Introduction
The majority of approved anticancer drugs are either
natural products or have been developed based on knowledge gained
from natural products, which have been important in the search for
novel anticancer drugs compared with other areas of drug
development (1–5). Traditional Chinese medicine has been
historically used to treat disease using natural products. Although
certain traditional Chinese herbs should no longer be used, other
traditional Chinese herbs that have been proven to be effective
have been gradually incorporated into modern medicine (6).
Among these traditional Chinese medicinal plants,
Artemisia sacrorum Ledeb (ASL; Compositae) is of
particular interest as it is widely used to prevent and treat
diverse diseases in the Yanbian area of Northeast China (7,8).
Previous studies have indicated that the water-soluble parts of the
A. sacrorum extract were protective against carbon
tetrachloride and acetaminophen-induced hepatotoxicity in mice, and
the underlying mechanisms have been investigated (9–11). In a
previous study, A. sacrorum was demonstrated to prevent
N-acetyl-p-aminophenol (APAP)-induced apoptosis and necrosis, as
indicated by liver histopathological and immunohistochemical
analysis, and DNA laddering (9–11).
According to the results from a western blot analysis, the ethanol
eluate precipitation (EEP) decreased APAP-induced caspase-3 and −8
protein expression levels in mouse livers (9–11).
Furthermore, ASL was able to inhibit adipocyte differentiation and
adipogenesis through the activation of 5′-adenosine
monophosphate-activated protein kinase (AMPK) in 3T3-L1 adipocytes
and hepatocellular carcinoma (HepG2) cells, respectively (12,13).
Petroleum ether fraction of A. sacrorum Ledeb, another
extract from A. sacrorum, inhibited glucose production via
the AMPK-glycogen synthase kinase-cAMP response element binding
protein signaling pathway in HepG2 cells (14). Notably, the cytotoxicities of the 9
fractions separated from A. sacrorum via the two extraction
methods was investigated in our previous study, and the results
demonstrated that the CH2Cl2 and 95% ethanol
eluate (EE) fractions revealed the strongest cytotoxic activities
against 3 human cancer cell lines. In addition, a subsequent study
demonstrated that 10 compounds, of which 2 were flavonoids, were
isolated from the CH2Cl2 fraction (15).
The present study aimed to investigate the isolation
and structure of the compounds from the 95% EE fraction of A.
sacrorum. The results demonstrated that this fraction was
markedly rich in flavonoids. Flavonoids are cancer-preventive
agents, and have been shown to be particularly important (16–18).
Therefore, the present study was further conducted to evaluate the
cytotoxic activities of all flavonoids from the
CH2Cl2 and 95% EE fractions.
Materials and methods
General experimental procedures
The nuclear magnetic resonance (NMR) spectra of
compounds 1, 2, 3, 5, 6, and 7 were recorded on a Bruker 500 MHz
NMR spectrometer (Bruker Corporation, Billerica, MA, USA),
operating at a frequency of 500 MHz for 1H and 125 MHz
for 13C nuclei at room temperature, and with TMS as the
internal standard. The NMR spectra of compounds 4 and 8 were
recorded on a Bruker 300 MHz NMR spectrometer, operating at 300 MHz
for 1H and 75 MHz for 13C nuclei at room
temperature with tetramethylsilane (TMS) as the internal standard.
Chemical shifts (δ) were expressed in parts per million (ppm)
relative to TMS. The chemical shifts (δ) and coupling constant
values were reported in ppm and Hz, respectively. Compounds 1, 2,
3, 4, 6, and 7 were dissolved in CD3OD, 5 was dissolved
in dimethyl sulfoxide (DMSO), and 8 was dissolved in
acetone-d6. Column chromatography (CC) was performed on
a Sephadex LH-20 (18–111 µm; GE Healthcare Biosciences, Pittsburgh,
PA, USA), YMC-GEL octadecyl (ODS)-A (50 µm; YMC Co., Ltd., Kyoto,
Japan) and silica gel (200–300 mesh, Qingdao Marine Chemical, Ltd.,
Qingdao, China) columns. D-101 macroporous absorption resin was
purchased from Tianjin Haiguang Chemical Co., Ltd. (Tianjin,
China). Silica gel 60 F254 plates (20×20 cm; 0.2 mm thick; Merck,
Darmstadt, Germany) and silica gel GF 254 (Qingdao Marine Chemical
Co., Ltd.) were used for thin-layer chromatography (TLC) analysis.
The CH3OH used for column chromatography was high
performance liquid chromatography-grade (Jiangsu Hanbang Science
and Technology Co., Ltd., Jiangsu, China). All other chemicals and
reagents used in the present study were of analytical grade.
Plant material
The aerial parts of ASL (Compositae) were
collected in July 2008 from Xidong, Yanji in Jilin. The plant was
taxonomically identified and authenticated by Professor Huizi Lv
(College of Pharmacy, Yanbian University of China, Yanji, China). A
specimen of the plant was deposited at the College of Pharmacy,
Yanbian University.
Extraction and isolation
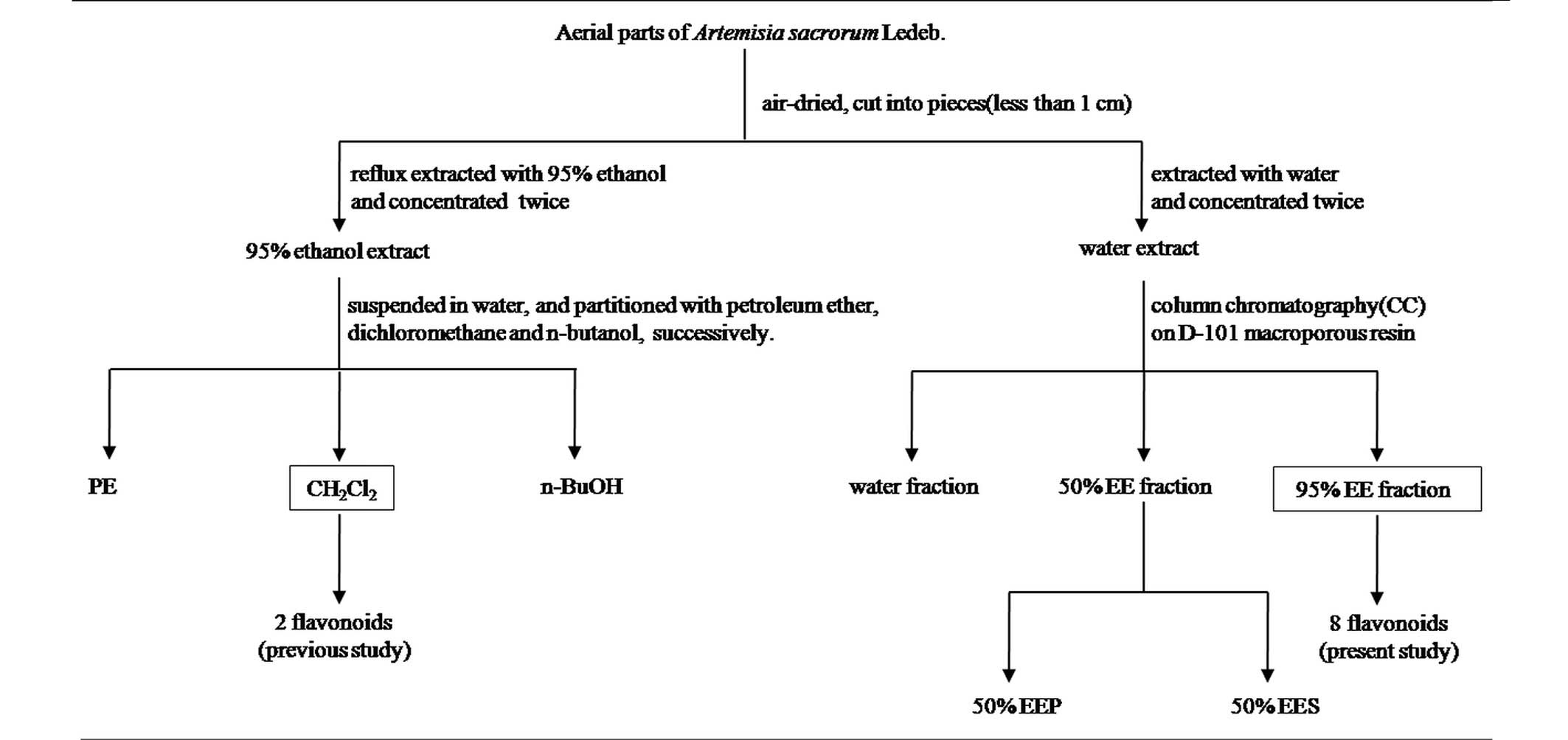
A total of 5 kg of the aerial parts of A.
sacrorum were collected, air-dried in the shade, and dissolved
in boiling water twice (Figs. 1 and
2). The extract was then separated
on a D-101 macroporous absorption resin to elute water, 50% EE and
95% EE fractions. From this separation, the 95% EE fraction (16.03
g) was chromatographed on a silica-gel column (200–300 mesh) with
petroleum ether-EtOAc in a stepwise gradient elution (50:1-0:100).
The eluates were combined based on the TLC results in order to
produce 8 fractions (1–8).
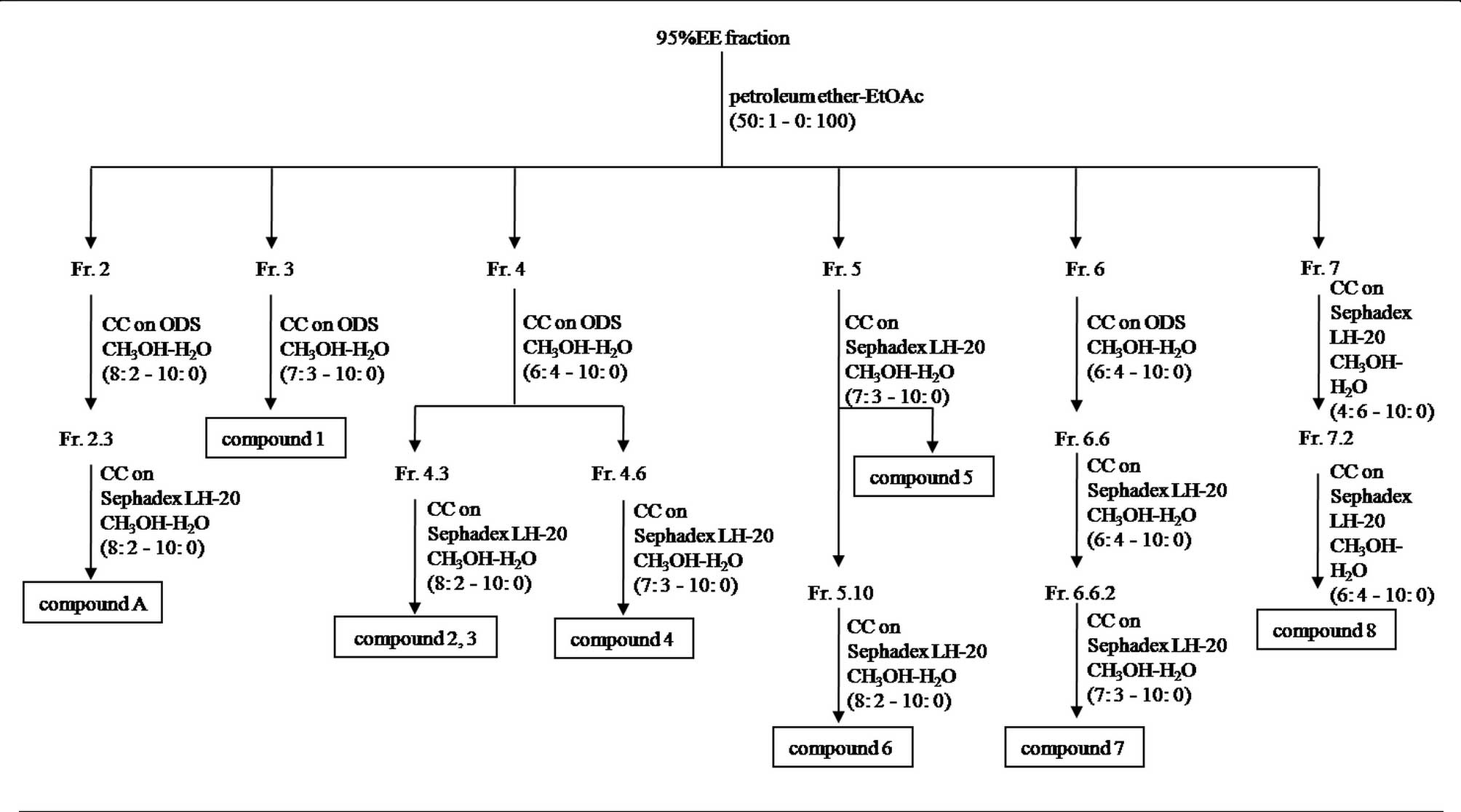
The fractions were chromatographed as follows:
Fraction 2 was chromatographed on an ODS column by eluting with
gradient mixtures of CH3OH and H2O (8:2 to
10:0) in order to obtain 7 subfractions (2.1–7). Subfraction 2.3
was rechromatographed on a Sephadex LH-20 column by eluting with
gradient mixtures of CH3OH and H2O (8:2 to
10:0) to obtain compound A (3.0 mg). Fraction 3 was
rechromatographed on an ODS column by eluting with gradient
mixtures of CH3OH and H2O (7:3 to 10:0) to
afford compound 1 (23.2 mg). Fraction 4 was rechromatographed on an
ODS column by eluting with gradient mixtures of CH3OH
and H2O (6:4 to 10:0) to obtain 11 subfractions
(4.1–11). Subfraction 4.3 was rechromatographed on a Sephadex LH-20
column by eluting with gradient mixtures of CH3OH and
H2O (8:2 to 10:0) to obtain compound 2 (2.6 mg) and
compound 3 (1.5 mg). Subfraction 4.6 was subjected to a Sephadex
LH-20 column by eluting with gradient mixtures of CH3OH
and H2O (7:3 to 10:0) to obtain compound 4 (8.2 mg).
Fraction 5 was rechromatographed on a Sephadex LH-20 column by
eluting with CH3OH-H2O (7:3 to 10:0) to
obtain compound 5 (2.0 mg) and 12 subfractions (5.1–12).
Subfraction 5.10 was subjected to a Sephadex LH-20 column by
eluting with gradient mixtures of CH3OH and
H2O (8:2 to 10:0) to obtain compound 6 (1.5 mg).
Fraction 6 was rechromatographed on an ODS column by eluting with
gradient mixtures of CH3OH and H2O (6:4 to
10:0) to obtain eight subfractions (6.1–8). Subfraction 6.6 was
subjected to a Sephadex LH-20 column repeatedly to obtain compound
7 (2.6 mg). Fraction 7 was rechromatographed on a Sephadex LH-20
column by eluting with gradient mixtures of
CH3OH-H2O (4:6 to 10:0) to produce 5
subfractions (7.1–5). Finally, subfraction 7.2 was subjected to a
Sephadex LH-20 column once more by eluting with gradient mixtures
of CH3OH and H2O (6:4 to 10:0) to afford
compound 8 (6.0 mg).
Cell culture and MTS assay
The two human cancer cell lines (SK-HEP-1 and HeLa)
and one normal human cell line (HEK293) used in the cytotoxic assay
were purchased from American Type Culture Collection (Manassas, VA,
USA). All cell lines were cultured in Dulbecco's modified Eagle
medium supplemented with 10% fetal bovine serum, 100 units/ml
penicillin and 100 mg/ml streptomycin and kept at 37°C in a
humidified atmosphere containing 5% CO2.
A 200-µl aliquot of adherent cells was used to seed
96-well cell culture plates at 3×104 cells/well and
allowed to adhere for 6 h prior to drug addition. Each cell line
was treated with 0, 5, 10, 25, 50, 100 and 200 µM of flavonoids for
48 h. Subsequently, 20 µl MTS reagent was added into each well and
incubated for 1 h. The cell viability was detected by the CellTiter
96 AQueous One solution Cell Proliferation Assay kit (Promega
Corporation, Madison, WI, USA).
Results and Discussion
To observe the cytotoxic activity of the herb, A.
sacrorum was extracted and isolated by 95% EtOH and water, as
described in our previous study (15). Following extraction with 95% EtOH
extract with various solvents, and separation of the water extract
with a D-101 macroporous resin column, 9 fractions of A.
sacrorum were obtained, of which the 95% EE and
CH2Cl2 fraction revealed the strongest
cytotoxity against 3 human cancer cell lines. In a subsequent
study, 2 flavonoids were isolated from the
CH2Cl2 fraction (15). In the present study, 9 compounds were
isolated from the 95% EE fraction.
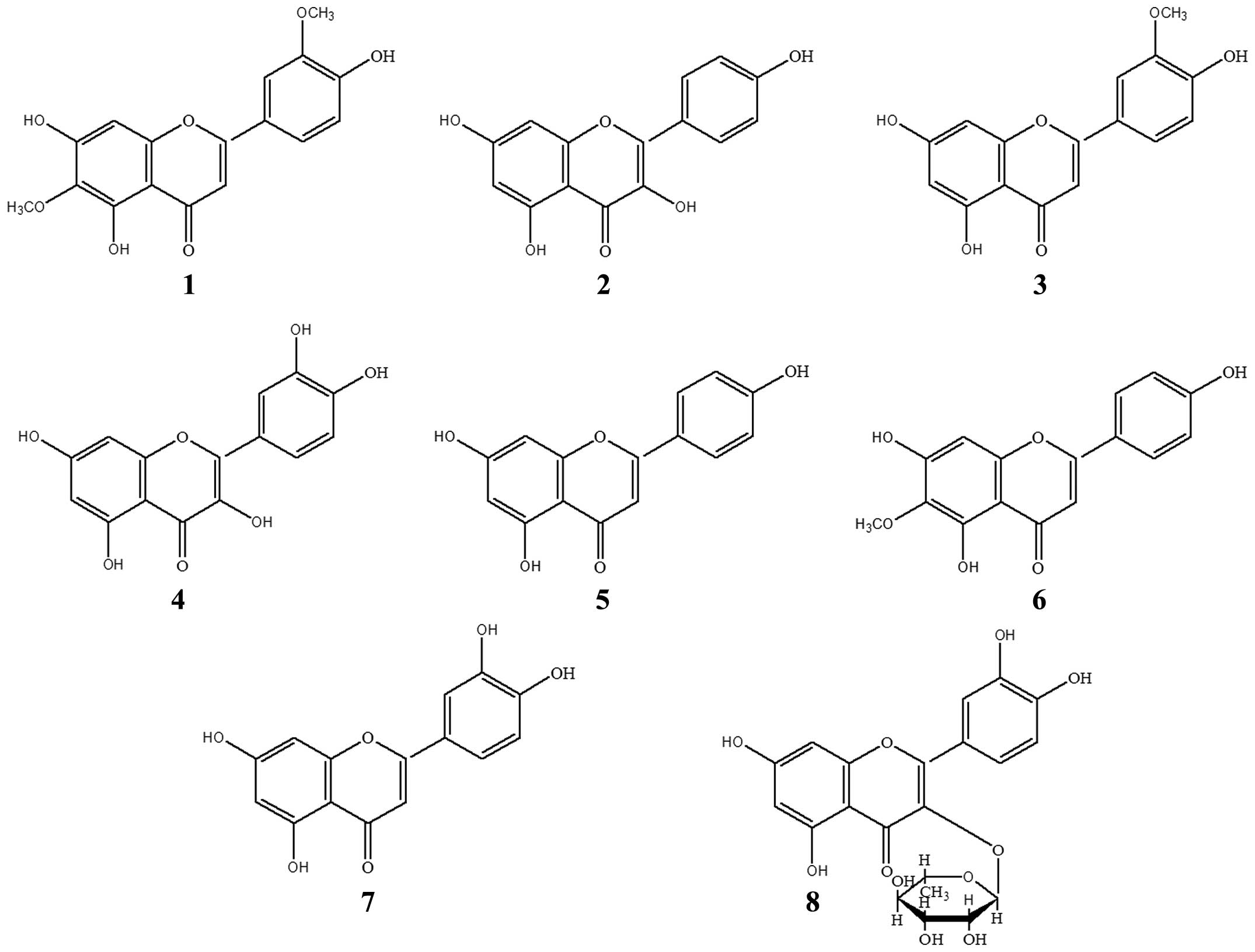
Compound 1 was isolated as yellow, needle-like
crystals (acetone). The 1H-NMR and 13C-NMR
spectra of the compound revealed the characteristic signals of a
flavonoid. The 1H-NMR spectrum exhibited two proton
signals at δ 6.56 (1H; s) and 6.61 (1H; s), which represented the
proton signals of C3-H and C8-H of the A ring, respectively.
Signals at δ 7.45 (1H; d; J=2.0 Hz), 6.92 (1H; d; J=8.35 Hz) and
7.49 (1H; dd; J=2.0; 8.35 Hz) represented the proton signals of
C2′-H, C5′-H and C6′-H of the B ring. δ 3.96 (3H; s) and 3.88 (3H;
s) corresponded to the proton signals of the 2 methoxy groups. The
13C-NMR spectrum showed 17 carbon signals of which
δC 184.23 was the carbonyl signal, whereas 56.71 and
60.94 were assigned to 2 methoxy groups. By analyzing these data
and comparing them with those in the literature (19), the structure of compound 1 was
determined as jaceosidin (Fig.
3).
Compound 2 was obtained as a yellow powder (MeOH).
The 1H-NMR spectrum demonstrated 2 meta-coupled
proton signals at δ 6.18 (1H; d; J=2.0 Hz; H-6) and 6.40
(1H; d; J=2.0 Hz; H-8) of the A ring. Two groups of
meta-coupled proton signals at δ 6.90 (2H; d; J=9.0
Hz; H-3′; H-5′) and 8.09 (2H; d; J=9.0 Hz; H-2′; H-6′) were
assigned to 4 protons in the B ring. The 13C-NMR
spectrum revealed carbon signals of δC 148.15, 137.17,
177.44, 158.35, 99.38, 165.77, 94.55, 162.53, and 104.56 for the A
ring, and 123.81, 130.69, 116.35, 160.57, 116.35, and 130.69 for
the B ring. Based on the above spectral data and comparison with a
previous study (20), the chemical
structure of compound 2 was determined as kaempferol.
Compound 6 was obtained as yellow, needle-like
crystals (MeOH). The 1H-NMR spectrum revealed 2 isolated
proton signals at δ 6.58 (1H; s; H-3) and 6.67 (1H; s; H-8), which
were attributed to H-3 (C-ring) and H-8 (A-ring), respectively. Two
groups of meta-coupled proton signals at δ 6.92 (2H; d;
J=8.75 Hz; H-3′; H-5′) and 7.84 (2H; d; J=8.75 Hz;
H-2′; H-6′) were assigned to 4 protons of the B ring. The
13C-NMR spectrum showed δC values of 164.46,
102.46, 184.31, 152.69, 131.70, 157.66, 94.17, 152.69, and 104.87
for the A ring, and 122.14, 128.45, 116.15, 161.32, 116.15, and
128.45 for the B ring. These spectroscopic data were characteristic
of a flavonoid. Based on the above spectral data and comparison
with previous studies (21,22), the chemical structure of compound 6
was determined as hispidulin.
Compound 8 was obtained as a yellow powder
(acetone). 1H-NMR revealed, in the aromatic region, 2
meta-coupled proton signals at δ 6.25 (1H; d; J=2.07
Hz; H-6) and 6.46 (1H; d; J=2.07 Hz; H-8) for the A ring.
1H-NMR also demonstrated 2 meta-coupled proton
signals at δ 7.49 (1H; d; J=2.07 Hz; H-2′), 7.39 (1H; dd;
J=2.07; 8.34 Hz; H-6′), and 1 isolated proton signal at δ
6.98 (1H; d; J=8.34 Hz; H-5′) for the B ring. These 3 proton
signals exhibited the typical three-spin system of the
1′,3′,4′-trisubstituted B ring. δ 5.28 (1H; d; J=1.5 Hz;
H-1′′) was the anomeric proton signal of sugar. The
13C-NMR spectrum revealed a carbonyl proton signal at
δC 179.18, which was assigned to C-4, and 17.63–102.57
ppm for a group of 6-carbon sugar signals revealing the presence of
an aglycone moiety. Based on the above spectral data and a
comparison with previous studies (23,24), the
chemical structure of compound 8 was determined as quercitrin.
In addition to the above-mentioned compounds, 5
other compounds were also identified as chrysoeriol (compound 3)
(25), quercetin (compound 4)
(26), apigenin (compound 5)
(27), luteolin (compound 7)
(28,29) and scopoletin (compound A) (30). These were identified by
interpretation of their spectroscopic data and comparison of the
data with the reported values. Among them, compounds 1–8 were
flavonoids, and flavonoids 1, 2, 4, 7 and 8 were isolated for the
first time from A. sacrorum. The structures of the 8
flavonoids are depicted in Fig. 3
and all 13C-NMR spectra data were listed in Table I.
 | Table I.13C-NMR spectra data of
compounds 1–8 (δ, ppm; compounds 4 and 8 were detected at 75 MHz,
others at 125 MHz; compounds 1, 2, 3, 4, 6 and 7 dissolved in
CD3OD, compound 5 dissolved in DMSO, and compound 8
dissolved in acetone-d6; OMe, OCH3). |
Table I.
13C-NMR spectra data of
compounds 1–8 (δ, ppm; compounds 4 and 8 were detected at 75 MHz,
others at 125 MHz; compounds 1, 2, 3, 4, 6 and 7 dissolved in
CD3OD, compound 5 dissolved in DMSO, and compound 8
dissolved in acetone-d6; OMe, OCH3).
| Compound no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|
| 2 | 166.24 | 148.15 | 160.89 | 147.99 | 164.17 | 164.46 | 164.73 | 158.15 |
| 3 | 103.80 | 137.17 | 104.15 | 137.24 | 103.04 | 102.46 | 104.62 | 135.63 |
| 4 | 184.23 | 177.44 | 183.79 | 177.34 | 181.77 | 184.31 | 183.66 | 179.18 |
| 5 | 154.70 | 158.35 | 163.13 | 158.23 | 157.48 | 152.69 | 162.33 | 163.06 |
| 6 | 132.97 | 99.38 | 123.75 | 99.22 | 98.90 | 131.70 | 99.78 | 99.35 |
| 7 | 158.90 | 165.77 | 166.09 | 162.52 | 163.88 | 157.66 | 165.61 | 164.77 |
| 8 |
95.38 |
94.55 |
95.31 |
94.39 |
94.16 |
94.17 |
94.75 |
94.35 |
| 9 | 154.70 | 162.53 | 156.19 | 165.58 | 161.22 | 152.69 | 158.25 | 157.63 |
| 10 | 105.78 | 104.56 | 105.39 | 104.52 | 103.77 | 104.87 | 103.89 | 105.75 |
| 1′ | 123.78 | 123.81 | 124.69 | 148.77 | 121.38 | 122.14 | 122.31 | 122.43 |
| 2′ | 110.75 | 130.69 | 110.70 | 115.98 | 128.56 | 128.45 | 114.22 | 115.99 |
| 3′ | 149.52 | 116.35 | 149.58 | 124.14 | 116.05 | 116.15 | 146.78 | 145.68 |
| 4′ | 152.13 | 160.57 | 152.52 | 146.22 | 161.06 | 161.32 | 150.71 | 148.85 |
| 5′ | 116.81 | 116.35 | 116.86 | 116.22 | 116.05 | 116.15 | 116.01 | 116.56 |
| 6′ | 121.76 | 130.69 | 121.77 | 121.67 | 128.56 | 128.45 | 120.46 | 122.73 |
|
OMe | 56.71 |
| 56.72 |
|
| 59.85 |
|
|
|
OMe | 60.94 |
|
|
|
|
|
|
|
|
1′′ |
|
|
|
|
|
|
| 102.57 |
|
2′′ |
|
|
|
|
|
|
| 71.26) |
|
3′′ |
|
|
|
|
|
|
| 71.97) |
|
4′′ |
|
|
|
|
|
|
| 72.86) |
|
5′′ |
|
|
|
|
|
|
| 71.19 |
|
6′′ |
|
|
|
|
|
|
| 17.63 |
A total of 8 flavonoids isolated from the 95% EE
fraction of A. sacrorum were designated as flavonoids 1–8,
and 2 flavonoids (genkwanin and acacetin) previously isolated from
the CH2Cl2 fraction were designated as
flavonoids 9 and 10. Although these 10 flavonoids were not novel
compounds, their possible biological activities should not be
ignored because they have previously been identified. Furthermore,
not every novel compound is examined for all its activities (even
in vitro experiments before it is considered to be old).
Conversely, the majority of novel compounds have only undergone
in vitro activity tests.
In the present study, the cytotoxic activities
against the 10 flavonoids on SK-HEP-1, HeLa and HEK293 cell growth
were determined using an MTS assay (31). Their IC50 values were
presented in Table II, in which
apigenin (flavonoid 5) and luteolin (flavonoid 7) exhibited strong
cytotoxic activities against human cervical cancer HeLa cells.
Conversely, they had no cytotoxic activity against normal human
embryonic kidney HEK293 cells.
 | Table II.Cytotoxities of 10 flavonoids
isolated from Artemisia sacrorum (IC50, µM). |
Table II.
Cytotoxities of 10 flavonoids
isolated from Artemisia sacrorum (IC50, µM).
| Flavonoids | SK-HEP1 | HeLa | HEK293 |
|---|
| 1 | >50 | >50 | 14.1380 |
| 2 | >50 | 45.809 | >50 |
| 3 | 47.19 | >50 | >50 |
| 4 |
45.96 | >50 | >50 |
| 5 | 46.56 | 6.092 | >50 |
| 6 | 49.93 | >50 | >50 |
| 7 | >50 | 16.254 | >50 |
| 8 | >50 | >50 | >50 |
| 9 | >50 | 47.834 | >50 |
| 10 | >50 | >50 | >50 |
The structures of the 10 flavonoids are similar.
However, certain flavonoids revealed different cytotoxic activity
against the two human liver cancer SK-HEP-1 and human cervical
cancer HeLa cells. Although certain flavonoids have similar
chemical structures, there are still compound-specific effects on
certain neoplasms that are relevant to adjust specific biochemical
processes to treat certain neoplasms differentially (32,33). In
the present study, the majority of the 10 flavonoids demonstrated
no cytotoxic activity against normal human embryonic kidney
cells.
Flavonoids may serve as clinically significant
chemotherapeutic agents in the treatment of cancer. Indeed,
flavonoids have been demonstrated to reveal cytotoxic activities
towards numerous human cancer cells, whilst having little or no
effect on normal cells. This has led to interest in the development
of potential flavonoid-based chemotherapeutics for anticancer
treatment (34–37). Traditional Chinese herbs, which
depend predominantly on empirical medication, have been used for
thousands of years in the treatment of numerous diseases, often
with few adverse effects. Furthermore, many of the active
flavonoids in traditional Chinese herbs have been the subject of
studies aiming to develop novel anti-cancer drugs by demonstrating
their activity through in vivo and in vitro
experiments (38–40). The diverse flavonoids in these herbal
medicines may be promising candidates in the development of novel
anti-cancer drugs.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81260669). The authors are
grateful to Mr. Guang-Hao Zheng and Mr. Zhi-Yong Li for their
support during the extraction and fractionation procedures. We also
thank Dr. Ming-Shan Zheng for her help in the chemical structure
elucidation of several compounds.
References
|
1
|
LiWeber M: New therapeutic aspects of
flavones: The anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Samarghandian S, Afshari JT and Davoodi S:
Chrysin reduces proliferation and induces apoptosis in the human
prostate cancer cell line pc-3. Clinics (Sao Paulo). 66:1073–1079.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tundis R, Loizzo MR and Menichini F,
Bonesi M, Colica C and Menichini F: In vitro cytotoxic activity of
extracts and isolated constituents of Salvia leriifolia Benth.
against a panel of human cancer cell lines. Chem Biodivers.
8:1152–1162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Newman DJ and Cragg GM: Natural products
as source of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong J: The relationship between
traditional chinese medicine and modern medicine. Evid Based
Complement Alternat Med. 2013:1531482013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piao MJ, Cui SN and Zhang DY: Korean
ethnic Materia Medica in China. Yanji, China: Yanbian People Press.
Yan Bian Ren Min Chu Ban She; pp. 244–245. 2012
|
|
8
|
Jia MR and Li XW: Chinese ethnic Materia
Medica. Zhong Guo Yi Yao Ke Ji Chu Ban She. 69:2005.
|
|
9
|
Piao GC and Quan YC: Protective effects of
extracts of Artemisia sacrorum Ledeb. on acute hepatic injury in
mice. Shi Zhen Guo Yi Guo Yao. 18:1646–1647. 2007.(In Chinese).
|
|
10
|
Yuan HD, Jin GZ and Piao GC: Protective
effects of the supernatant of ethanol eluate from Artemisia
sacrorum Ledeb. against acetaminophen-induced liver injury in mice
[corrected]. Biol Pharm Bull. 32:1683–1688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan HD, Jin GZ and Piao GC:
Hepatoprotective effects of an active part from Artemisia sacrorum
Ledeb. against acetaminophen-induced toxicity in mice. J
Ethnopharmacol. 127:528–533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan HD and Piao GC: An active part of
Artemisia sacrorum Ledeb. inhibits adipogenesis via the AMPK
signaling pathway in 3T3-L1 adipocytes. Int J Mol Med. 27:531–536.
2011.PubMed/NCBI
|
|
13
|
Yuan HD, Yuan HY, Chung SH, Jin GZ and
Piao GC: An active part of Artemisia sacrorum Ledeb. attenuates
hepatic lipid accumulation through activating AMP-activated protein
kinase in human HepG2 cells. Biosci Biotechnol Biochem. 74:322–328.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan HD and Piao GC: An active part of
Artemisia sacrorum Ledeb. inhibits adipogenesis via the AMPK
signaling pathway in 3T3-L1 adipocytes. Int J Mol Med. 27:531–536.
2011.PubMed/NCBI
|
|
15
|
Piao GC, Li YX, Yuan HD and Jin GZ:
Cytotoxic fraction from Artemisia sacrorum Ledeb. against three
human cancer cell lines and separation and identification of its
compounds. Nat Prod Res. 26:1483–1491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chien CS, Shen KH, Huang JS, Ko SC and
Shih YW: Antimetastatic potential of fisetin involves inactivation
of the PI3K/Akt and JNK signaling pathways with downregulation of
MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell
Biochem. 333:169–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung CS, Jiang Y, Cheng D and Birt DF:
Impact of adenomatous polyposis coli (APC) tumor supressor gene in
human colon cancer cell lines on cell cycle arrest by apigenin. Mol
Carcinog. 46:773–782. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takagaki N, Sowa Y, Oki T, Nakanishi R,
Yogosawa S and Sakai T: Apigenin induces cell cycle arrest and
p21/WAF1 expression in a p53-independent pathway. Int J Oncol.
26:185–189. 2005.PubMed/NCBI
|
|
19
|
Wang QH, Wu XL and Wang JH: Study on
chemical constituents of Artemisia frigida (II). Chin Tradit Herb
Drug. 42:1075–1078. 2011.
|
|
20
|
Yu DQ and Yang JS: Analytical Chemistry
Handbook, Vol 7. 2nd edition. Beijing Chemical industry Press. 820.
Beijing: pp. 8201999
|
|
21
|
Hazekamp A, Verpoorte R and Panthong A:
Isolation of a bronchodilator flavonoid from the Thai medicinal
plant Clerodendrum petasites. J Ethnopharmacol. 78:45–49. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou LD, Yu JG, Guo J and Yang SL:
Compounds from roots of Chirita fimbrisepala Hand.-Mazz. Zhongguo
Zhong Yao Za Zhi. 26:114–117. 2001.(In Chinese). PubMed/NCBI
|
|
23
|
Wang HP, Cao F and Yang XW: Chemical
constituents in aerial parts of Polygonum capitatum. Zhong Cao Yao.
44:24–30. 2013.
|
|
24
|
Lu J and Kong LY: Studies on the
Constituents of Hypericum japonicum Thunb. Ex Murray. Zhong Guo
Xian Dai Zhong Yao. 9:12–13. 2007.
|
|
25
|
Wei JH, Chen J, Cai SF, Lu RM and Lin SW:
Chemical constituents in whole herb of Cardiospermum halicacabum
(I). Zhong Cao Yao. 42:1509–1511. 2011.
|
|
26
|
Luo QH, Bu XY, Liu HW, Fan M, Ding AS and
Yao XS: Studies on the anti-hypoxia active Constituents of
Artemisia scoparia. Zhong Cao Yao Zeng Kan. 37:199–201. 2006.
|
|
27
|
Nan HH, Zhang S and Wu J: Chemical
constituents from Clerodendrum inerme. Zhong Cao Yao. 36:492–494.
2005.
|
|
28
|
Wei TM, Yan YN, Guan XL, Liu YF and Wei
DH: Analysis of the chemical constituents of the ground part of
Sedum sarmentosum. J B Univ TCM. 26:59–61. 2003.
|
|
29
|
Xu ZH, Wu HX, Wei XY, Feng SX and Hu TM:
Flavonoids from Lespedeza davurica. Acta Bot Boreal-Occident Sin.
30:1485–1489. 2010.
|
|
30
|
Xie T, Liang JY, Liu J, Wang M, Wei XL and
Yang CH: Chemical Study on Artemisia scoparia. J China Pharm Univ.
35:401–403. 2004.
|
|
31
|
Kometani T, Yoshino I, Miura N, Okazaki H,
Ohba T, Takenaka T, Shoji F, Yano T and Maehara Y: Benzo[a]pyrene
promotes proliferation of human lung cancer cells by accelerating
the epidermal growth factor receptor signaling pathway. Cancer
Lett. 278:27–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonham M, Posakony J, Coleman I,
Montgomery B, Simon J and Nelson PS: Characterization of chemical
constituents in Scutellaria baicalensis with antiandrogenic and
growth-inhibitory activities toward prostate carcinoma. Clin Cancer
Res. 11:3905–3914. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng Q, Hirose Y, Yoshimi N, Murakami A,
Koshimizu K, Ohigashi H, Sakata K, Matsumoto Y, Sayama Y and Mori
H: Further investigation of the modifying effect of various
chemopreventive agents on apoptosis and cell proliferation in human
colon cancer cells. J Cancer Res Clin Oncol. 128:539–546. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu XF, Cai BL, Guan SM, Li Y, Wu JZ, Wang
Y and Liu B: Baicalin induces human mucoepidermoid carcinoma Mc3
cells apoptosis in vitro and in vivo. Invest New Drugs. 29:637–645.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen WY, Hsieh YA, Tsai CI, Kang YF, Chang
FR, Wu YC and Wu CC: Protoapigenone, a natural derivative of
apigenin, induces mitogen-activated protein kinase-dependent
apoptosis in human breast cancer cells associated with induction of
oxidative stress and inhibition of glutathione S-transferase TT.
Invest New Drugs. 29:1347–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Plochmann K, Korte G, Koutsilieri E,
Richling E, Riederer P, Rethwilm A, Schreier P and Scheller C:
Structure-activity relationships of flavonoid-induced cytotoxicity
on human leukemia cells. Arch Biochem Biophys. 460:1–9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
BenSghaier M, Skandrani I, Nasr N, Franca
MG, ChekirGhedira L and Ghedira K: Flavonoids and sesquiterpenes
from Tecurium ramosissimum promote antiproliferation of human
cancer cells and enhance antioxidant activity: A structure-activity
relationship study. Environ Toxicol Pharmacol. 32:336–348. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo Y, Sun G, Dong X, Wang M, Qin M, Yu Y
and Sun X: Isorhamnetin attenuates atherosclerosis by inhibiting
macrophage apoptosis via PI3K/AKT activation and HO-1 induction.
PLoS One. 10:e01202592015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen X, Zhang B, Yuan X, Yang F, Liu J,
Zhao H, Liu L, Wang Y, Wang Z and Zheng Q:
Isoliquiritigenin-induced differentiation in mouse melanoma B16F0
cell line. Oxid Med Cell Longev. Dec 10–2012.(Epub ahead of print)
doi: 10.1155/2012/534934. View Article : Google Scholar
|
|
40
|
Wang B: General research on effects of
flavonoids ingredients on Chinese herbs on anti-cancer. Zhe Jiang
Zhong Yi Yao Da Xue Xue. 36:838–840. 2012.(In Chinese).
|