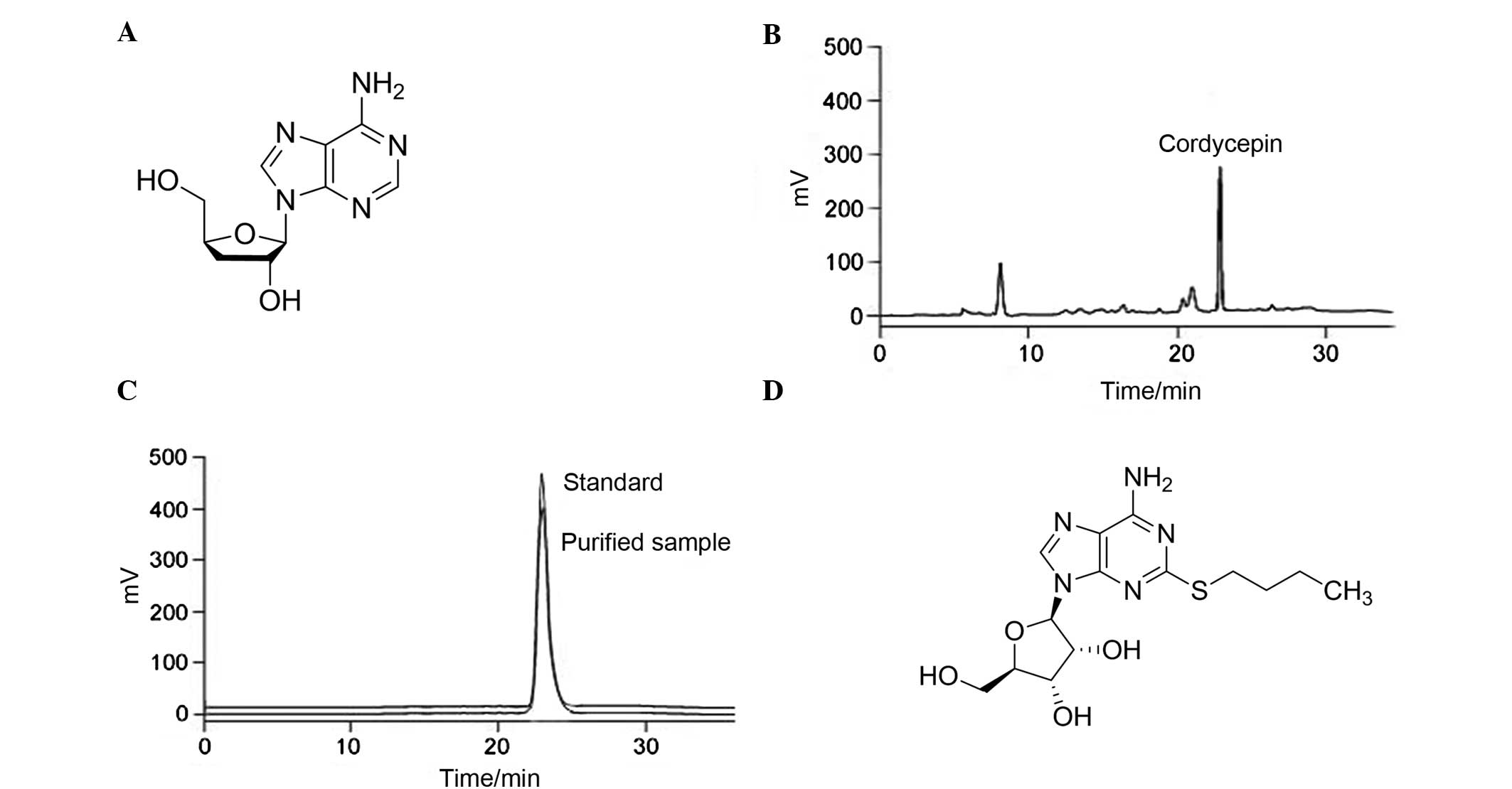
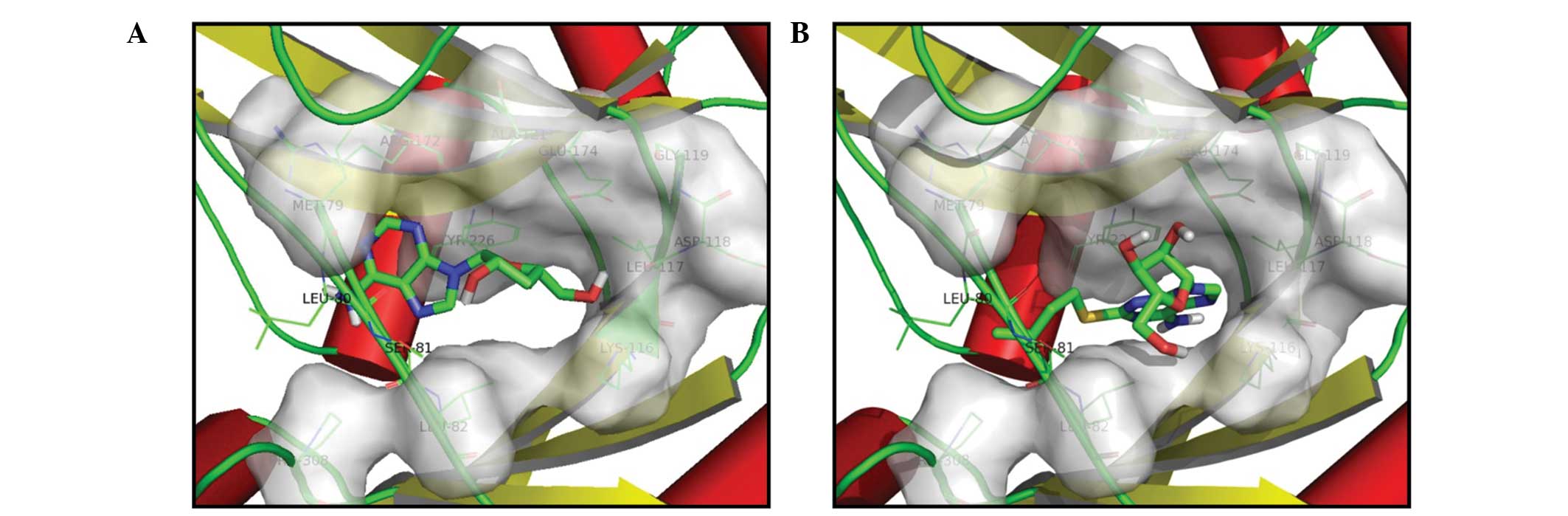
|
1
|
Cunningham KG, Manson W, Spring FS and
Hutchinson SA: Cordycepin, a metabolic product isolated from
cultures of Cordyceps militaris (Linn.) Link. Nature.
166:9491950. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaczka EA, Trenner NR, Arison B, Walker RW
and Folkers K: Identification of cordycepin, a metabolite of
Cordyceps militaris, as 3′-deoxyadenosine. Biochem Biophys
Res Commun. 14:456–457. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frederiksen S, Malling H and Klenow H:
Isolation of 3′-deoxyadenosine (cordycepin) from the liquid medium
of Cordyceps militaris (L. ex Fr.) Link. Biochim Biophys
Acta. 95:189–193. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kodama EN, McCaffrey RP, Yusa K and
Mitsuya H: Antileukemic activity and mechanism of action of
cordycepin against terminal deoxynucleotidyl transferase-positive
(TdT+) leukemic cells. Biochem Pharmacol. 59:273–281.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen LS, Stellrecht CM and Gandhi V:
RNA-directed agent, cordycepin, induces cell death in multiple
myeloma cells. Br J Haematol. 140:682–691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang W, Lim S, Song H, Kim HJ, Cha MJ,
Sung JM, Kim TW and Hwang KC: Cordycepin inhibits vascular smooth
muscle cell proliferation. Eur J Pharmacol. 597:64–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomadaki H, Tsiapalis CM and Scorilas A:
The effect of the polyadenylation inhibitor cordycepin on human
Molt-4 and Daudi leukaemia and lymphoma cell lines. Cancer
Chemother Pharmacol. 61:703–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn YJ, Park SJ, Lee SG, Shin SC and Choi
DH: Cordycepin: Selective growth inhibitor derived from liquid
culture of Cordyceps militaris against Clostridium
spp. J Agric Food Chem. 48:2744–2748. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugar AM and McCaffrey RP: Antifungal
activity of 3′-deoxyadenosine (cordycepin). Antimicrob Agents
Chemother. 42:1424–1427. 1998.PubMed/NCBI
|
|
10
|
King RW, Zecher M and Jefferies MW:
Inhibition of the replication of a hepatitis C virus-like RNA
template by interferon and 3′-deoxycytidine. Antivir Chem
Chemother. 13:363–370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Luo L, Dressel W, Shadier G,
Krumbiegel D, Schmidtke P, Zepp F and Meyer CU: Cordycepin is an
immunoregulatory active ingredient of Cordyceps sinensis. Am
J Chin Med. 36:967–980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiapeng T, Yiting L and Li Z: Optimization
of fermentation conditions and purification of cordycepin from
Cordyceps militaris. Prep Biochem Biotechnol. 44:90–106.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mao XB, Zhu LH, Li G and Wang XG:
Investigation of cordycepin adsorption kinetics by cation exchange
resin. Appl Chem Ind. 37:272–274. 2008.
|
|
14
|
Ni H, Zhou XH, Li HH and Huang WF: Column
chromatographic extraction and preparation of cordycepin from
Cordyceps militaris waster medium. J Chromatogr B Analyt
Technol Biomed Life Sci. 877:2135–2141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ling JY, Zhang GY, Lin JQ, Cui ZJ and
Zhang CK: Supercritical fluid extraction of cordycepin and
adenosine from Cordyceps kyushuensis and purification by
high-speed counter-current chromatography. Sep Purif Technol.
66:625–629. 2009. View Article : Google Scholar
|
|
16
|
Colin H: Preparative chromatography today.
Analusis. 26:M15–M17. 1998. View Article : Google Scholar
|
|
17
|
Lee YW: Comparison between
ultra-performance liquid chromatography with tandem mass
spectrometry and a chemiluminescence immunoassay in the
determination of cyclosporine A and tacrolimus levels in whole
blood. Exp Ther Med. 6:1535–1539. 2013.PubMed/NCBI
|
|
18
|
Taylor RD, Jewsbury PJ and Essex JW: A
review of protein-small molecule docking methods. J Comput Aided
Mol Des. 16:151–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reis FS, Barros L, Calhelha RC, Cirić A,
van Griensven LJ, Soković M and Ferreira IC: The methanolic extract
of Cordyceps militaris (L.) Link fruiting body shows
antioxidant, antibacterial, antifungal and antihuman tumor cell
lines properties. Food Chem Toxicol. 62:91–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bensona EL, Tomichb PK, Wolfec ML, Choi
GH, Hagadom JC, Mutchler VT and Garlick RL: A high-throughput
resonance energy transfer assay for Staphylococcus aureus
DNA ligase. Anal Biochem. 324:298–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen XC, Hentz NG, Hubbard F, Meier TI,
Sittampalam S and Zhao G: Development of a fluorescence resonance
energy transfer assay for measuring the activity of
Streptococcus pneumoniae DNA ligase, an enzyme essential for
DNA replication, repair and recombination. Anal Biochem.
309:232–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mills SD, Eakin AE, Buurman ET, Newman JV,
Gao N, Huynh H, Johnson KD, Lahiri S, Shapiro AB, Walkup GK, et al:
Novel bacterial NAD+-dependent DNA ligase inhibitors
with broad-spectrum activity and antibacterial efficacy in vivo.
Antimicrob Agents Chemother. 55:1088–1096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen J, Guan Y, Zhang J, Tang J, Lu X and
Zhang C: Application of microarray technology for the detection of
intracranial bacterial infection. Exp Ther Med. 7:496–500.
2014.PubMed/NCBI
|
|
24
|
Siev M, Weingerg R and Penman S: The
selective interruption of nucleolar RNA synthesis in HeLa cells by
cordycepin. J Cell Biol. 41:510–520. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kondrashov A, Meijer HA, Barthet-Barateig
A, Parker HN, Khurshid A, Tessier S, Sicard M, Knox AJ, Pang L and
De Moor CH: Inhibitor of polyadenylation reduces inflammatory gene
induction. RNA. 18:2236–2250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang GJ, Wang YB, Li DN, Li C and Deng BB:
Expression of tissue factor pathway inhibitor-2 in gastric stromal
tumor and its clinical significance. Exp Ther Med. 7:513–517.
2014.PubMed/NCBI
|