Introduction
One-lung ventilation (OLV) has become a standard
procedure for many interventions in thoracic surgery (1), and is particularly mandatory in
thoracoscopic surgery. Since it is not physiological, OLV may lead
to lung injury in both lungs (2–4). This is
one of the causes of postoperative pulmonary complications which
contribute substantially to the risks associated with surgery and
anesthesia, with major impact on health outcomes and are a
financial burden to health systems (5). Therefore, there are numerous animal and
clinical studies investigating OLV.
Rabbits, pigs, dogs and rats are commonly used
animals in this area of study (6–9). Due to
their relatively low cost and convenience in raising and operation,
rabbits are very popular among researchers. Rabbits are used in OLV
modeling in vivo and in vitro (isolated or perfused
lung model) (10,11). As the right lung is larger than the
left lung, oxygenation is better during right-OLV than left-OLV
(12), therefore right-OLV is often
selected when oxygenation is concerned. In our pre-experimental
work, it was relatively easy to build a left-OLV model based on the
present method of deeply intubating the endotracheal tube into the
left main bronchus. Therefore, in the present study we specifically
focused on right-OLV.
Several different methods are used to build in
vivo right-OLV model in rabbits, including deeply intubating
the endotracheal tube into the right main bronchus (13), clamping the left main bronchus with a
clip (14) and other methods, such
as inserting an artificial double-lumen endotracheal tube (DLT)
into the trachea, and inserting a bronchial blocker through the
ventilation tube into the left primary bronchus (6,15).
However, these methods each have disadvantages. The objective of
the present study was to compare a novel method with two other
methods in a number of variables: Circulation, oxygenation, airway
pressure, lung injury caused by OLV and time, blood loss and
success rate of modeling.
Materials and methods
Experimental protocol
Forty healthy New Zealand white rabbits (Jinling
Shuto Field, Nanjing, China) were studied following approval by the
Ethics Review Committee for Animal Experimentation of the
Affiliated Hospital of Nanjing Medical University (Nanjing,
China).
Induction of anesthesia was by intravenous
injections (4 ml/kg, i.v.) of 30% urethane (Shanghai Green Analysis
of Chemical Technology Co., Shanghai, China), after which animals
were placed on an operating table (DWV-II; On Haishao Feng
Laboratory Animal Equipment Co., Ltd, Shanghai, China) in the
supine position. Following initial anesthesia, urethane was
maintained at 5–8 ml/kg/h and tracheotomy was performed aseptically
at the level of 2–3 tracheal cartilage using a midline incision of
the neck. After infiltration with 1 ml lidocaine (2%; China Otsuka
Pharmaceutical Co., Ltd., Tianjin, China) and intravenous injection
of cisatracurium (2 mg/kg; Jiangsu Hengrui Medical Co., Ltd.,
Lianyungang, China), the trachea was intubated with a 3.0-mm
uncuffed endotracheal tube (Mallinckrodt Medical, Athlone,
Ireland), to 4 cm deep. Breathing sound and peak pressure (Ppeak)
were checked and the left lung was observed through a hole (0.5 cm)
between the 7–8 ribs of the left thoracic wall using a fiberscope
(FI-9RBS Portable Intubation Fiberscope; Pentax Canada, Inc., ON,
Canada). Then the trachea was tied to prevent air leak and 0.5 h of
baseline TLV (DW3000B small animal ventilation machine; ZS Dichuang
Science and Technology Development Co., Ltd., Beijing, China) was
started in all animals. Rabbits received volume controlled
ventilation with the following setting: Tidal volume
(VT) of 10 ml/kg, respiratory rate (RR) of 40
breaths/min, inspiratory:expiratory ratio (I:E) of 1:2, inspired
oxygen fraction (FiO2) of 0.6 and no PEEP. The right
femoral artery was cannulated (22, 24 G, BD arterial cannula; BD
Biosciences, Franklin Lakes, NJ, USA) for arterial blood pressure
and arterial blood gas analysis.
The room temperature was set at 25°C. Maintenance
anesthesia consisted of 30% urethane 1 ml/kg/h after tracheotomy
and cisatracurium 2 mg/kg/h was administered to achieve muscle
relaxation. Ringer's solution was infused at 10 ml/kg/h
continuously throughout the procedure. Depth of anesthesia was
assessed according to changes in vital signs (PM-9000 Express
Patient Monitor; Mindray Medical International Limited, Shenzen,
China) as primary criteria.
After 30 min of baseline TLV, rabbits were randomly
divided into four groups: Sham group (TLV for 3 h to be a
contrast), deep intubation group (right-OLV for 3 h by deeply
intubating the tube into the right main bronchus), clamp group
(right-OLV for 3 h by clamping the left main bronchus) and blocker
group (right-OLV for 3 h by deeply intubating the self-made
bronchial blocker into the left main bronchus). Breathing sound and
Ppeak were checked and the collapsed left lung was observed before
and after rabbits were turned to the right-lateral position, which
was used to simulate surgical position. Subsequently, breath sound,
Ppeak and the collapsed left lung were checked every 30 min.
After arterial blood gas analysis at the end point
(3 h of OLV), anesthesia was deepened with boluses of urethane
(30%; 3 ml), the animal was euthanized by exsanguination. After the
position of the deeply intubated endotracheal tube, the clamp,
self-made bronchial blocker, collapsed left lung and ventilated
right lung were checked, specimens were surgically obtained
immediately which includes trimmed lung-tissue block concerning
~1.5×1×0.3 cm3 at the same location of different lung
individually. The tissue block was fixed in 10% neutral-buffered
formaldehyde and embedded in paraffin.
Data collection
Arterial blood samples were measured (i-STAT
portable clinical analyzer; Abbott Laboratories, Inc., Lake Bluff,
IL, USA) at 30 min of TLV (T=0); 10 min (T=10 min), 30 min (T=30
min), 1 h (T=1 h), 2 h (T=2 h), 3 h (T=3 h) of OLV (arterial blood
gas analysis was measured at same time after changing to the
right-lateral position for the sham group).
The length of the left and right main bronchus and
the trachea from the carina to the tracheal cut were recorded.
During the right-OLV modeling, time, blood loss, first-time and
final success rate of right-OLV modeling were all recorded.
The paraffin-embedded tissue blocks were sliced to 5
µm sections. Routine hematoxylin and eosin staining was performed
on each section for histopathological examination, and were
evaluated by two individual pathologists being ignored by
experimental protocols. Random fields from each slide were
digitally imaged for qualitative analysis (BX40; Olympus
Corporation, Tokyo, Japan) and photographed using a Panasonic
WV-CP450 camera (Panasonic Corporation, Osaka, Japan). Lung injury
was scored on the basis of the following four morphological
features: i) Alveolar congestion; ii) hemorrhage; iii)
infiltrations or aggregations of neutrophils in airspace or the
vessel wall; and iv) thickness of the alveolar wall/hyaline
membrane formation. Each item was graded according to a five-point
scale, as described previously: 0=Minimal (little) damage, 1=mild
damage, 2=moderate damage, 3=severe damage and 4=maximal damage
(16).
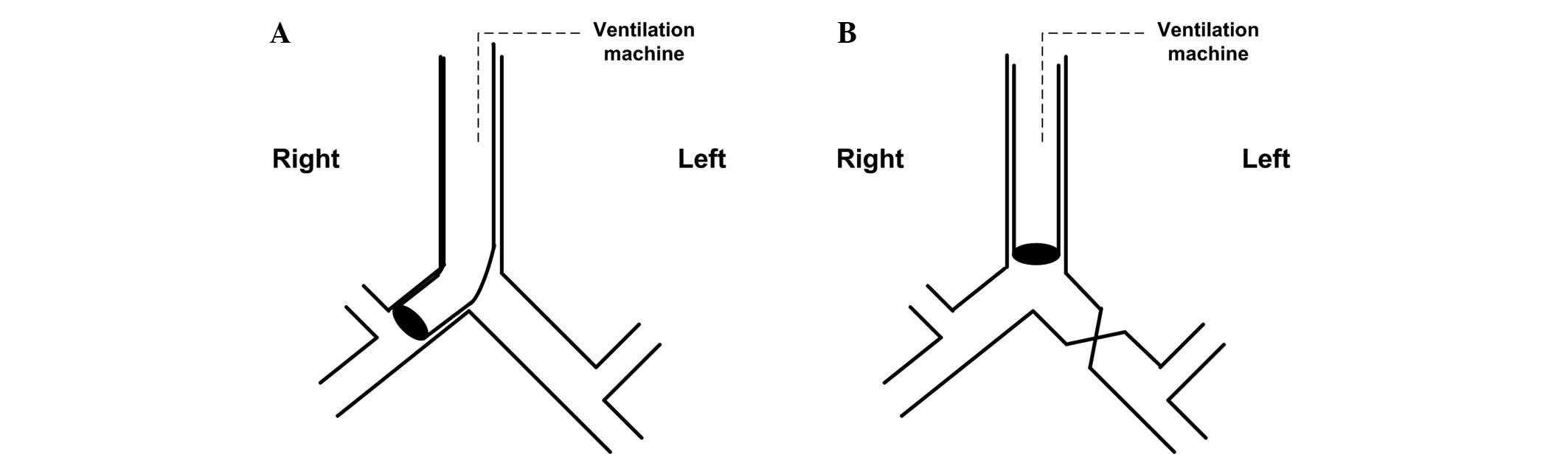
Right-OLV modeling
Deep intubation group (Fig. 1A): Loosen the tie of the trachea,
turn the rabbit's head to the left, deeply intubate the
endotracheal tube to the right main bronchus until feeling
resistance, then slightly regulate the tube position by checking
breathing sound, Ppeak and observing the collapsed left lung.
Finally, tie the trachea again to prevent air leak and tube
movement.
Clamp group (Fig.
1B): Perform thoracotomy by cutting between the fourth and
fifth ribs, separate the left main bronchus, then completely clamp
the left main bronchus with a clip, determine the effect of the
clamp through checking breathing sound, Ppeak and observing the
collapsed left lung.
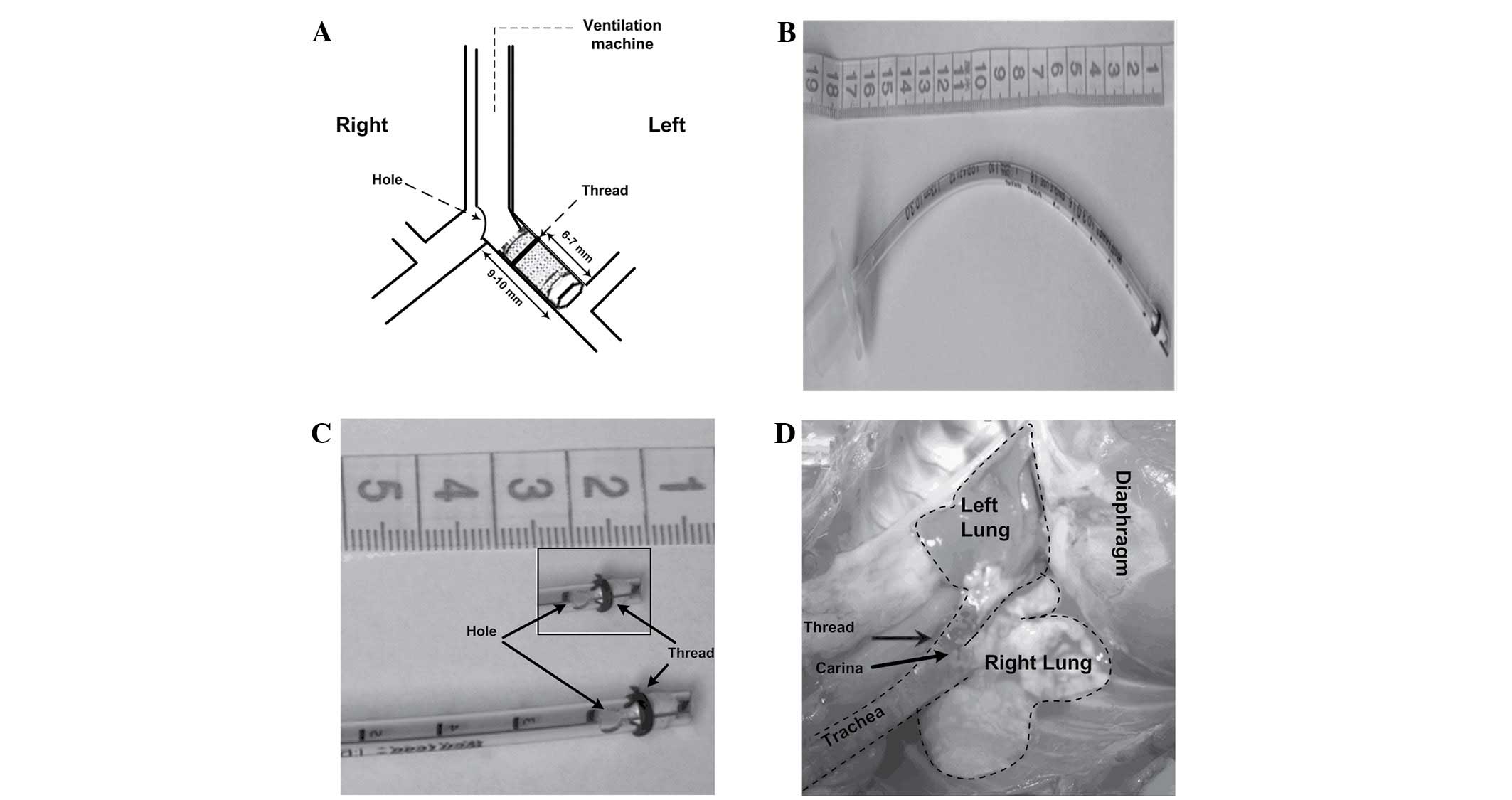
Blocker group (Fig.
2): Loosen the tie of the trachea, turn the rabbit head to the
right, deeply intubate the self-made bronchial blocker to the left
main bronchus until resistance is felt, then slightly regulate the
tube position by checking breathing sound, Ppeak and observing the
collapsed left lung. Finally, tie the trachea again to prevent air
leak and tube movement. The bronchial blocker was made as follows:
First, an uncuffed endotracheal tube (3.0 mm, endotracheal tube;
Mallinckrodt Medical, Athlone, Ireland) was blocked at the tip with
a 5-mm endotracheal tube core wrapped up with several levels of
water-repellent film; Second, a leaking test was conducted. Connect
the blocked tube to the ventilation machine with the following
settings: VT of 10 ml/kg, RR of 40 breaths/min, I:E of
1:2, FIO2 of 0.6 and no PEEP. No bubble was
found when the blocked tube was put in the water while the small
animal ventilation machine showed the Ppeak of 4.2 cm
H2O (~2x of the highest Ppeak observed in the
experiment); Third, thread (3–0, 2 metric, SA84G, Mersilk, silk
braided non-absorbable suture; Johnson & Johnson Medical China
Ltd., Shanghai, China) was wound around the blocker at 6–7 mm from
the tip to make the blocker attach tightly to the left bronchial
wall and an elliptical hole (4×3 mm) was made (9–10 mm from the
head) to be adjacent to the opening of the right main bronchus.
Statistical analysis
All statistical analyses were performed using the
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Results were
expressed as mean ± standard deviation for quantitative variables
(all the results except ALI scores and success rate), and ALI
scores were presented as the mean rank. Quantitative variables were
analyzed by general linear model followed by multivariate test.
Lung injury scores were analyzed by general linear model followed
by univariate test after rank transform. First-time and final
success rate of modeling were analyzed by crosstabs with
χ2. P<0.05 was considered to indicate a statistically
significant difference.
Results
Rabbit treatment and anatomy
index
The rabbits in the four groups had similar weight,
total dosage of urethane, cisatracurium and Ringer's solution
(Table I). Based on anatomy, the
rabbits in four groups had similar length from the carina to the
tracheal cut (2–3 tracheal cartilage), and similar length of the
left and right main bronchus (Table
II).
 | Table I.Weight, total usage of urethane,
cisatracurium and Ringer's solution. |
Table I.
Weight, total usage of urethane,
cisatracurium and Ringer's solution.
| Group | Weight (kg) | Urethane (g) | Cisatracurium
(mg) | Ringer's solution
(ml) |
|---|
| Sham | 2.07±0.17 | 20±3 | 12.9±4.3 | 117±15 |
| Deep intubation | 1.97±0.10 | 20±5 | 12.6±4.1 | 127±9 |
| Clamp | 2.00±0.14 | 23±4 | 16.0±3.9 | 116±9 |
| Blocker | 2.06±0.14 | 19±3 | 15.4±4.1 | 125±15 |
 | Table II.Anatomy index of rabbit airway. |
Table II.
Anatomy index of rabbit airway.
| Group | Length from the
carina to the tracheal cut (cm) | Length of the left
main bronchus (cm) | Length of the right
main bronchus (cm) |
|---|
| Sham | 6.10±0.42 | 0.97±0.14 | 0.53±0.08 |
| Deep intubation | 6.04±0.57 | 1.00±0.10 | 0.55±0.11 |
| Clamp | 6.37±0.55 | 0.95±0.12 | 0.55±0.08 |
| Blocker | 5.97±0.51 | 1.00±0.11 | 0.52±0.06 |
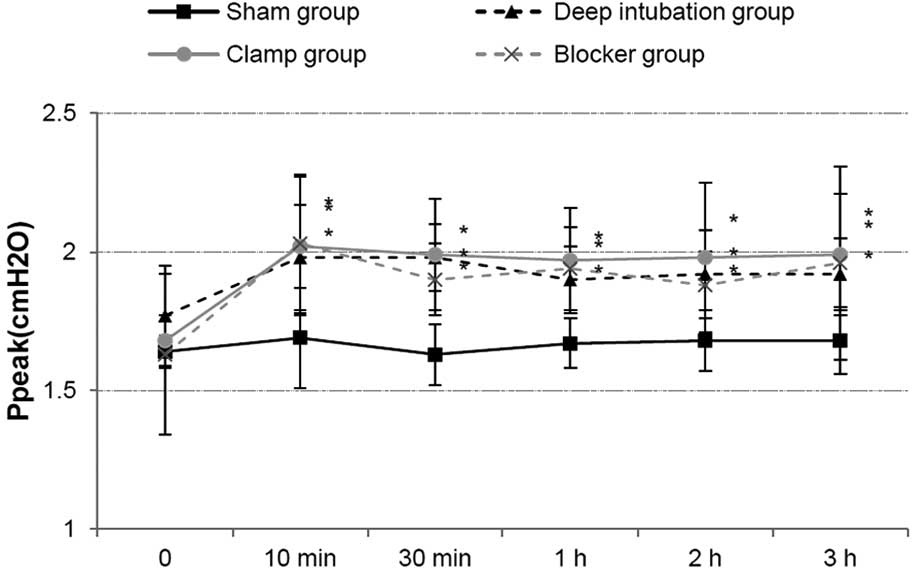
Circulation, oxygenation and airway
pressure
HR and MAP showed no differences at all time points
among the three OLV groups. Ppeak increased in three OLV groups
after OLV, compared with sham group, and it showed no differences
among three OLV groups (Fig. 3).
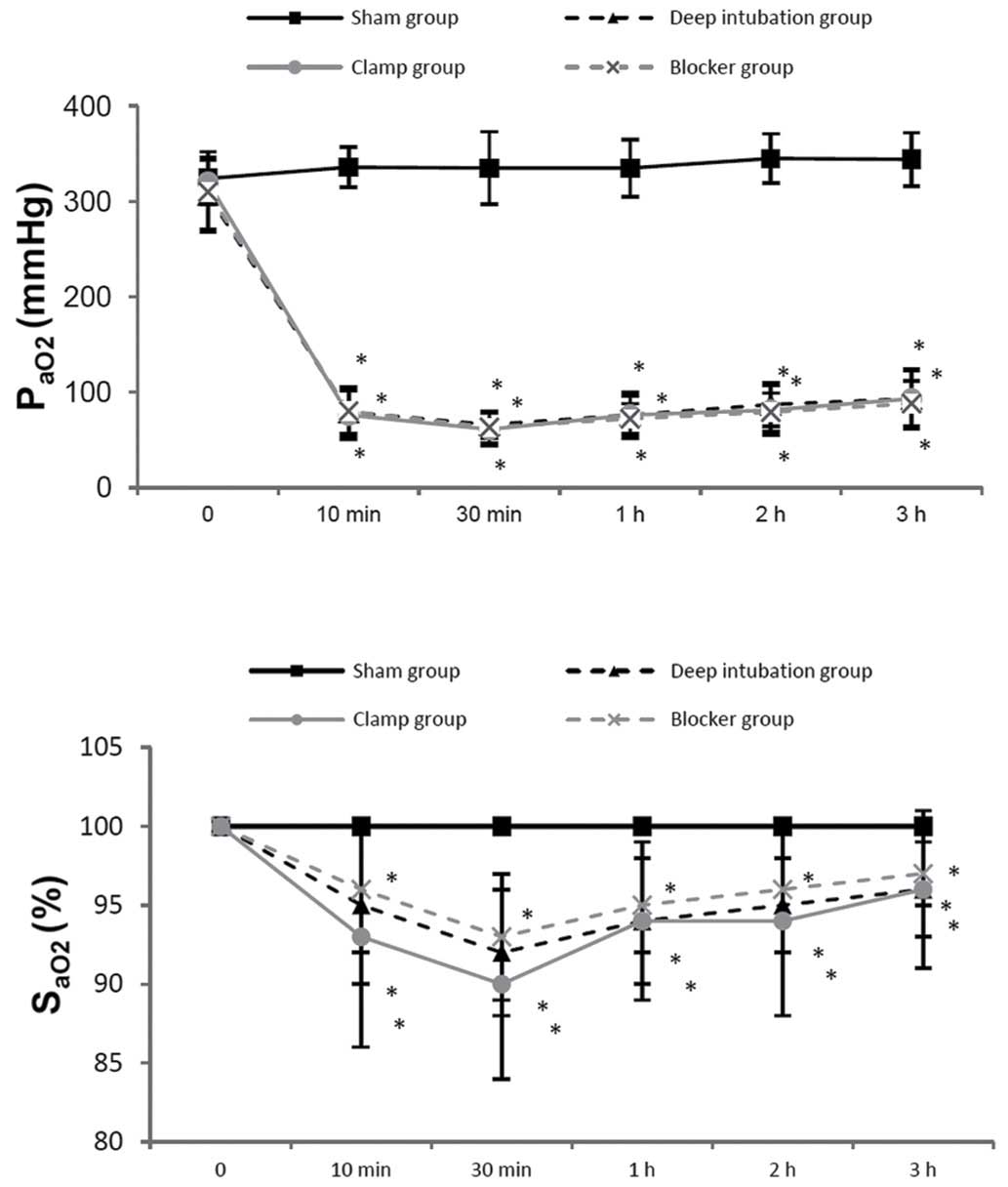
PaO2 and SaO2 were lower in three OLV groups
at 10 min, 30 min, 1 h, 2 h and 3 h of OLV, compared with the sham
group, and it showed no differences among three OLV groups
(Fig. 4). PH and PaCO2
showed no differences among the three OLV groups.
Lung injury caused by OLV
Sham group showed nearly normal lung histology. The
three OLV groups showed edema, thickening of the alveolar wall,
infiltration of inflammatory cells into alveolar spaces and
interstitial spaces at 3 h of OLV (Fig.
5). Lung injury score was higher at 3 h of OLV in three OLV
groups (Deep intubation group/left: 2.4±1.1, P<0.05, right:
2.4±1.1, P<0.01; Clamp group/left: 2.7±1.1, P<0.01, right:
2.3±1.5, P<0.05; Blocker group/left: 2.1±1.4, P<0.05, right:
2.4±1.1, P<0.01) compared with sham group (left: 1.0±0.7, right:
0.9±0.7), and no differences were detected among three OLV
groups.
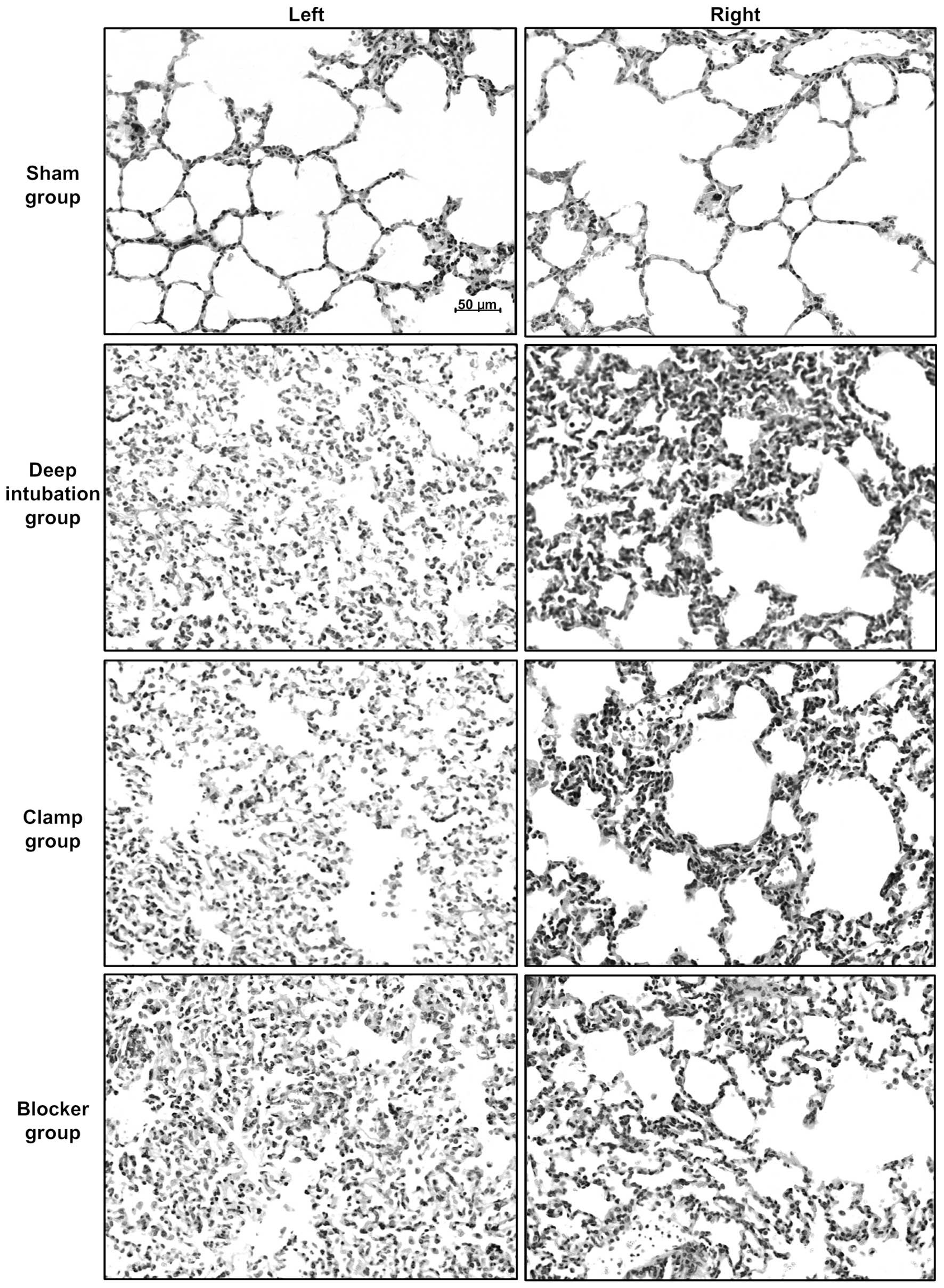 | Figure 5.Representative photographs of
histological sections of rabbit lungs at 3 h of one-lung
ventilation (OLV). Lung histology specimens from the left/right
lungs of the sham, deep intubation, clamp and blocker groups. Three
OLV groups showed edema, thickening of the alveolar wall,
infiltration of inflammatory cells into alveolar spaces and
interstitial spaces, compared with sham group. All images are at
×200 magnification. Lung injury score was higher at 3 h of OLV in
three OLV groups (deep intubation group/left: 2.4±1.1, P<0.05,
right: 2.4±1.1, P<0.01; clamp group/left: 2.7±1.1, P<0.01,
right: 2.3±1.5, P<0.05; blocker group/left: 2.1±1.4, P<0.05,
right: 2.4±1.1, P<0.01) compared with sham group (left: 1.0±0.7,
right: 0.9±0.7), and it showed no differences among three OLV
groups. |
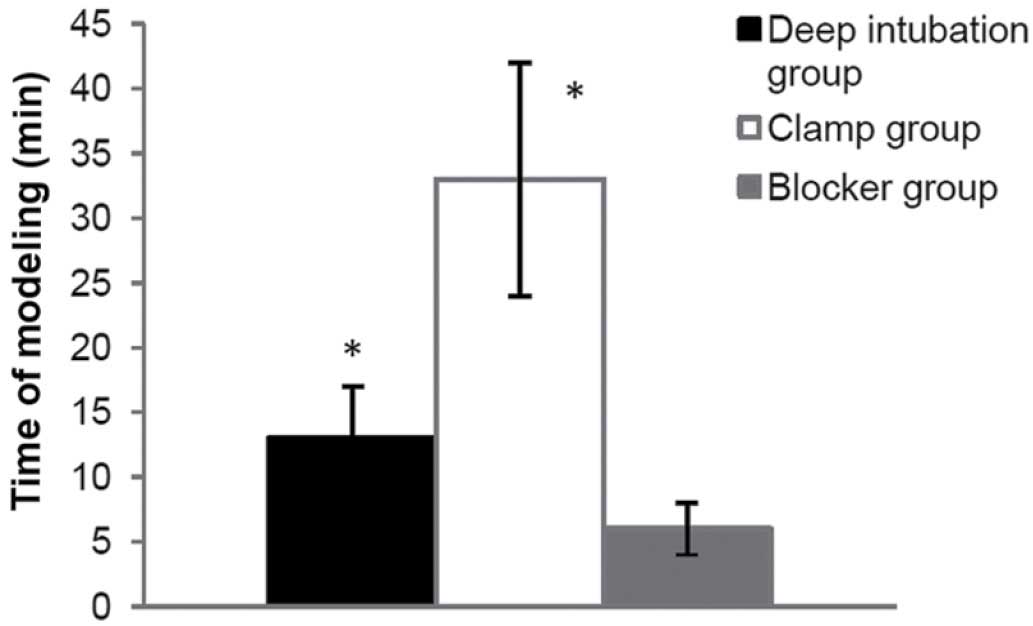
Time of modeling
(build right-OLV model after 30 min of TLV, and
check breathing sound, Ppeak and the collapsed left lung
immediately after changed to lateral position). Less time was spent
in blocker group (6±2 min), compared with the other two OLV groups
(13±4 min in the deep intubation group, P<0.05; 33±9 min in the
clamp group, P<0.001) (Fig.
6).
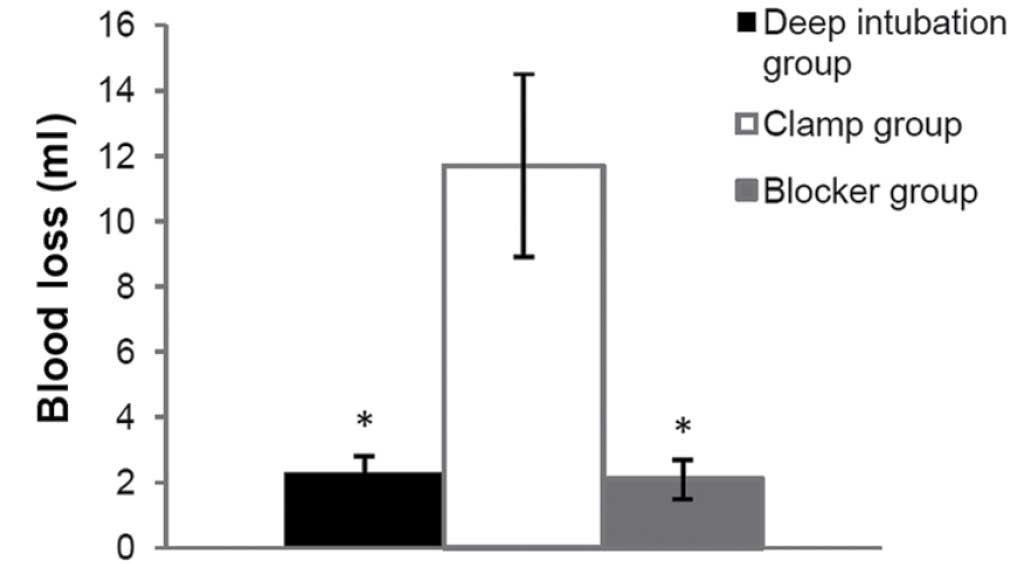
Blood loss during modeling
During right-OLV modeling, more blood loss was
observed in the clamp group (11.7±2.8 ml), compared with the other
two OLV groups (2.3±0.5 ml in deep intubation group, P<0.001;
2.1±0.6 ml in blocker group P<0.001) (Fig. 7).
Success rate
The first-time success rate of right-OLV modeling
showed no differences among three OLV groups (50% in deep
intubation group, 70% in clamp group and 70% in blocker group). The
final success rate of right-OLV modeling also showed no differences
among three OLV groups (70% in deep intubation group, 70% in clamp
group and 80% in blocker group).
Discussion
The present study presents a novel method for
establishing a right-OLV model in rabbits. Rabbits, dogs, pigs and
rats are the most commonly used animals in an building OLV model.
Dogs and pigs have relatively bigger airway size, which makes it
easy and convenient to build both left and right OLV models using a
DLT or endobronchial blocker (7,8,17). However, they are expensive and
difficult to raise, so they are less commonly selected once
research funding and lab qualification are taken into
consideration. By contrast, rabbits are cheap and easy to raise,
and compared with rats, rabbits have a median airway size and are
convenient for building OLV models.
As the right lung is larger than the left lung, it
is not surprising that oxygenation during right-OLV is better than
during left-OLV (12). In
Schwarzkopf et al (18), they
found that while ventilating with 1.0 FiO2,
PaO2 during OLV was ~280 mmHg during left-sided thoracic
surgery as compared with ~170 mmHg during right-sided operations.
Slinger et al (19) using
regression analysis, found the side of operation to be one of the
important factors in predicting hypoxemia during OLV. Therefore,
right-OLV is safer than left-OLV when oxygenation is concerned,
numerous researchers select right-OLV in their studies (20,21).
In our pre-experimental work, it was easier to build
left-OLV model in rabbits by deeply intubating the endotracheal
tube into the left main bronchus which is ~1 cm long. However, the
right main bronchus is ~0.5-cm long according to our anatomical
examination, and the right upper pulmonary lobe is easily blocked
by deeply intubated tube. Based on these reasons, we particularly
focused on right-OLV rabbit model in the present study.
Furthermore, there are several methods to build right-OLV in
rabbits, which each have disadvantages.
One method to build right-OLV is deeply intubating
the tracheal tube into the right main bronchus (13). In the present study, the right main
bronchus of rabbit (~0.5 cm long) was found to be markedly shorter
than the left main bronchus. Thus, it was difficult to fix the
deeply intubated endotracheal tube in the right place and required
a longer time (13±4 min), compared with the blocker group (6±2 min,
P<0.05). Moreover, the endotracheal tube is easier to slide to
the wrong position after the rabbits turned from supine to right
lateral position. We failed to establish a right-OLV model in half
of the rabbits (five out of ten rabbits in the deep intubation
group: part of the left lung was ventilated in three rabbits, part
of the right upper lung was collapsed in two rabbits, failure rate:
50%) in the first time. Following regulation, the final failure
rate decreased to 30% (two cases with partial ventilated left lung
were corrected, the other three failure cases were certified until
final anatomy), so the final success rate was 70%.
Another method is to clamp the left main bronchus.
After a thoracotomy was performed, the left main bronchus was
separated and completely clamped with a clip (14). Since thoractomy and bronchus
separation should be performed, this method was accompanied by
further trauma and surgical interference. In our pre-experimental
work, it took us a relatively long time to learn this method under
a surgeon's guidance, compared with the other two OLV group
methods. More blood loss was observed in the clamp group (11.7±2.8
ml), compared with the other two OLV groups (2.3±0.5 ml in deep
intubation group, P<0.001; 2.1±0.6 ml in blocker group,
P<0.001), and bleeding was the predominant cause of failure
(failure rate: 30%). Also it took longer time (33±9 min) to
complete the whole procedure, compared with the other two OLV
groups (13±4 min in deep intubation group, P<0.001; 6±2 min in
blocker group, P<0.001).
Our self-made bronchial blocker may be easily
constructed, with no special materials or technique required. The
material includes a 3.0 mm endotracheal tube, 3–0 silk, small
scissors, common tube core which is used clinically during adult
intubation, water-repellent film and wire cutters, which are common
materials purchasable in hardware stores. A single person may
construct our bronchial blocker in ~10 min.
Technically, the present novel method relies on two
key factors: Blocking and ventilation. The tube core was wrapped up
with several layers of water-repellent film before blocking the
tube, then leaking test was performed. These guaranteed effective
air-block from inside the blocker. The 3.0-mm uncuffed endotracheal
tube was selected based on pre-experimental work and measurement of
the diameter of the rabbit trachea and left/right main bronchus.
The reason we gave up the 3.0-mm cuffed endotracheal tube is that
the length from the tip to the cuff is >1 cm even part of the
cuff is tied; thus, the cuff is up the level of carina instead of
completely inside the left main bronchus. The experimental results
indicate that the performance of 3.0-mm uncuffed endotracheal tube
was adequate and only one of the rabbits had a thinner left
bronchus, in which a 2.5-mm endotracheal tube was used. Besides the
appropriate size of the blocker, thread was wound around the
blocker at 6–7 mm from the tip to attach the blocker tightly to the
left bronchial wall, and the thread was in the left main bronchus
beyond the carina in those successful cases shown by anatomy.
Meanwhile, immediately after endpoint, the left main bronchus was
cut beyond the blocker and put in water with ventilation machine
still running, and no air bubbles were found. These all guaranteed
effective air-block from between the blocker and the left main
bronchus. Thus, from inside to outside the blocker, the blocking
effect is certified.
The hole was made 9–10 mm from the tip to face the
opening of the right main bronchus. The hole was up the carina
shown by anatomy after endpoint. These ensured effective
ventilation of the right lung. In conclusion, all the sizes were
selected on the basis of anatomy, and little difference was found
between the rabbits which did not influence our modeling so much.
During OLV, breathing sound, Ppeak and the collapsed left lung were
examined. Ultimately, the position of the blocker, the ventilated
right lung and collapsed left lung were checked through anatomy,
these all guaranteed the reliability of our modeling.
In the present study, Ppeak increased,
PaO2, SaO2 decreased in three OLV groups at
10 min, 30 min, 1 h, 2 h, 3 h of OLV, compared with sham group. The
three OLV groups showed more lung injury with higher lung injury
score at 3 h of OLV, compared with sham group, which was consistent
with previous studies (22–24), indicating the reliability of the OLV
model in all three OLV groups. Meanwhile, the three OLV groups had
similar oxygenation, circulation, airway pressure and acute lung
injury score, which meant there were no differences among the three
OLV models built by three different methods. Furthermore, our novel
method (self-made bronchial blocker) required less time and
resulted in reduced blood loss during modeling.
There are other methods used in OLV modeling in
rabbits. One method recently proposed by You et al involves
inserting a self-made artificial DLT into the trachea (6). A specific technique is required to
construct the artificial DLT, which has limited its use. Meanwhile,
DLT has two thinner individual lumens, the airway pressure is
higher and the lumen is more easily blocked by sputum. Another
method previously advanced is a 4F bronchial blocker through the
ventilation tube into the left main bronchus with the blocker cuff
placed precisely distal to the carina (15). The disadvantage of this method is
similar to that of the artificial DLT. The outer diameter of 4F
bronchial blocker is 1.33 mm, the inner diameter of 3.0 mm
endotracheal tube is 3.0 mm. After the 4F bronchial blocker is
inserted through the 3.0 mm endotracheal tube, the airway pressure
will be increased and the tube is more easily blocked by airway
secretion. As these methods are infrequently used, they were not
included in the present study.
In conclusion, the present study suggests that the
use of our self-made bronchial blocker to block the left main lung
may be an effective, convenient and reliable method for
establishing a right-OLV model in rabbits.
Acknowledgements
The present study was subsidized by grants from the
Department of Anesthesiology, Jiangsu Cancer Hospital. The authors
thank the epidemiologist Mr. Suping Li for assistance with the
statistical work in the present study and thoracic surgeon Dr
Dongjie Feng for assistance with rabbit modeling.
Glossary
Abbreviations
Abbreviations:
|
OLV
|
one-lung ventilation
|
|
TLV
|
two lung ventilation
|
|
HR
|
heart rate
|
|
MAP
|
mean arterial pressure
|
|
PaO2
|
arterial partial pressure of
oxygen
|
|
SaO2
|
arterial hemoglobin oxygen
saturation
|
|
Ppeak
|
peak pressure
|
|
VT
|
tidal volume
|
|
RR
|
respiratory rate
|
|
DLT
|
double-lumen endotracheal tube
|
References
|
1
|
Della Rocca G and Coccia C: Ventilatory
management of one-lung ventilation. Minerva Anestesiol. 77:534–536.
2011.PubMed/NCBI
|
|
2
|
Eguchi T, Hamanaka K, Kondo R, Saito G,
Shiina T, Koizumi T and Yoshida K: Lung re-expansion following
one-lung ventilation induces neutrophil cytoskeletal rearrangements
in rats. Ann Thorac Cardiovasc Surg. 20:276–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kozian A, Schilling T, Fredén F, Maripuu
E, Röcken C, Strang C, Hachenberg T and Hedenstierna G: One-lung
ventilation induces hyperperfusion and alveolar damage in the
ventilated lung: An experimental study. Br J Anaesth. 100:549–559.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kallet RH and Matthay MA: Hyperoxic acute
lung injury. Respir Care. 58:123–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Abreu MG and Pelosi P: How can we
prevent postoperative pulmonary complications? Curr Opin
Anaesthesiol. 26:105–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You Z, Feng D, Xu H, Cheng M, Li Z, Kan M
and Yao S: Nuclear factor-kappa B mediates one-lung
ventilation-induced acute lung injury in rabbits. J Invest Surg.
25:78–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schilling T, Kretzschmar M, Hachenberg T,
Hedenstier-Na G and Kozian A: The immune response to
one-lung-ventilation is not affected by repeated alveolar
recruitment manoeuvres in pigs. Minerva Anestesiol. 79:590–603.
2013.PubMed/NCBI
|
|
8
|
Adami C, Axiak S, Rytz U and Spadavecchia
C: Alternating one lung ventilation using a double lumen
endobronchial tube and providing CPAP to the non-ventilated lung in
a dog. Vet Anaesth Analg. 38:70–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siegl S and Uhlig S: Using the one-lung
method to link p38 to pro-inflammatory gene expression during
overventilation in C57BL/6 and BALB/c mice. PLoS One. 7:e414642012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siempos II, Maniatis NA, Kopterides P,
Magkou C, Glynos C, Roussos C and Armaganidis A: Pretreatment with
atorvastatin attenuates lung injury caused by high-stretch
mechanical ventilation in an isolated rabbit lung model. Crit Care
Med. 38:1321–1328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu R, Yang Y, Li Y, Li J, Ma Q, Zhao Y
and Wang D: Effects of sevoflurane on pulmonary cytosolic
phospholipase A2 and clara cell secretory protein
expressions inrabbits with one-lung ventilation-induced lung
injury. Nan Fang Yi Ke Da Xue Xue Bao. 33:469–473. 2013.PubMed/NCBI
|
|
12
|
Katz Y, Zisman E, Isserles SA and
Rozenberg B: Left, but not right, one-lung ventilation causes
hypoxemia during endoscopic transthoracic sympathectomy. J
Cardiothorac Vasc Anesth. 10:207–209. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schreiber T, Niemann C, Schmidt B and
Karzai W: A novel model of selective lung ventilation to
investigate the long-term effects of ventilation-induced lung
injury. Shock. 26:50–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura M, Fujishima S, Sawafuji M,
Ishizaka A, Oguma T, Soejima K, Matsubara H, Tasaka S, Kikuchi K,
Kobayashi K, et al: Importance of interleukin-8 in the development
of reexpansion lung injury in rabbits. Am J Respir Crit Care Med.
161:1030–1036. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Groh J, Kuhnle GE, Sckell A, Ney L and
Goetz AE: Isoflurane inhibits hypoxic pulmonary vasoconstriction.
An in vivo fluorescence microscopic study in rabbits.
Anesthesiology. 81:1436–1444. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu D, Zeng BX and Shang Y: Decreased
expression of peroxisome proliferator-activated receptor gamma in
endotoxin-induced acute lung injury. Physiol Res. 55:291–299.
2006.PubMed/NCBI
|
|
17
|
Mayhew PD, Culp WT, Pascoe PJ, Kass PH and
Johnson LR: Evaluation of blind thoracoscopic-assisted placement of
three double-lumen endobronchial tube designs for one-lung
ventilation in dogs. Vet Surg. 41:664–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwarzkopf K, Klein U, Schreiber T,
Preussler NP, Bloos F, Helfritsch H, Sauer F and Karzai W:
Oxygenation during one-lung ventilation: The effects of inhaled
nitric oxide and increasing levels of inspired fraction of oxygen.
Anesth Analg. 92:842–847. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Slinger P, Suissa S and Triolet W:
Predicting arterial oxygenation during one-lung anaesthesia. Can J
Anaesth. 39:1030–1035. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kozian A, Schilling T, Fredén F, Maripuu
E, Röcken C, Strang C, Hachenberg T and Hedenstierna G: One-lung
ventilation induces hyperperfusion and alveolar damage in the
ventilated lung: An experimental study. Br J Anaesth. 100:549–559.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhatia R, Shaffer TH, Hossain J, Fisher
AO, Horner LM, Rodriguez ME, Penfil S and Theroux MC: Surfactant
administration prior to one lung ventilation: Physiological and
inflammatory correlates in a piglet model. Pediatr Pulmonol.
46:1069–1078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe S, Noguchi E, Yamada S, Hamada N
and Kano T: Sequential changes of arterial oxygen tension in the
supine position during one-lung ventilation. Anesth Analg.
90:28–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin K, Gribbin E, Emanuel S, Orndorff R,
Walker J, Weese J and Fallahnejad M: Histochemical alterations in
one lung ventilation. J Surg Res. 137:16–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Della Rocca G and Coccia C: Acute lung
injury in thoracic surgery. Curr Opin Anaesthesiol. 26:40–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|