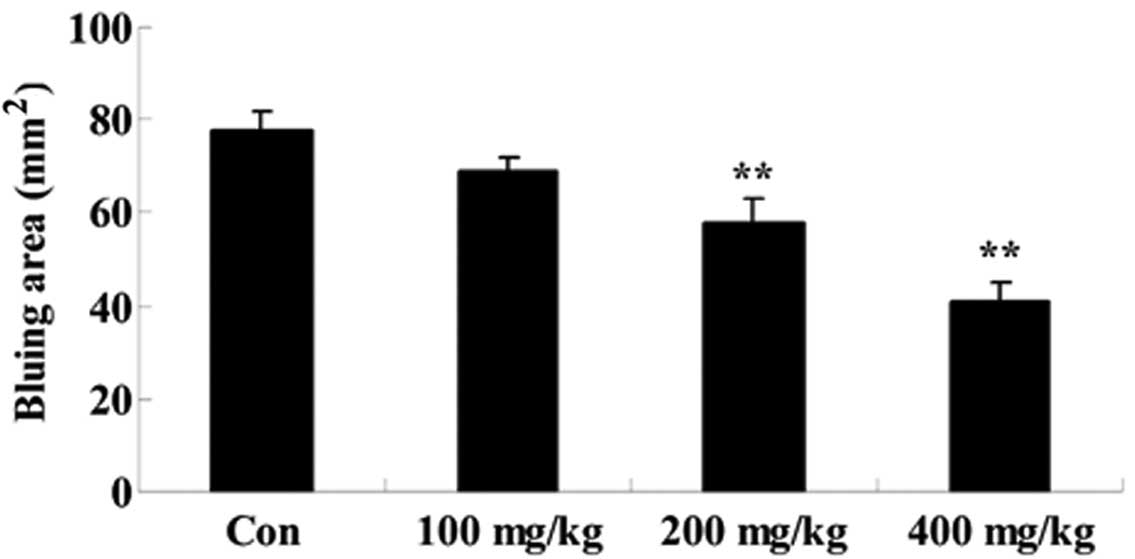
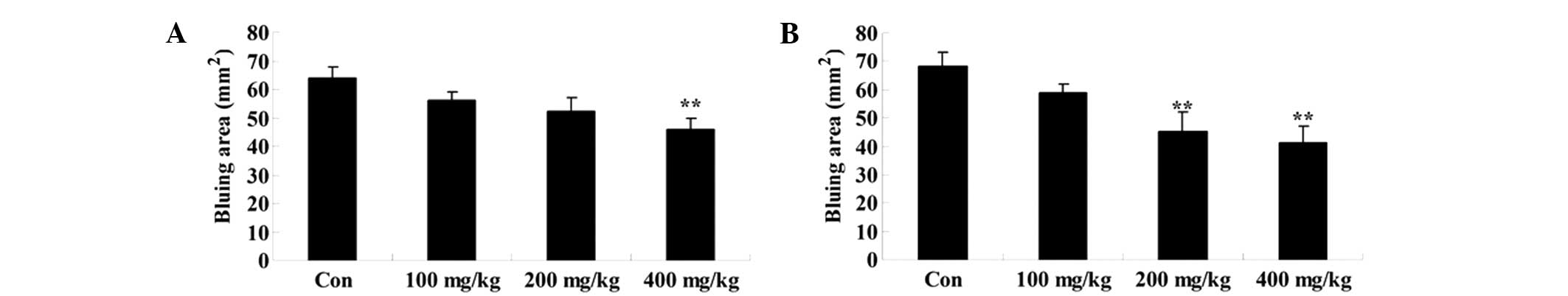
Effect of Artemisia extract on the
vascular permeability induced by compound 48/80
To assess the effect of Artemisia extract on
the vascular permeability of allergic model rats, the vascular
permeability was evaluated following 0.5 mg/0.02 ml compound 48/80
injection. Compound 48/80 effectually augmented the vascular
permeability of allergic model rats (Fig. 3). The Artemisia extract
pretreatment (200 and 400 mg/kg) significantly reduced the vascular
permeability of allergic model rats (Fig. 3).
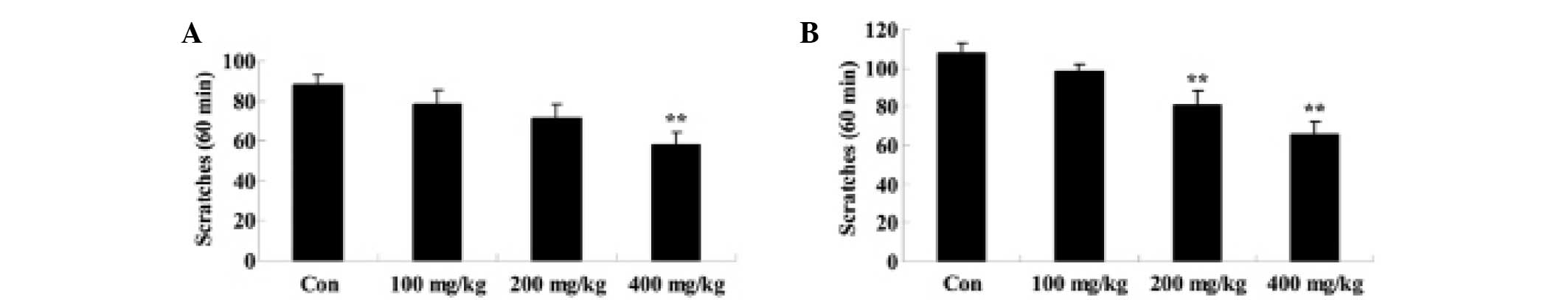
Effects of Artemisia extract on the
scratching behavior induced by histamine and serotonin
To examine the effect of Artemisia extract on
the scratching behavior of allergic model rats, scratching behavior
was induced with 100 nmol/0.02 ml histamine or serotonin injection.
Histamine or serotonin significanlty increased the scratching
behavior of allergic model rats (Fig. 4A
and B). The Artemisia extract pretreatment (400 mg/kg)
significantly reduced the scratching behavior induced by histamine
(Fig. 4A). Furthermore, the
Artemisia extract pretreatment (200 and 400 mg/kg)
significantly reduced the scratching behavior induced by serotonin
(Fig. 4B).
Effect of artemisia extract on the
vascular permeability induced by histamine and serotonin
To analyze the effect of Artemisia extract on
the vascular permeability of allergic model rats, the vascular
permeability was investigated after 10 nmol/0.02 ml histamine or
serotonin injection. Histamine or serotonin markedly increased the
vascular permeability of allergic model rats (Fig. 5A and B). The Artemisia extract
pretreatment (400 mg/kg) significantly reduced the vascular
permeability induced by histamine (Fig.
5A). Furthermore, the Artemisia extract pretreatment
(200 and 400 mg/kg) significantly reduced the vascular permeability
induced by serotonin (Fig. 5B).
Discussion
Hypersensitivity reaction is a type of B-type
adverse reaction that is an abnormal reaction unrelated to dose and
conventional pharmacological effects, and is unpredictable in
preclinical experiments, with low incidence and high mortality
(12). Drug-induced allergic
reactions may be serious, as anaphylactic shock may lead to patient
fatality (13). Establishing a
sensitive and reliable animal model for the study of allergic
anaphylaxis is necessary; as a basis for screening and discovering
drug allergens in addition to the investigation of drug-induced
allergic reaction mechanisms, that are relevant to the prevention
and treatment of clinical allergic reactions to drugs (14). However, during the preclinical safety
evaluation of drugs, allergic reaction is among the most difficult
factors to predict due to the lack of appropriate models for
predicting clinical allergic reactions (15). The establishment of appropriate
animal models for allergic reaction prediction, and their
successful application to predicting drug allergies are required
(16,17). The present results demonstrated that
Artemisia extract pretreatment is capable of reducing the
systemic anaphylactic shock, histamine release, scratching behavior
and vascular permeability induced by compound 48/80. Previously,
Kim et al suggested that Artemisia asiatica extract
improved airway inflammation of allergic asthma in mice (18).
The safety of various traditional Chinese medicines,
particularly with regard to clinical allergic reaction, has led to
increased their preclinical re-evaluation in China to guarantee
drug safety of patients (19). The
traditional Chinese medicine A. apiacea is also known as
Artemisia annua L (20). Its
extract is anti-allergy medicine, which has been proposed as a
treatment of disease due to its speculated anti-illness effects,
limited side effects and low cost (21). Recent studies show that the extract
is a physiologically active traditional medicine, with
anti-allergic, anti-oxidative and anti-inflammatory effects
(22–24). In the present study, the
anti-allergic effect of Artemisia extract markedly
suppressed the scratching behavior and vascular permeability
induced by histamine and serotonin.
In conclusion, the present findings suggest that
the extract obtained from the dried flowering tips of A.
apiacea exerts an anti-allergic effect on the allergic reaction
induced by compound 48/80 in rats. This study provides an
experimental basis for the investigation of Artemisia
extract in the treatment of allergic diseases.
Acknowledgements
This study was supported by The National Natural
Science Fund (grant no. 81202704).
References
|
1
|
Kiss B, Szántó M, Szklenár M, Brunyánszki
A, Marosvölgyi T, Sárosi E, Remenyik É, Gergely P, Virág L, Decsi
T, et al: Poly (ADP) ribose polymerase-1 ablation alters eicosanoid
and docosanoid signaling and metabolism in a murine model of
contact hypersensitivity. Mol Med Rep. 11:2861–2867.
2015.PubMed/NCBI
|
|
2
|
Roth-Walter F, Gomez-Casado C, Pacios LF,
Mothes-Luksch N, Roth GA, Singer J, Diaz-Perales A and
Jensen-Jarolim E: Bet v 1 from birch pollen is a lipocalin-like
protein acting as allergen only when devoid of iron by promoting
Th2 lymphocytes. J Biol Chem. 289:17416–17421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitoshi M, Kuriyama I, Nakayama H,
Miyazato H, Sugimoto K, Kobayashi Y, Jippo T, Kuramochi K, Yoshida
H and Mizushina Y: Suppression of allergic and inflammatory
responses by essential oils derived from herbal plants and citrus
fruits. Int J Mol Med. 33:1643–1651. 2014.PubMed/NCBI
|
|
4
|
Kim JH, Yi JS, Gong CH and Jang YJ:
Development of Aspergillus protease with ovalbumin-induced allergic
chronic rhinosinusitis model in the mouse. Am J Rhinol Allergy.
28:465–470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brandys J, Grimsøen A, Nilsen BM, Paulsen
BS, Park HS and Hong CS: Cross-reactivity between pollen extracts
from six artemisia species. Planta Med. 59:221–228. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olsen OT, Frølund L, Heinig J, Jacobsen L
and Svendsen UG: A double-blind, randomized study investigating the
efficacy and specificity of immunotherapy with Artemisia
vulgaris or Phleum pratense/Betula verrucosa.
Allergol Immunopathol (Madr). 23:73–78. 1995.PubMed/NCBI
|
|
7
|
Subiza J, Subiza JL, Alonso M, Hinojosa M,
Garcia R, Jerez M and Subiza E: Allergic conjunctivitis to
chamomile tea. Ann Allergy. 65:127–132. 1990.PubMed/NCBI
|
|
8
|
Ryu JC, Park SM, Hwangbo M, Byun SH, Ku
SK, Kim YW, Kim SC, Jee SY and Cho IJ: Methanol extract of
Artemisia apiacea hance attenuates the expression of
inflammatory mediators via NF-κB inactivation. Evid Based
Complement Alternat Med. Feb 22–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
9
|
Shin TY, Park JH and Kim HM: Effect of
Cryptotympana atrata extract on compound 48/80-induced
anaphylactic reactions. J Ethnopharmacol. 66:319–325. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noori A, Amjad L and Yazdani F: The
effects of Artemisia deserti ethanolic extract on pathology
and function of rat kidney. Avicenna J Phytomed. 4:371–376.
2014.PubMed/NCBI
|
|
11
|
Kuraishi Y, Nagasawa T, Hayashi K and
Satoh M: Scratching behavior induced by pruritogenic but not
algesiogenic agents in mice. Eur J Pharmacol. 275:229–233. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du Q, Gu X, Cai J, Huang M and Su M:
Chrysin attenuates allergic airway inflammation by modulating the
transcription factors T-bet and GATA-3 in mice. Mol Med Rep.
6:100–104. 2012.PubMed/NCBI
|
|
13
|
Cheng LJ, Liu B, Ning B, Ming H, Wang C
and Wan LX: High-intensity focused ultrasound for the treatment of
allergic rhinitis using nasal endoscopy. Exp Ther Med. 5:320–322.
2013.PubMed/NCBI
|
|
14
|
Hui Y, Li L, Qian J, Guo Y and Zhang X and
Zhang X: Efficacy analysis of three-year subcutaneous
SQ-standardized specific immunotherapy in house dust mite-allergic
children with asthma. Exp Ther Med. 7:630–634. 2014.PubMed/NCBI
|
|
15
|
Hollander SM, Joo SS and Wedner HJ:
Factors that predict the success of cyclosporine treatment for
chronic urticaria. Ann Allergy Asthma Immunol. 107:523–528. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HH, Choi PH, Yoo JS, Jeon H, Chae BS,
Park JS, Kim SH and Shin TY: Ripe fruit of Rubus coreanus
inhibits mast cell-mediated allergic inflammation. Int J Mol Med.
29:303–310. 2012.PubMed/NCBI
|
|
17
|
Yong J, Chen GQ, Huang B and Wu S:
Correlation between the ratio of T-bet/GATA-3 and the levels of
IL-4 and IFN-γ in patients with allergic asthma. Mol Med Rep.
4:663–666. 2011.PubMed/NCBI
|
|
18
|
Kim JY, Kim DY, Lee YS, Lee BK, Lee KH and
Ro JY: DA-9601, Artemisia asiatica herbal extract,
ameliorates airway inflammation of allergic asthma in mice. Mol
Cells. 22:104–112. 2006.PubMed/NCBI
|
|
19
|
Guo H and Liu MP: Mechanism of traditional
Chinese medicine in the treatment of allergic rhinitis. Chin Med J
(Engl). 126:756–760. 2013.PubMed/NCBI
|
|
20
|
Zhang F, Fu X, Lv Z, Shen Q, Yan T, Jiang
W, Wang G, Sun X and Tang K: Type 2C phosphatase 1 of Artemisia
annua L. Is a negative regulator of ABA signaling. Biomed Res Int.
2014:5217942014.PubMed/NCBI
|
|
21
|
Yin Y, Gong FY, Wu XX, Sun Y, Li YH, Chen
T and Xu Q: Anti-inflammatory and immunosuppressive effect of
flavones isolated from Artemisia vestita. J Ethnopharmacol.
120:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rasool R, Ganai BA, Akbar S and Kamili AN:
Free radical scavenging potential of in vitro raised and greenhouse
acclimatized plants of Artemisia amygdalina. Chin J Nat Med.
11:377–384. 2013.PubMed/NCBI
|
|
23
|
Park JM, Hahm KB, Kwon SO and Kim EH: The
Anti-inflammatory effects of acidic polysaccharide from
Artemisia capillaris on Helicobacter pylori
infection. J Cancer Prev. 18:161–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Puc M and Wolski T: Forecasting of the
selected features of Poaceae (R. Br.) Barnh.,
Artemisia L. and Ambrosia L. pollen season in
Szczecin, north-western Poland, using Gumbel's distribution. Ann
Agric Environ Med. 20:36–47. 2013.PubMed/NCBI
|