Introduction
Globally, subarachnoid hemorrhage (SAH) is a
prominent pathological occurrence which is involved in the etiology
of 5–7% of all strokes cases that involve high mortality and
functional loss (1). Despite
advances in medical treatment and diagnosis, the mortality and
morbidity rates of SAH have not been decreased significantly
(2). Furthermore, the outcome of
treatment in SAH patients remains poor, with a mortality rate of
>50% and high morbidity among the survivors (3–5). Due to
its complex pathology, researchers have deepened their interest in
understanding the mechanisms underlying SAH at molecular level
(6).
In the event of SAH, two clinical scenarios have
been addressed primarily; vasospasm and early brain injury (EBI)
(7). Arterial narrowing during SAH
elicits fatal complications such as cerebral ischemia, and hence
targeting the vasospasm has been a key target in the treatment of
SAH among neurosurgeons in the past years (4). However, little success has been
achieved in improving outcome following SAH (8,9).
Additionally, accumulating studies have indicated that the
administration of clazosentan in SAH patients does not improve
patient outcome, despite reducing vasoconstriction (8,10).
Therefore, studies have investigated the involvement of cardinal
factors such as ischemia, disruption of the blood brain barrier
(BBB), inflammatory reactions and cortical spreading depression in
the early stages following SAH (11,12).
Previous studies have suggested that the EBI period
(24–72 h) following SAH elicits a series of events that may lead to
poor prognosis (13,14). Furthermore, prior reports indicate
that oxidative stress and brain edema are involved in EBI after SAH
(15,16). Furthermore, reactive oxygen species
(ROS) and reactive nitrogen species have been implicated in the
occurrence of brain injury after SAH (17).
During EBI, brain edema occurs due to the disruption
of the BBB (18,19) more than as a consequence of vasospasm
(16). Thus, in clinical diagnosis,
global edema has been proposed as a sole independent risk factor
for fatal complications after SAH (16). Furthermore, noxious oxidative assault
has been closely associated with brain edema (19). Therapeutic approaches have focussed
on inhibiting ROS-induced apoptosis and inflammation as reasonable
choices for the treatment of brain injury (20). Among an array of therapeutic
interventions, a potential approach to boost or combat endogenous
defense against oxidative stress is through dietary or
pharmacological intake of antioxidants (21).
3,4-Dihydroxyphenylethanol (DOPET) is a phenol
extracted from olive oil and grape juice, and is an endogenous
metabolite of dopamine (22). DOPET
has a good safety margin (23), and
has been suggested to exert neuroprotective (24,25),
cardioprotective (26),
uroprotective (27), renoprotective
(28), hepatoprotective (29), anti-diabetic and antiobesity
(30), anti-osteoporotic (31), anti-inflammatory (32), anti-atherosclerotic (33), anticarcinogenic (34) and anti-virus effects (35) in animal studies. Notably, earlier
reports indicate the neuroprotective potential of DOPET in rats and
in vitro (36–38). On the basis of these preliminary
findings, we investigated whether that DOPET may be an effective
molecule in the in the mitigation of SAH in a rat model.
Materials and methods
Animals
A total of 21 male Sprague-Dawley rats (weight,
170–200 g; age, 9 weeks) were obtained from the animal facility of
Tongcheng People's Hospital (Xianning, China). The animals were
maintained under standard laboratory conditions of relative
humidity (55±5%), temperature (25±2°C) and light (12-h light/dark).
The rats were fed standard diet pellets and water was provided
ad libitum.
Animal grouping
Sprague-Dawley rats were divided into three groups
(n=7 per group): Sham-operated rats (sham group); SAH rats treated
with saline (SAH group); and SAH rats treated with DOPET (10 mg/kg)
orally (SAH + DOPET group).
Administration of DOPET
DOPET was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and dissolved in 0.9% saline at a concentration of 3%. In
the SAH + DOPET rats, DOPET (10 mg/kg) was injected
intraperitoneally at 5 min and 6 h after SAH induction. In the SAH
group, the rats underwent SAH-induction and were treated with an
equal volume of 0.9% saline. No treatment was applied in the
sham-operated animals.
Induction of SAH
SAH in rats was induced using an endovascular
perforation technique, as described previously (39). Briefly, in anesthetized rats (5%
isoflurane; Sigma-Aldrich) the left carotid artery and its branches
were exposed and transected distally and reflected caudally in line
with the internal carotid artery (ICA). Then, a blunted 4-0
monofilament nylon suture (Ethicon, San Angelo, TX, USA) was placed
in the external carotid artery and advanced through the ICA until
resistance was detected at 18–20 mm from the common carotid artery
bifurcation. Next, the suture was advanced for ~3 mm to perforate
the ICA near its intracranial bifurcation and removed after 15
sec.
Neurological test
The neurological evaluation was performed at 24 h
after SAH surgery using the Garcia scoring method (40). In this evaluation, spontaneous
activity, symmetry in the movement of four limbs, forepaw
outstretching, climbing, body proprioception and response to
vibrissae touch were assessed. These six tests were each scored
from 0 to 3. Overall scores were graded as a minimum of 0 and the
maximum as 18.
Brain water content
Rats were sacrificed by CO2 inhalation 24
h after SAH. The whole brain was removed and immediately weighed to
obtain the wet weight, and then dried at 105°C for 24 h to obtain
the dry weight. The brain water content was calculated as: [(Wet
weight-dry weight)/wet weight] × 100% (41).
Tissue harvesting
Following the evaluation of neurological score, the
rats (n=7) were anesthetized using 5% isoflurane and the brains
were removed for biochemical analysis. The olfactory bulb, pons and
medulla were discarded and the cerebral cortex was dissected,
weighed and chilled using liquid nitrogen until homogenization.
These procedures lasted up to 3 min. The cerebral cortex was
homogenized in 10 volumes (1:10 w/v) of cold saline. Brain samples
were homogenized and centrifuged at 4,000 × g at 4°C for 10 min.
Supernatant aliquots were used to assay various biochemical
parameters.
Estimation of lipid peroxidation and
oxidative stress
The activities of malondialdehyde (MDA; A003-1),
glutathione (GSH; A006), glutathione peroxidase (GPx; A007) and
superoxide dismutase (SOD; A001-1) in the cerebral cortex
homogenate were measured respectively using commercial kits
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China),
according to the manufacturer's instructions. Briefly, lipid
peroxidation was estimated using the level of MDA (ε=155
mM−1cm−1), which was determined
spectrophotometrically at A532. A yellow complex is produced during
the reaction between 5,5′-dithio-bis-(2-nitrobenzoic acid) and a
sulfhydryl compound. Through spectrophotometry, GSH levels were
detected. Activity of GPx was calculated by the reduction of GSH.
The color of 5-thio-dinitrobenzoic acid anion produced by the
reaction between GSH and 5,5′-dithio-bis-(2-nitrobenzoic acid) is
yellow and the absorbance is measured at 412 nm via
spectrophotometry. The method of SOD determination involves
generation of superoxide radical by photoreduction of riboflavin
and its detection by nitrite formation from hydroxylamine
hydrochloride at 543 nm. One unit of SOD activity was defined as
the amount of enzyme capable of inhibiting 50% of nitrite formation
under assay conditions. All standards and samples were run in
duplicate. Tissue protein concentrations were determined using a
BCA Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Reverse transcription quantitative
polymerase chain reaction
Total RNA was extracted from cerebral cortex tissue
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA) according to manufacturer's instructions. A
total of 10 µl RNA was reverse transcribed using Moloney murine
leukemia virus RT (Thermo Fisher Scientific, Inc.) in a 30 µl
reaction mixture. The resultant cDNA (20 ng) was amplified using an
iCycler IQ real-time detection system (Bio-Rad Laboratories, Inc)
using IQ Supermix with 0.5X SYBR-Green (Bio-Rad Laboratories,
Inc.). β-actin served as an endogenous control. Rat-specific
primers for caspase-3 and caspase-9 were synthesized by Shanghai
Shine Gene Molecular Biotech, Inc., (Shanghai, China), and the
sequences were as follows: Caspase-3, forward
5′-GGTATTGAGACAGACAGTGG-3′ and reverse 5′-CATGGGATCTGTTTCTTTGC-3′;
caspase-9, forward 5′-ACAAGGCCTTCGACAGTG-3′ and reverse
5′-GTACCAGGAACCGCTCTT-3′; and β-actin, forward
5′-ATCTGGCACCACACCTTC-3′ and reverse 5′-AGCCAGGTCCAGACGCA-3′.
Thermocycling conditions were as follows: Initial denaturation at
94°C for 2 min, followed by 35 cycles of denaturation at −95°C for
15 sec, annealing at −58°C for 45 sec and extension at −60°C for
30–45 sec, with final extension at 72°C for 5 min. mRNA expression
levels were normalized to the β-actin internal reference gene and
the relative expression levels were calculated using the
2−ΔΔCq method (42) and
CFX Manager software (Bio-Rad Laboratories, Inc). Reactions were
performed in triplicate.
Western blot analysis
Western blotting was performed as described
previously (43). Briefly, the left
basal cortical sample facing the blood clot was weighed,
homogenized, and centrifuged at 1,000 × g for 10 min at 4°C. The
resulting supernatants were further centrifuged. Samples were
transferred to sterile tubes containing cold TCAAEB [acetone
containing 10% (w/v) TCA and 0.07% mercaptoethanol], and the
proteins were precipitated for 1 h at −20°C, followed by
centrifugation at 18,900 × g for 15 min at 4°C. The supernatant was
decanted, and the pellet was washed twice with chilled wash buffer
(acetone containing 0.07% mercaptoethanol, 2 mM EDTA and EDTA-free
proteinase inhibitor cocktail tablets (Roche Diagnostics GmbH,
Mannheim, Germany), followed by the removal of the acetone. The
pellet was subsequently solubilized in LB-TT [7 M urea, 2 M
thiourea, 4% (w/v) CHAPS, 18 mM Tris-HCl (pH 8.0), 14 mM trizma
base, EDTA-free proteinase inhibitor cocktail, 0.2% (v/v) Triton
X-100 (R), containing 50 mM dithiothreitol]. The protein content
was measured using a DC protein assay kit (Bio-Rad Laboratories,
Inc.) prior to electrophoresis. An equal quantity of protein (60
µg) from each sample was resuspended in loading buffer (Bio-Rad
Laboratories, Inc.), denatured at 95°C for 5 min, separated by
10–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis,
and transferred onto polyvinylidene fluoride membranes (both
Bio-Rad Laboratories, Inc.). The membranes were blocked with
non-fat dry milk buffer for 2 h and incubated overnight at 4°C with
primary antibodies against interleukin (IL)-1β (cat. no. sc-7884;
1:500), IL-6 (cat. no. sc-13026; 1:800), tumor necrosis factor
(TNF)-α (cat. no. sc-1351; 1:800) and β-actin (cat. no. sc-47778;
1:2,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The
membranes were processed with horseradish peroxidase-conjugated
chicken anti-rabbit IgG secondary antibodies (1:500; Santa Cruz
Biotechnology, Inc.) at room temperature for 3 h.
Statistic analysis
Data are presented as the mean ± standard error of
the mean. SPSS, version 12.0 (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis of the data. All data were subjected
to one-way analysis of variance followed by the Tukey test for
multiple comparisons. P<0.05 was considered to be statistically
significant.
Results
Effect of SAH and DOPET on
neurological score
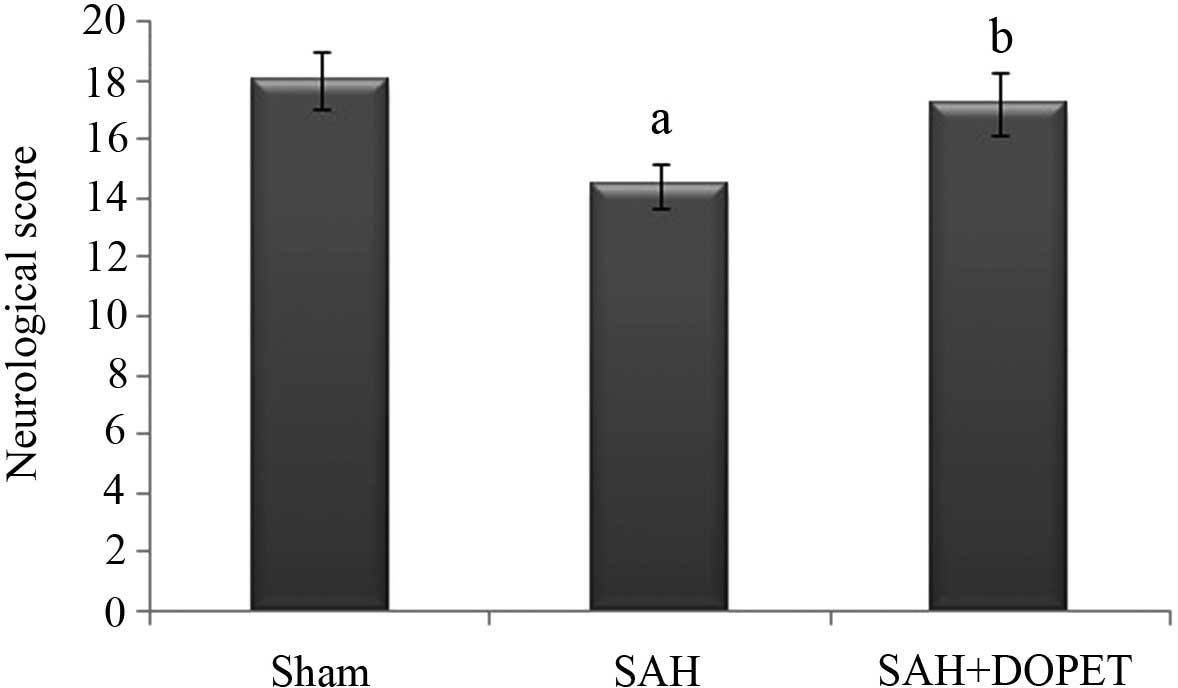
The neurological score was significantly (P<0.05)
decreased in the SAH group compared to the sham group. After DOPET
treatment, neurological deficits were reduced compared to that of
the SAH (P<0.05) (Fig. 1).
Effect of SAH and DOPET on brain water
content
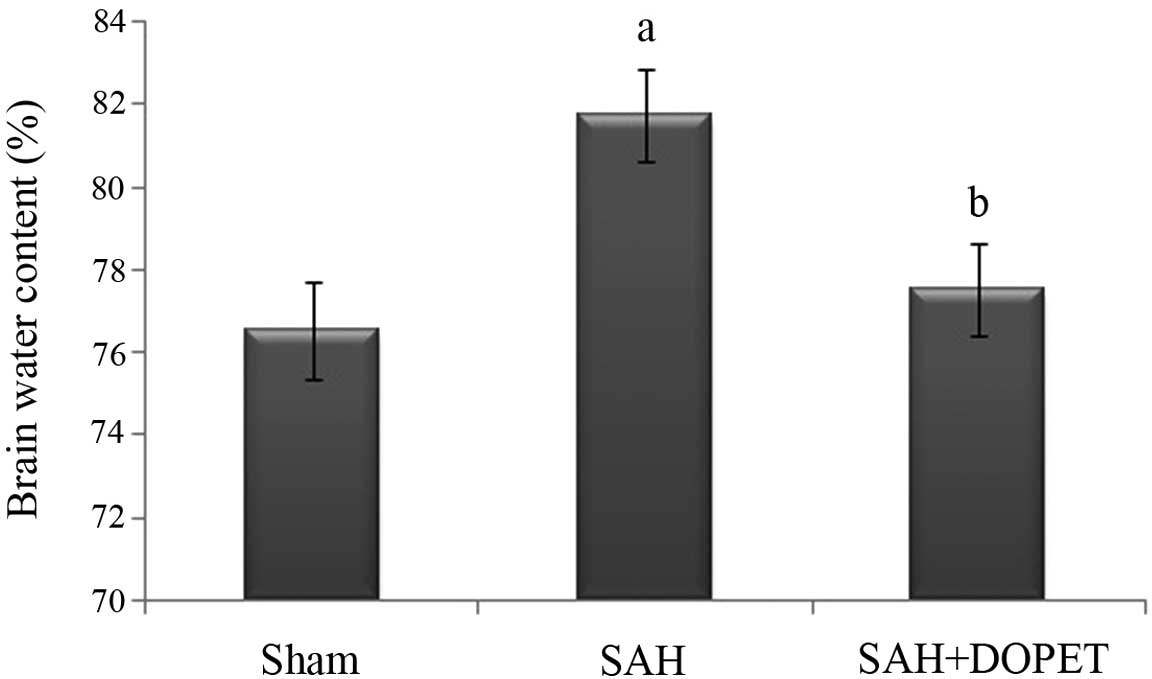
As shown in Fig. 2,
brain water content was significantly (P<0.05) elevated in SAH
group compared to the sham group at 24 h after SAH. Brain edema was
attenuated significantly (P<0.05) reduced in the SAH + DOPET
group compared with the SAH group.
Effect of SAH and DOPET on lipid
peroxidation in brain cortex homogenate
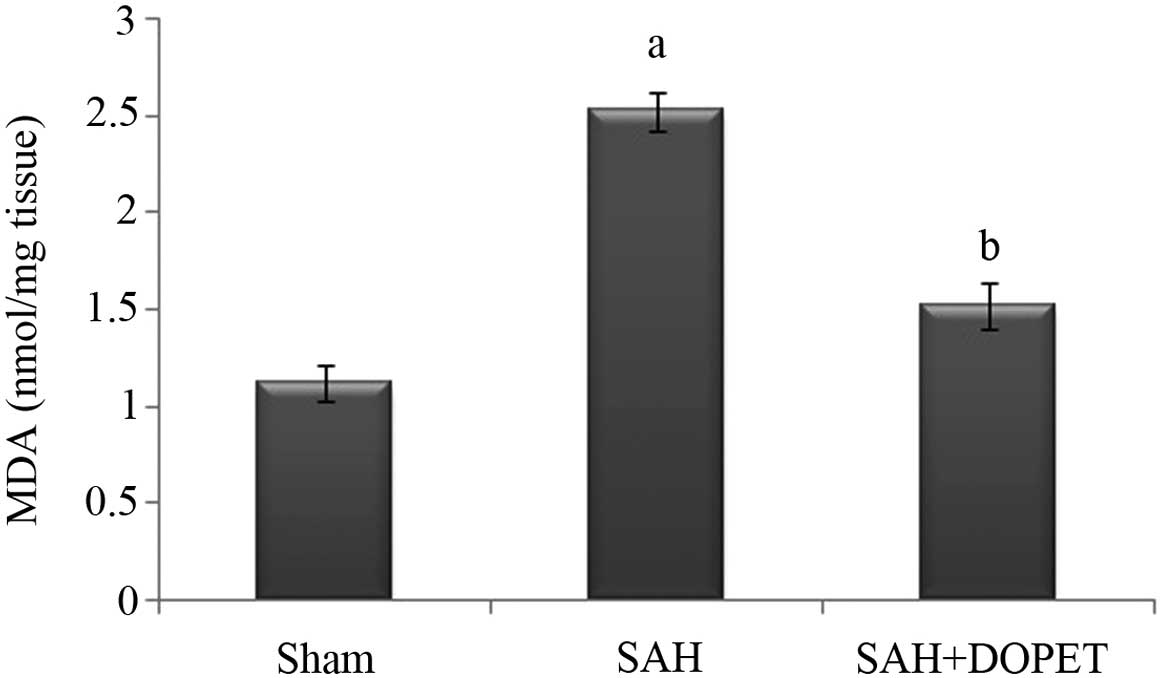
Lipid peroxidation in the cerebral cortex was
quantified by measuring MDA levels (Fig.
3). The level of MDA in the brain of SAH rats was significantly
higher compared with in the sham rat group (P<0.05). The
increase in lipid peroxidation indicates an elevated in vivo
oxidative stress in the brain of SAH rats, which was significantly
decreased by treatment with DOPET compared to SAH rats
(P<0.05).
Effect of SAH and DOPET on oxidative
stress
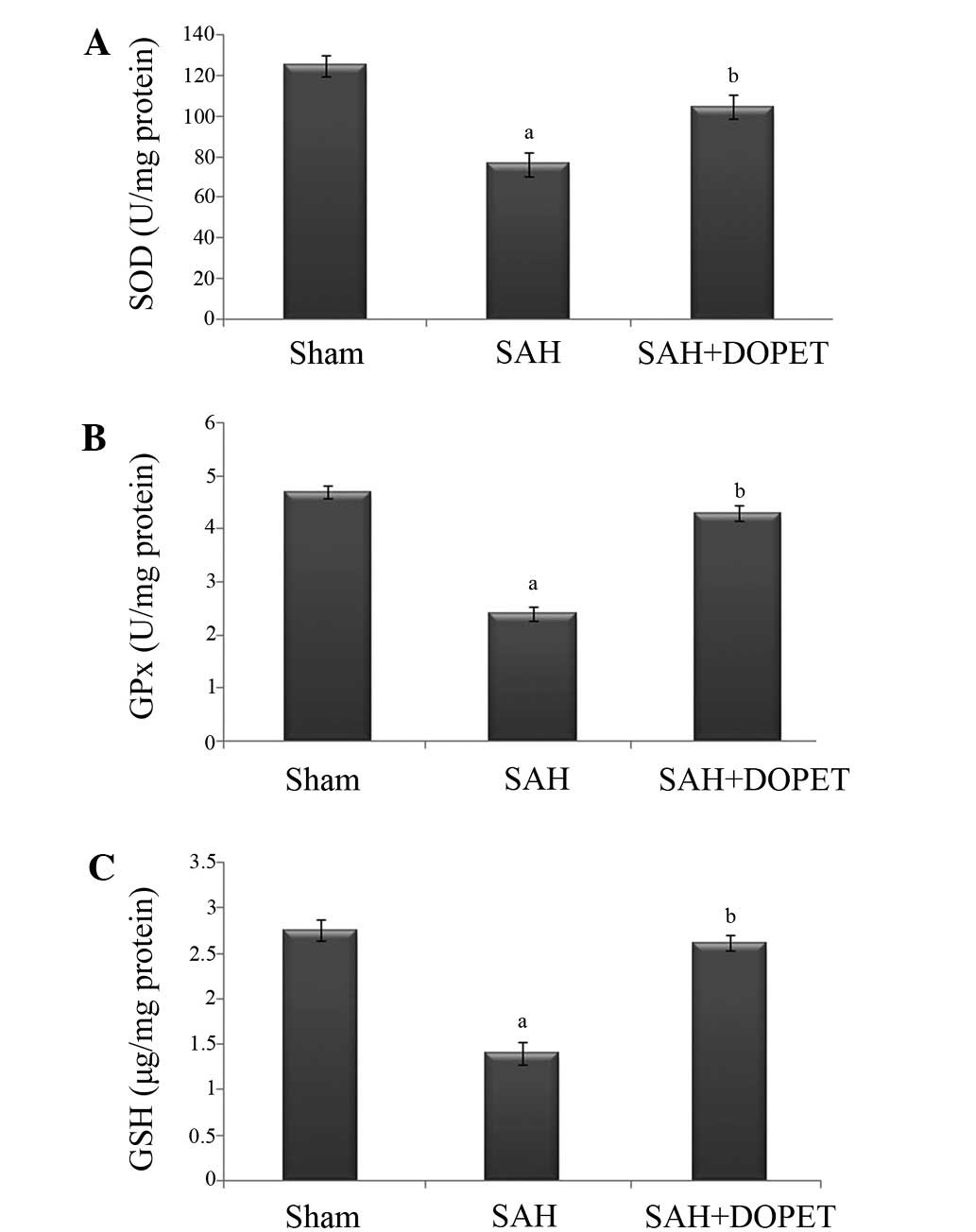
The concentration of ROS is determined by the
balance between the rate of production and the rate of clearance by
various antioxidant compounds and enzymes. In the present study,
post SAH there was a significant (P<0.05) decline in the level
of antioxidants (GSH, SOD, and GPx) when compared to the sham rats.
Treatment with DOPET significantly (P<0.05) increased the level
of antioxidant in brain through its anti lipid peroxidative effect
(Fig. 4).
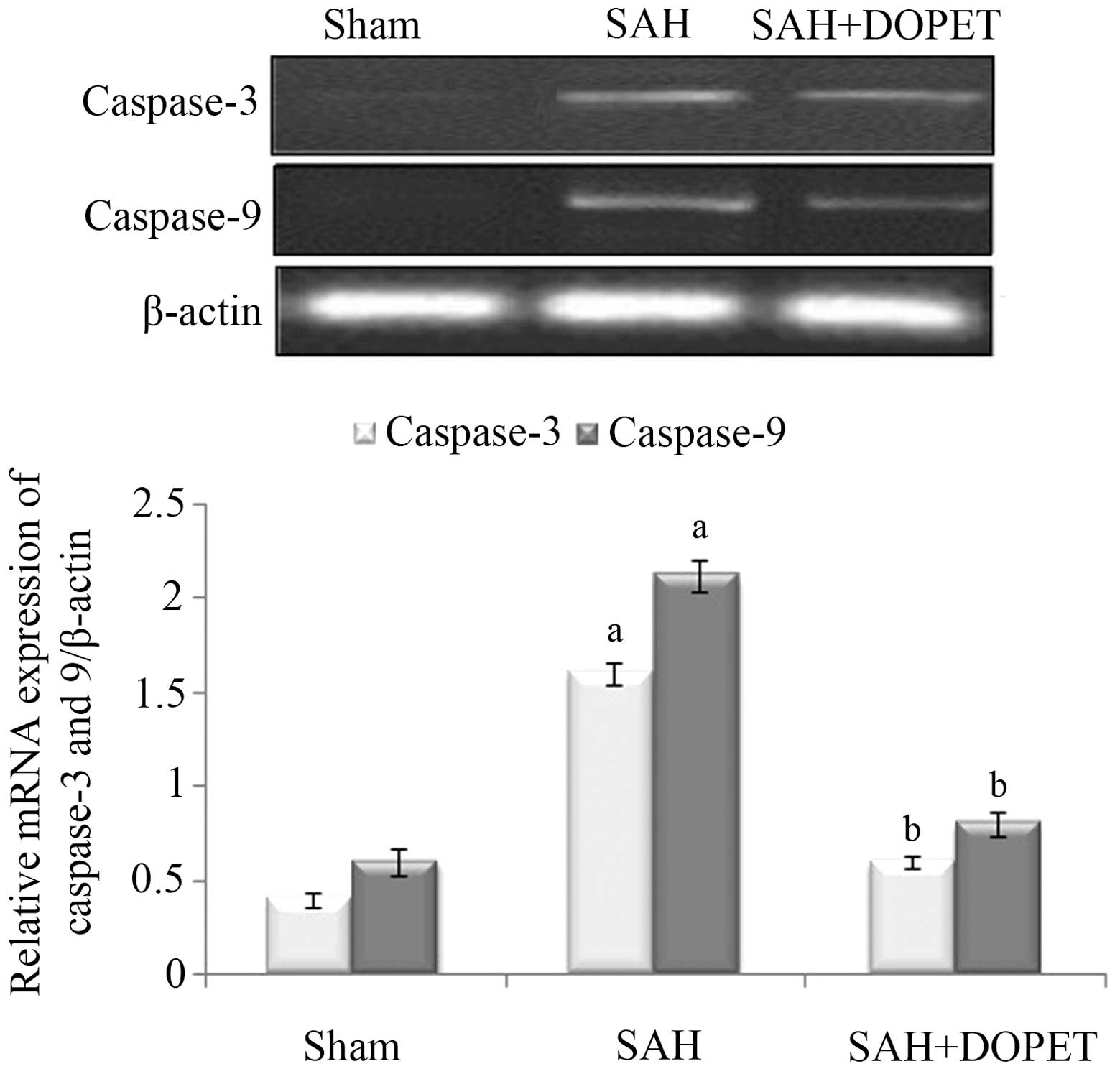
Effect of SAH and DOPET on caspase-3
and caspase-9 mRNA expression
In the experimental SAH model, the caspase-3 and
caspase-9 mRNA expression levels in the cerebral cortex were
significantly increased (P<0.05) when compared with the
sham-operated rats. However, therapeutic intervention with DOPET
downregulated the mRNA levels of caspase-3 and caspase-9 when
compared with the SAH rats, and thus attenuated the apoptosis
(Fig. 5).
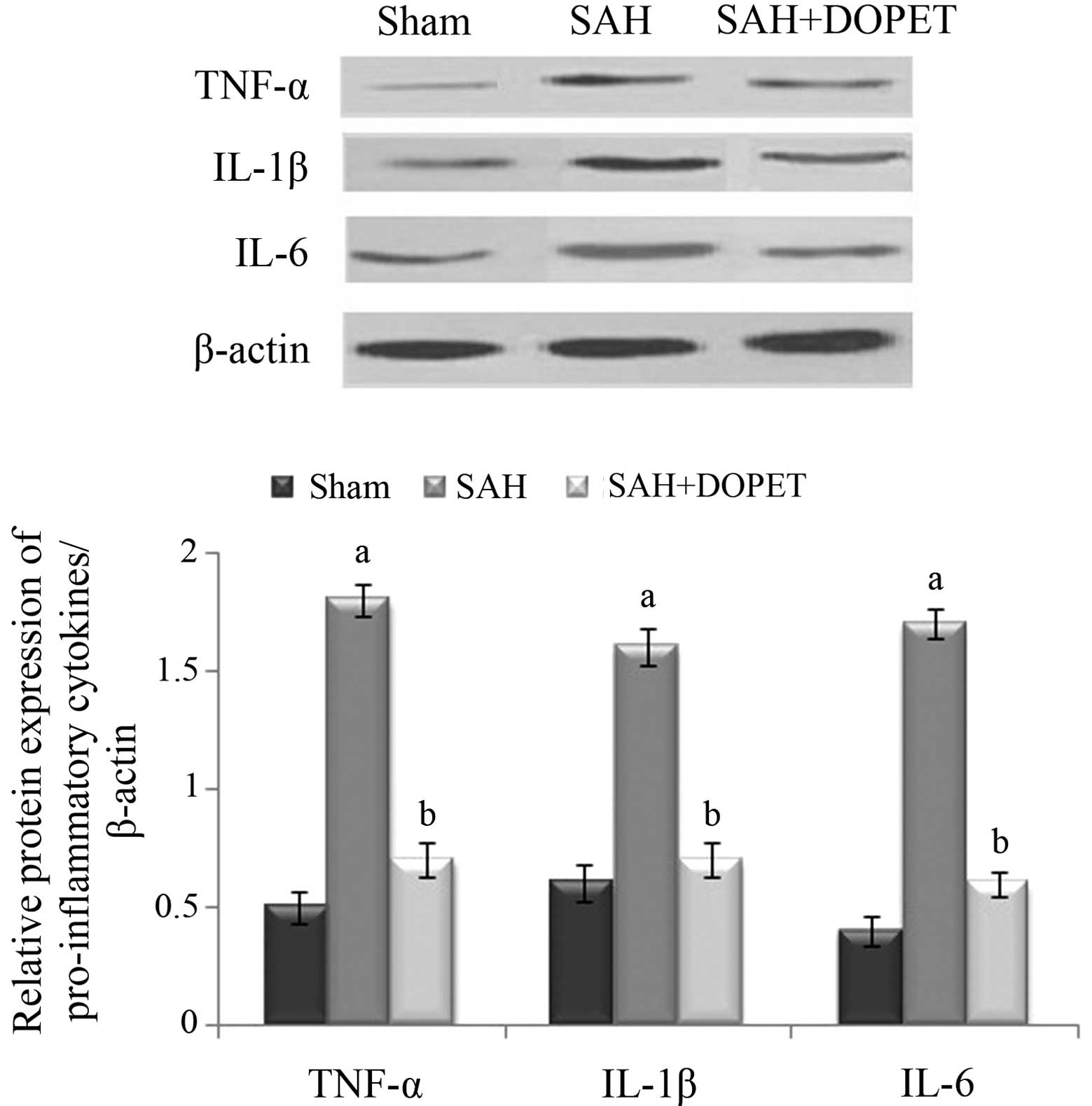
Effect of SAH and DOPET on protein
expression of proinflammatory cytokines
Western blot analysis was used to evaluate the
protein expression levels of TNF-α, IL-6 and IL-1β. Compared with
the sham group, levels of the three inflammatory cytokines were
significantly increased 24 h after SAH in the SAH group
(P<0.05), whereas DOPET administration significantly reduced the
levels of TNF-α, IL-6 and IL-1β compared with the SAH group
(P<0.05). These results show that administration of DOPET
downregulates the cortical expressions of pro-inflammatory
cytokines 24 h after SAH (Fig.
6).
Discussion
Oxidative stress is a biological event which emerges
from the potent cellular oxidizing ability of abundant ROS or free
radicals (44,45). Following SAH, increased generation of
oxidative stress occurs and prior results suggest that oxidative
stress is a prime mediator of brain injury (15). During SAH, clot derived hemoglobin
(Hb) triggers free radicals, including O−2•,
H2O2 and •OH, which subsequently
react. Auto-oxidation of Hb produces O−2• and
dismutation of two O−2• forms
H2O2, which is the source of highly reactive
•OH in the reaction catalyzed by ferric ion (46). Amongst these oxidants, •OH
is highly potent and attacks the nucleic acids, lipids and proteins
to produce a marked cytotoxic effect (47,48).
Thus, the generation of •OH free radicals from
extravasated Hb (49), loss of
mitochondrial integrity (50) and
depletion of endogenous antioxidant system (51) have been elsewhere reported in
experimental or human SAH.
Lipid peroxidation (LPO) is a noxious biological
event induced by the free radicals such as •OH,
ONOO− and H2O2 resulting in
structural alterations of membranes and functional impairment of
cellular components. MDA, the end product of LPO, attacks the
polyunsaturated fatty acids of the cell membrane and thus serves as
an effective marker of free radical damage (52). Similarly, in the present study,
elevated MDA levels were observed in the cortex of SAH rats, which
is in corroboration with a previous report (53). Treatment with DOPET significantly
mitigated the elevated MDA level. The anti-lipid peroxidative
effect of DOPET may be due to its lipophilic and hydrophilic nature
(54). The phenolic group may imbed
in the membrane, acting as a chain-breaking inhibitor of lipid
peroxidation (55).
Furthermore, the downregulation of antioxidant
defense system may be crucially involved in the pathology of SAH
(56). In the antioxidant defense
mechanism, the primary protection is performed by SOD against
oxidative stress and LPO (15). In
the oxidative stress cascade, the superoxide radical is initially
generated and converted into H2O2 and
molecular oxygen by catalase or GPx (57). Thus, vital organs and tissues are
more prone to oxidative stress attack, which may be due to reduced
antioxidant levels (58). The
non-enzymic antioxidant reduced GSH, terminates the vicious cycle
of ROS by reacting with the single oxygen and hydroxide radical and
thus prevents tissue damage (59).
In the present investigation, SAH rats displayed diminished
glutathione, SOD, GPx and levels in cortex tissue. However,
treatment with DOPET restored the altered antioxidant status to
normal which may be due to the scavenging of free radicals and
inhibition of LPO (60).
Delayed global edema has been displayed as an
independent predictor of mortality (16). Furthermore, post SAH provoked
cerebral edema may prelude elevated intracranial pressure (ICP) and
brain herniation, leading to irreversible brain damage or mortality
(61). Clinically, brain edema are
underscored as cytotoxic or vasogenic edema (62). The characteristic features of
cytotoxic edema include swelling with intracellular fluid
accumulation which resembles astrocyte swelling (63). In cases of vasogenic edema,
disruption of BBB occurs which may lead to the accumulation of
fluid surrounding the cells (64).
Furthermore, studies suggest that the altered expression levels of
aquaporins, BBB disruption, clot derived substances, secondary
noxious events like elevated ICP and hypertension are actively
involved in the progression of brain edema after SAH, and
hypertension are involved in the pathogenesis of brain edema
(65,66). Turbulence in the BBB permeability is
a key event during the brain injury after SAH (66). Furthermore, in SAH patients with
vasogenic edema, a direct noxious effect after BBB rupture have
been proved clinically, as well as in experimental studies
(67). Furthermore, the edema
increases the brain volume and thus extends the elevated ICP after
SAH (68). Consequently, there is an
elevation in ICP, which further reduces cerebral blood flow,
leading to increased ischemia (65).
In the present study, it was found that the brain water content
increased obviously after SAH and administration of DOPET abated
brain edema significantly. Previous reports suggest that DOPET
mitigates brain edema in ischemic rats by reducing of BBB
permeability (69).
However, oxidative stress can induce changes of
enzymes which are apoptosis-related, including p53, caspase-3 and
caspase-9 (70). Caspase-9 is an
essential protein involved in the breakdown of procaspase-3 to
caspase-3 (71). During SAH,
caspase-3 was overexpressed in the cortical neurons and the
upregulation of caspase-3 led to the apoptosis of neural cells and
brain edema (72). The present data
showed that the expression of caspase-3 and caspase-9 increased
significantly in the experimental SAH group, while these expression
levels may be reversed by DOPET administration. These results
suggest that DOPET could inhibit proapoptotic enzymes via its
antioxidant activity and exertion of a neuroprotection effect.
In conclusion, DOPET treatment significantly
attenuated the toxic manifestation of SAH by preserving BBB
integrity, inhibition of lipid peroxidation and restoration of
antioxidant levels. Furthermore, the mRNA expression levels of the
apoptotic markers caspase-3 and caspase-9 and the protein
expression of proinflammatory cytokines TNF-α, IL-6 and IL-1β were
downregulated DOPET intervention. Further studies on DOPET are
required to elucidate the neuroprotective mechanism involved in its
protective effect against SAH trauma.
Acknowledgements
The present study was supported by Tongcheng
People's Hospital (grant no. TCYY-20140402).
References
|
1
|
Ansar S, Maddahi A and Edvinsson L:
Inhibition of cerebrovascular raf activation attenuates cerebral
blood flow and prevents upregulation of contractile receptors after
subarachnoid hemorrhage. BMC Neurosci. 12:1072011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lantigua H, Ortega-Gutierrez S, Schmidt
JM, Lee K, Badjatia N, Agarwal S, Claassen J, Connolly ES and
Stephan A: Mayercorresponding author. Subarachnoid hemorrhage: Who
dies, and why? Crit Care. 19:3092015.
|
|
3
|
Schievink WI, Riedinger M, Jhutty TK and
Simon P: Racial disparities in subarachnoid hemorrhage mortality:
Los Angeles county, california, 1985–1998. Neuroepidemiology.
23:299–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Gijn J and Rinkel GJ: Subarachnoid
haemorrhage: Diagnosis, causes and management. Brain. 124:249–278.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hop JW, Rinkel GJ, Algra A and van Gijn J:
Changes in functional outcome and quality of life in patients and
caregivers after aneurysmal subarachnoid hemorrhage. J Neurosurg.
95:957–963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen S, Feng H, Sherchan P, Klebe D, Zhao
G, Sun X, Zhang J, Tang J and Zhang JH: Controversies and evolving
new mechanisms in subarachnoid hemorrhage. Prog Neurobio.
115:64–91. 2014. View Article : Google Scholar
|
|
7
|
Pluta RM, Hansen-Schwartz J, Dreier J,
Vajkoczy P, Macdonald RL, Nishizawa S, Kasuya H, Wellman G, Keller
E, Zauner A and Dorsch N: Cerebral vasospasm following subarachnoid
hemorrhage: Time for a new world of thought. Neurol Res.
31:151–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cahill J, Calvert JW and Zhang JH:
Mechanisms of early brain injury after subarachnoid hemorrhage. J
Cereb Blood Flow Metab. 26:1341–1353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vajkoczy P, Meyer B, Weidauer S, Raabe A,
Thome C, Ringel F, Breu V and Schmiedek P: Clazosentan
(AXV-034343), a selective endothelin A receptor antagonist, in the
prevention of cerebral vasospasm following severe aneurysmal
subarachnoid hemorrhage: Results of a randomized, double-blind,
placebo-controlled, multicenter phase IIa study. J Neurosurg.
103:9–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macdonald RL, Kassell NF, Mayer S,
Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S and Pasqualin
A: CONSCIOUS-1 Investigators: Clazosentan to overcome neurological
ischemia and infarction occurring after subarachnoid hemorrhage
(CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2
dose-finding trial. Stroke. 39:3015–3021. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen-Schwartz J, Vajkoczy P, Macdonald
RL, Pluta RM and Zhang JH: Cerebral vasospasm: Looking beyond
vasoconstriction. Trends Pharmacol Sci. 28:252–256. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rabinstein AA: Secondary brain injury
after aneurysmal subarachnoid haemorrhage: More than vasospasm.
Lancet Neurol. 10:593–595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Broderick JP, Brott TG, Duldner JE,
Tomsick T and Leach A: Initial and recurrent bleeding are the major
causes of death following subarachnoid hemorrhage. Stroke.
25:1342–1347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sehba FA and Bederson JB: Mechanisms of
acute brain injury after subarachnoid hemorrhage. Neurol Res.
28:381–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaetani P, Pasqualin A, Rodriguezy Baena
R, Borasio E and Marzatico F: Oxidative stress in the human brain
after subarachnoid hemorrhage. J Neurosurg. 89:748–754. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Claassen J, Carhuapoma JR, Kreiter KT, Du
EY, Connolly ES and Mayer SA: Global cerebral edema after
subarachnoid hemorrhage: frequency, predictors and impact on
outcome. Stroke. 33:1225–1232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cahill J and Zhang JH: Subarachnoid
hemorrhage: Is it time for a new direction? Stroke. 40(Suppl 3):
S86–S87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
László FA, Varga C and Dóczi T: Cerebral
oedema after subarachnoid haemorrhage. Pathogenetic significance of
vasopressin. Acta Neurochir (Wien). 133:122–133. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dóczi T, Joó F, Adám G, Bozóky B and
Szerdahelyi P: Blood-brain barrier damage during the acute stage of
subarachnoid hemorrhage, as exemplified by a new animal model.
Neurosurgery. 18:733–739. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palade C, Ciurea AV, Nica DA, Savu R and
Moisa HA: Interference of apoptosis in the pathophysiology of
subarachnoid hemorrhage. Asian J Neurosurg. 8:106–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gilgun-Sherki Y, Rosenbaum Z, Melamed E
and Offen D: Antioxidant therapy in acute central nervous system
injury: Current state. Pharmacol Rev. 54:271–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de la Torre R, Covas MI, Pujadas MA, Fitó
M and Farré M: Is dopamine behind the health benefits of red wine?
Eur J Nutr. 45:307–310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Auñon-Calles D, Canut L and Visioli F:
Toxicological evaluation of pure hydroxytyrosol. Food Chem Toxicol.
55:498–504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
González-Correa JA, Navas MD,
Lopez-Villodres JA, Trujillo M, Espartero JL and De La Cruz JP:
Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate
in rat brain slices subjected to hypoxia-reoxygenation. Neurosci
Lett. 446:143–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ristagno G, Fumagalli F,
Porretta-Serapiglia C, Orrù A, Cassina C, Pesaresi M, Masson S,
Villanova L, Merendino A, Villanova A, et al: Hydroxytyrosol
attenuates peripheral neuropathy in streptozotocin-induced diabetes
in rats. J Agric Food Chem. 60:5859–5865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Granados-Principal S, El-Azem N, Pamplona
R, Ramirez-Tortosa C, Pulido-Moran M, Vera-Ramirez L, Quiles JL,
Sanchez-Rovira P, Naudí A, Portero-Otin M, et al: Hydroxytyrosol
ameliorates oxidative stress and mitochondrial dysfunction in
doxorubicin-induced cardiotoxicity in rats with breast cancer.
Biochem Pharmacol. 90:25–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rouissi K, Hamrita B, Kouidi S, Messai Y,
Jaouadi B, Hamden K, Medimegh I, Ouerhani S, Cherif M and Elgaaied
AB: In vivo prevention of bladder urotoxicity: Purified
hydroxytyrosol ameliorates urotoxic effects of cyclophosphamide and
buthionine sulfoximine in mice. Int J Toxicol. 30:419–427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Capasso G, Di Gennaro CI, Della Ragione F,
Manna C, Ciarcia R, Florio S, Perna A, Pollastro RM, Damiano S,
Mazzoni O, et al: In vivo effect of the natural antioxidant
hydroxytyrosol on cyclosporine nephrotoxicity in rats. Nephrol Dial
Transplant. 23:1186–1195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan S, Liu L, Pan H, Ma Y, Wang D, Kang K,
Wang J, Sun B, Sun X and Jiang H: Protective effects of
hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol
Nutr Food Res. 57:1218–1227. 2003. View Article : Google Scholar
|
|
30
|
Cao K, Xu J, Zou X, Li Y, Chen C, Zheng A,
Li H, Li H, Szeto IM, Shi Y, et al: Hydroxytyrosol prevents
diet-induced metabolic syndrome and attenuates mitochondrial
abnormalities in obese mice. Free Radic Biol Med. 67:396–407. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hagiwara K, Goto T, Araki M, Miyazaki H
and Hagiwara H: Olive polyphenol hydroxytyrosol prevents bone loss.
Eur J Pharmacol. 662:78–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de la Puerta R, Ruiz Gutierrez V and Hoult
JR: Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin
olive oil. Biochem Pharmacol. 57:445–449. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
González-Santiago M, Martín-Bautista E,
Carrero JJ, Fonollá J, Baró L, Bartolomé MV, Gil-Loyzaga P and
López-Huertas E: One month administration of hydroxytyrosol,
phenolic antioxidant present in olive oil, to hyperlipemic rabbits
improves blood lipid profile, antioxidant status and reduces
atherosclerosis development. Atherosclerosis. 188:35–42. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao B, Ma Y, Xu Z, Wang J, Wang F, Wang
D, Pan S, Wu Y, Pan H, Xu D, et al: Hydroxytyrosol, a natural
molecule from olive oil, suppresses the growth of human
hepatocellular carcinoma cells via inactivating AKT and nuclear
factor-kappa B pathways. Cancer Lett. 347:79–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee-Huang S and Huang PL, Zhang D, Lee JW,
Bao J, Sun Y, Chang YT, Zhang J and Huang PL: Discovery of
small-molecule HIV-1 fusion and integrase inhibitors oleuropein and
hydroxytyrosol: Part I. fusion (corrected) inhibition. Biochem
Biophys Res Commun. 354:872–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cabrerizo S, De La Cruz JP,
López-Villodres JA, Muñoz-Marín J, Guerrero A, Reyes JJ, Labajos MT
and González-Correa JA: Role of the inhibition of oxidative stress
and inflammatory mediators in the neuroprotective effects of
hydroxytyrosol in rat brain slices subjected to hypoxia
reoxygenation. J Nutr Biochem. 24:2152–2157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
St-Laurent-Thibault C, Arseneault M,
Longpré F and Ramassamy C: Tyrosol and hydroxytyrosol, two main
components of olive oil, protect N2a cells against
amyloid-β-induced toxicity. Involvement of the NF-κB signaling.
Curr Alzheimer Res. 8:543–551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schaffer S, Podstawa M, Visioli F, Bogani
P, Müller WE and Eckert GP: Hydroxytyrosol-rich olive mill
wastewater extract protects brain cells in vitro and ex vivo. J
Agric Food Chem. 55:5043–5049. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park S, Yamaguchi M, Zhou C, Calvert JW,
Tang J and Zhang JH: Neurovascular protection reduces early brain
injury after subarachnoid hemorrhage. Stroke. 35:2412–2417. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garcia JH, Wagner S, Liu KF and Hu XJ:
Neurological deficit and extent of neuronal necrosis attributable
to middle cerebral artery occlusion in rats. Statistical
validation. Stroke. 26:627–634; discussion 635. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xi G, Hua Y, Keep RF, Younger JG and Hoff
JT: Brain edema after intracerebral Hemorrhage: The effects of
systemic complement depletion. Acta Neurochir Suppl. 81:253–256.
2002.PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsubokawa T, Jadhav V, Solaroglu I,
Shiokawa Y, Konishi Y and Zhang JH: Lecithinized superoxide
dismutase improves outcomes and attenuates focal cerebral ischemic
injury via antiapoptotic mechanisms in rats. Stroke. 38:1057–1062.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wallace DC: A mitochondrial paradigm of
metabolic and degenerative diseases, aging and cancer: A dawn for
evolutionary medicine. Annu Rev Genet. 39:359–407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Winterbourn CC: Biological reactivity and
biomarkers of the neutrophil oxidant, hypochlorous acid.
Toxicology. 181-182:223–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Macdonald RL and Weir BK: Cerebral
vasospasm and free radicals. Free Radic Biol Med. 16:633–643. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohsawa I, Ishikawa M, Takahashi Watanabe
M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S and Ohta
S: Hydrogen acts as a therapeutic antioxidant by selectively
reducing cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Floyd RA and Carney JM: Free radical
damage to protein and DNA: mechanisms involved and relevant
observations on brain undergoing oxidative stress. Ann Neurol.
32(Suppl): S22–S27. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Asano T: Oxyhemoglobin as the principal
cause of cerebral vasospasm: A holistic view of its actions. Crit
Rev Neurosurg. 9:303–318. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rodriguez y Baena R, Gaetani P, Silvani V,
Spanu G and Marzatico F: Effect of nimodipine on mitochondrial
respiration in different rat brain areas after subarachnoid
haemorrhage. Acta Neurochir Suppl (Wien). 43:177–181.
1988.PubMed/NCBI
|
|
51
|
Kaynar MY, Tanriverdi T, Kafadar AM,
Kacira T, Uzun H, Aydin S, Gumustas K, Dirican A and Kuday C:
Detection of soluble intercellular adhesion molecule-1 and vascular
cell adhesion molecule-1 in both cerebrospinal fluid and serum of
patients after aneurysmal subarachnoid hemorrhage. J Neurosurg.
101:1030–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rossi R, Dalle-Donne I, Milzani A and
Giustarini D: Oxidized forms of glutathione in peripheral blood as
biomarkers of oxidative stress. Clin Chem. 52:1406–1414. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Erşahin M, Ozsavcı D, Sener A, Ozakpınar
OB, Toklu HZ, Akakin D, Sener G and Yeğen BÇ: Obestatin alleviates
subarachnoid haemorrhage-induced oxidative injury in rats via its
anti-apoptotic and antioxidant effects. Brain Inj. 27:1181–1189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Faine LA, Rodrigues HG, Galhardi CM, Ebaid
GM, Diniz YS, Padovani CR and Novelli EL: Effects of olive oil and
its minor constituents on serum lipids, oxidative stress, and
energy metabolism in cardiac muscle. Can J Physiol Pharml.
84:239–245. 2006. View Article : Google Scholar
|
|
55
|
Deiana M, Incani A, Rosa A, Corona G,
Atzeri A, Loru D, Paola Melis M and Assunta Dessì M: Protective
effect of hydroxytyrosol and its metabolite homovanillic alcohol on
H(2)O(2) induced lipid peroxidation in renal tubular epithelial
cells. Food Chem Toxicol. 46:2984–2990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Erşahin M, Toklu HZ, Erzik C, Cetinel S,
Akakin D, Velioğlu-Oğünç A, Tetik S, Ozdemir ZN, Sener G and Yeğen
BC: The anti-inflammatory and neuroprotective effects of ghrelin in
subarachnoid hemorrhage-induced oxidative brain damage in rats. J
Neurotrauma. 27:1143–1155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidant therapeutic options. Curr
Neuropharmacol. 7:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kandhare AD, Raygude KS, Ghosh P, Ghule AE
and Bodhankar SL: Neuroprotective effect of naringin by modulation
of endogenous biomarkers in streptozotocin induced painful diabetic
neuropathy. Fitoterapia. 83:650–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pastore A, Federici G, Bertini E and
Piemonte F: Analysis of glutathione: Implication in redox and
detoxification. Clinica Chim Acta. 333:19–39. 2003. View Article : Google Scholar
|
|
60
|
Gutierrez VR, de la Puerta R and Catalá A:
The effect of tyrosol, hydroxytyrosol and oleuropein on the
non-enzymatic lipid peroxidation of rat liver microsomes. Mol Cell
Biochem. 217:35–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Graham DI, McIntosh TK, Maxwell WL and
Nicoll JA: Recent advances in neurotrauma. J Neuropathol Exp
Neurol. 59:641–651. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Katzman R, Clasen R, Klatzo I, Meyer JS,
Pappius HM and Waltz AG: Report of joint committee for stroke
resources. IV. Brain edema in stroke. Stroke. 8:512–540. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kimelberg HK: Current concepts of brain
edema. Review of laboratory investigations. J Neurosurg.
83:1051–1059. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Feillet-Coudray C, Sutra T, Fouret G,
Ramos J, Wrutniak-Cabello C, Cabello G, Cristol JP and Coudray C:
Oxidative stress in rats fed a high-fat high-sucrose diet and
preventive effect of polyphenols: Involvement of mitochondrial and
NAD(P)H oxidase systems. Free Radic Biol Med. 46:624–632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fukuhara T, Douville CM, Eliott JP, Newell
DW and Winn HR: Relationship between intracranial pressure and the
development of vasospasm after aneurysmal subarachnoid hemorrhage.
Neurol Med Chir (Tokyo). 38:710–715; discussion 716–717. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Imperatore C, Germanò A, d'Avella D,
Tomasello F and Costa G: Effects of the radical scavenger AVS on
behavioral and BBB changes after experimental subarachnoid
hemorrhage. Life Sci. 66:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dóczi T: The pathogenetic and prognostic
significance of blood-brain barrier damage at the acute stage of
aneurysmal subarachnoid haemorrhage. Clinical and experimental
studies. Acta Neurochir (Wien). 77:110–132. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fornezza U, Carraro R, Demo P, Zamperetti
N, Volpin L, Landi A, De Luca GP and Benedetti A: The transcranial
Doppler ultrasonography in the evaluation of vasospasm and of
intracranial hypertension after subarachnoid hemorrhage.
Agressologie. 31:259–261. 1990.PubMed/NCBI
|
|
69
|
Mohagheghi F, Bigdeli MR, Rasoulian B,
Zeinanloo AA and Khoshbaten A: Dietary virgin olive oil reduces
blood brain barrier permeability, brain edema and brain injury in
rats subjected to ischemia-reperfusion. Scientific World Journal.
10:1180–1191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ayer RE and Zhang JH: Oxidative stress in
subarachnoid haemorrhage: Signifi-cance in acute brain injury and
vasospasm. Acta Neurochir Suppl. 104:33–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cregan SP, MacLaurin JG, Craig C,
Robertson GS, Nicholson DW, Park DS and Slack RS: Bax-dependent
caspase-3 activation is a key determinant in p53-induced apoptosis
in neurons. J Neurosci. 19:7860–7869. 1999.PubMed/NCBI
|
|
72
|
Simard JM, Geng Z, Woo SK, Ivanova S,
Tosun C, Melnichenko L and Gerzanich V: Glibenclamide reduces
inflammation, vasogenic edema and caspase-3 activation after
subarachnoid hemorrhage. J Cereb Blood Flow Metab. 29:317–330.
2009. View Article : Google Scholar : PubMed/NCBI
|