Introduction
Renal ischemia is commonly observed in patients with
cardiovascular surgery, trauma, shock, burn and those that have
undergone organ transplantation (1,2).
Reperfusion of ischemic tissue is associated with increased
production of oxygen radicals, subsequently leading to endothelial
barrier dysfunction and tissue injury (3).
Ischemia-reperfusion (I/R) injury may result in a
molecular and cellular inflammatory response within the kidney,
which can induce the activation of the inflammation associated
transcription factor nuclear factor-κB (NF-κB), which is crucially
involved in the pathogenesis of I/R injury (4,5).
Notably, the increased activation of NF-κB in the I/R-challenged
kidney further contributes to kidney tissue damage, frequently
causing systemic a inflammatory response and subsequent leading to
acute kidney failure (5). Therefore,
suppressing NF-κB mediated inflammation may be an effective measure
to attenuate renal ischemia reperfusion injury.
The Janus kinase/signal transducer 2 and activator
of transcription 3 (JAK2/STAT3) pathway is a classical signaling
pathway that transduces cellular signals from the plasma membrane
to the nucleus, and has an important role in regulating
NF-κB-mediated inflammation (6).
Osthole is a naturally-derived component of
coumarin, which has been widely used clinically owing to its
multiple biochemical and pharmacological effects (7). Furthermore, previous studies have
demonstrated that osthole has a protective effect in cerebral
intestinal I/R injury by exerting anti-inflammatory effects
(7,8).
However, the role and potential molecular mechanisms
of osthole in modulation of I/R-induced inflammatory response in
the kidney remain unclear. Therefore, the present study aimed to
investigate the effects and potential mechanisms of osthole in
modulation of I/R-induced inflammatory response in rats kidney.
Materials and methods
Ethical approval
The experimental protocol was approved by the
Institutional Animal Care and Use Committee at the First Affiliated
Hospital of Chongqing Medical University (Chongqing, China).
Drug
Osthole (purity, >98%) was purchased from the
National Institute for Food and Drug Control (Beijing, China).
Osthole was dissolved in a 1:9 (v/v) mixture of Tween 80 (Google
Biological Technology Co., Ltd., Wuhan, China) and 0.9% sodium
chloride (Google Biological Technology Co., Ltd.).
Animals
Male Sprague-Dawley rats (weight, 180–240 g) were
purchased from Hua Fukang Experimental Animal Center (Beijing,
China). The rats were housed in a specific pathogen-free facility
and fed with laboratory chow and ad libitum water. After a
minimum seven days of acclimation, the rats were randomly allocated
into seven groups (n=10 per group): i) Sham-operated group (sham),
in which the rats were subjected to identical surgical procedure
without occlusion of renal pedicles; ii) I/R-vehicle group (IRI),
in which the rats were subjected to renal ischemia for 45 min; and
I/R-osthole group (osthole), in which the rats were administered
osthole (0, 5, 10, 20 or 40 mg/kg, intravenously) 45 min prior to
I/R induction. The dosage of osthole was based on a previous study
(9).
I/R induction in the kidneys
The rats were first anesthetized with an
intraperitoneal injection of 1% sodium pentobarbital solution (65
mg/kg; Google Biological Technology Co., Ltd.) and a rectal probe
was inserted to monitor body temperature, which was maintained at
37±1°C using a heating blanket. A midline laparotomy was performed
and the abdominal cavity was fully exposed.
Bilateral renal pedicles were carefully isolated
without damaging the ureter and clamped by non-traumatic
microvascular clamps to effect complete cessation of renal arterial
blood flow. After 45 min, the clamps were removed to allow return
of blood flow to the kidneys. Successful ischemia or reperfusion
was judged by observing the change in tissue color from red to dark
blue or from dark blue to bright red respectively, the
contralateral kidney was removed. Middle abdominal incisions were
closed in two layers and covered with antibiotic ointment when the
operation finished. The animals were allowed to recover from
anesthesia, remaining 24 h in a controlled-environment room with
food and water freely available. Rats in the sham group underwent
laparotomy without performing renal ischemia, as a control
population. The rats were sacrificed with an intraperitoneal
injection of 1% sodium pentobarbital solution (120 mg/kg body
weight) 24 h after reperfusion, and the kidneys were harvested for
further analysis.
Assessment of renal function
Serum creatinine (Cr) and blood urea nitrogen (BUN)
were used as indicators of impaired renal function. Blood samples
were obtained from the inferior vena cava 24 h after reperfusion
and were placed in the refrigerator at 4°C for 20 min and
centrifuged (6,000 × g for 3 min) to separate the serum. The
biochemical parameters (BUN and Cr levels) were analyzed
photometrically with an autoanalyzer (AU5800; Beckman Coulter, Inc,
Brea, CA, USA) in the core laboratory of the First Affiliated
Hospital of Chongqing Medical University for assessment of renal
function.
Histological analysis
Renal samples were fixed in formalin and then
embedded in paraffin, and renal sections were next prepared and
subjected to hematoxylin and eosin (HE) staining, as reported
previously (10). The
histopathological changes in the cortex and medulla were evaluated
by a pathologist in a blinded fashion using a five-point
quantitative scale according to the degree of tubular necrosis,
hemorrhage and cast formation, as follows: 0, <10%; 1, 10–25%;
2, 25–50%; 3, 50–75%; and 4, 75–100% (11).
Western blot analysis
The renal tissue was lysed in ice-cold
radioimmunoprecipitation assay buffer [50 mM Tris (pH 7.4), 150 mM
NaCl, 1% Triton, 0.5% deoxycholate, 0.1% SDS, 1 mM EDTA, 10 mM NaF,
and 0.1 mM phenylmethylsulfonyl fluoride] to obtain the total
proteins. The protein concentration was detected using a
bicinchoninic acid protein assay (Beyotime Institute of
Biotechnology, Shanghai, China). Equal amounts of protein samples
(50 µg protein/lane) were separated by 10–12% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. The
non-specific antibodies were blocked with 5% non-fat dried milk in
phosphate-buffered saline for 2 h at room temperature. The
membranes were then incubated overnight at 4°C with primary
antibodies directed against JAK2 (cat. no. ab39636; 1:1,000; Abcam,
Cambridge, UK), p-JAK2 (cat. no. ab32101; 1:500, Abcam), STAT3
(cat. no. ab68153; 1:1,000; Abcam), p-STAT3 (cat. no. ab76351;
1:500; Abcam), p65 (cat. no. ab16502; 1:1,000; Abcam) and p-p65
(cat. no. ab86299; 1:500; Abcam). The membranes were then washed
with Tris-buffered saline with Tween 20 three times and further
incubated with horseradish peroxidase conjugated secondary antibody
(1:3,000; Jackson ImmunoResearch Laboratories, Inc, West Grove, PA,
USA) for 1 h at room temperature. Following washing, the membranes
were processed using an electrochemiluminescence reagent (GE
Healthcare Bio-Sciences, Pittsburg, PA, USA) and the light emission
was captured on X-ray film. The signals were visualized by
chemiluminescent horseradish peroxidase substrate and then
subjected to a densitometric analysis and normalized to β-actin
(1:3,000; Abmart Co, Ltd., Shanghai, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from renal tissue using
RNAiso Plus reagent (Takara Bio, Inc, Otsu, Japan). RNA was treated
with RNase-free DNase I to remove gDNA. Absorbances at 260 and 280
nm were measured for RNA quantification and quality control. All
RNA samples exhibited high quality RNA and were subsequently
reverse transcribed to cDNA using a PrimeScript™ RT reagent kit
(Perfect Real Time; Takara Bio, Inc.) according to the
manufacturer's instructions. Subsequently, qPCR was conducted to
determine the levels of mRNA expression using an ABI Prism 7000
sequence detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in triplicate in 96-well plates
in a final volume of 20 µl under standard conditions. qPCR was
conducted on cDNA samples using the SYBR Green method with
SYBR® Premix Ex Taq™ (Tli RNaseH Plus; Takara Bio,
Inc.). Reaction mixtures contained 10 µl 2X SYBR Green mastermix, 1
µl (6 µM) forward primer, 1 µl (6 µM) reverse primer, 6 µl water
and 2 µl (5 ng/µl) cDNA. qPCR was performed as follows: Initial
denaturation at 95°C for 30 sec for activation of AmpliTaq Cold DNA
polymerase (Applied Biosystems; Thermo Fisher Scientific, Inc.),
followed by 40 cycles of denaturation at 95°C for 5 sec, annealing
at 60°C for 30 sec, and extension at 95°C for 15 sec. Forward and
reverse primer sequences are listed in Table I and were synthesized by Takara Bio,
Inc. To normalize each sample, a control gene (β-actin) was used,
and the arbitrary intensity threshold of amplification was
computed. The 2−ΔΔCq method was used to calculate the
relative expression of each target gene, as described previously
(12), and analyzed using SPSS 12.0
software (SPSS, Inc, Chicago, IL, USA).
 | Table I.Primers used for quantitative
polymerase chain reaction analysis. |
Table I.
Primers used for quantitative
polymerase chain reaction analysis.
| Gene | Sense strand
sequence | Anti-sense strand
sequence |
|---|
| TNF-α |
CTGAACTTCGGGGTGATCGG |
GGCTTGTCACTCGAATTTTGAGA |
| IL-6 |
AGCTTCCTTGTGCAAGTGTCT |
GACAGCCCAGGTCAAAGGTT |
| IL-8 |
CTGCAAGAGACTTCCATCCAG |
AGTGGTATAGACAGGTCTGTTGG |
| β-actin |
AGAGGGAAATCGTGCGTGAC |
CAATAGTGATGACCTGGCCGT |
ELISA analysis
Levels of the inflammatory mediators TNF-α (Bosde
Biotechnology, Wuhan, China; QN-PS1726), IL-6 (Bosde Biotechnology;
EK0526) and IL-8 (Bosde Biotechnology; BA-3381) in the serum were
quantified using specific ELISA kits for mice according to the
manufacturer's instructions (BioSource International, Inc.,
Camarillo, CA, USA).
Statistical analysis
All results are expressed as the mean ± standard
error of the mean of three independent experiments. Differences
among groups were assessed using a one way analysis of variance.
Least significant difference t tests were used when a single
control group was compared with all other groups. All statistical
analyses were conducted using SPSS 13.0 software (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
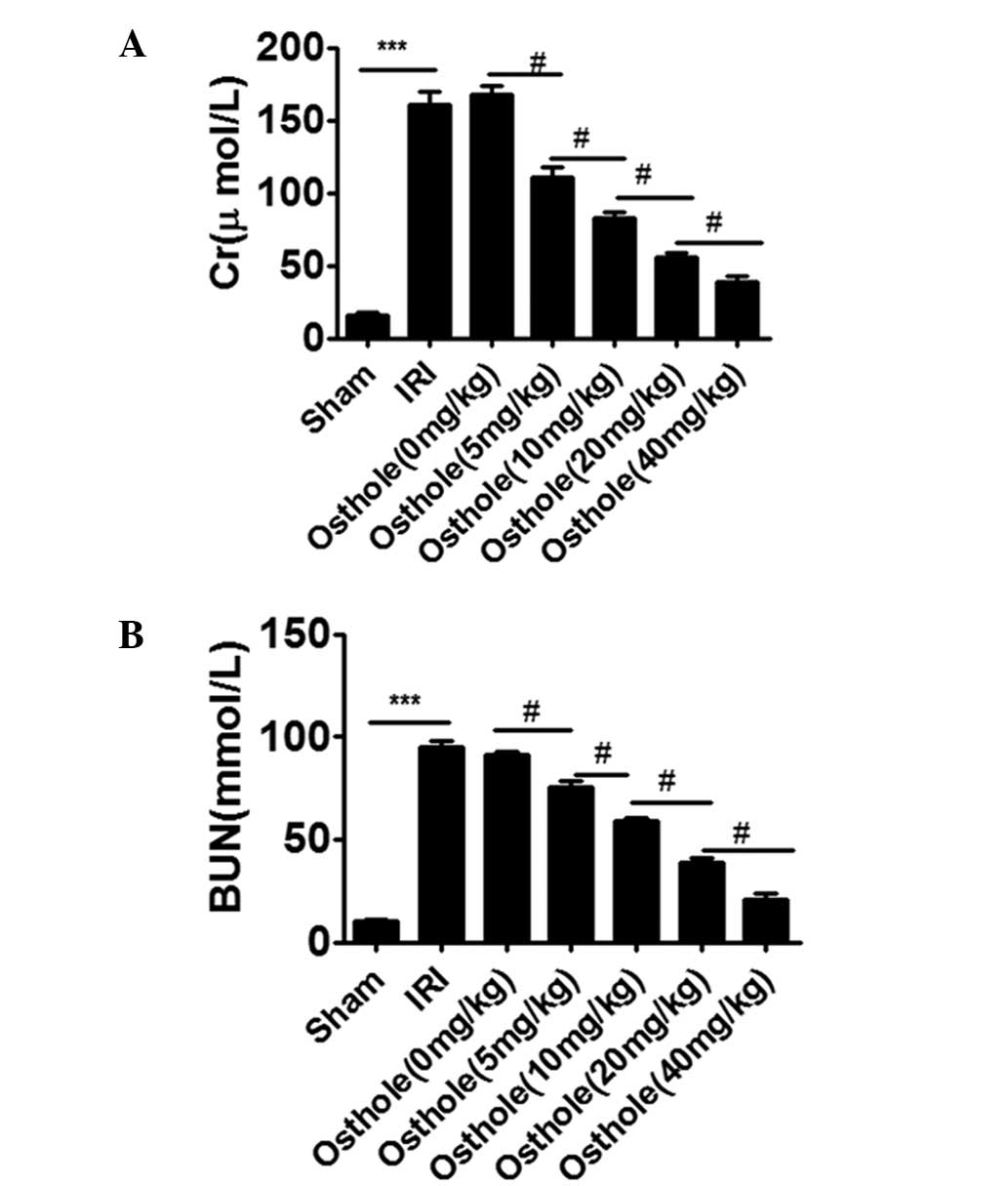
Osthole pretreatment decreased renal
dysfunction after I/R-induced renal injury
As shown in Fig. 1A and
B, compared with the sham group, rats in IRI group showed a
significant increase in the levels of Cr and BUN (P<0.001).
However, osthole pretreatment significantly decreased the levels of
Cr and BUN induced by renal I/R injury in a dose-dependent manner.
The optimal concentration of osthole for the protective effect was
40 mg/kg (P<0.001).
Osthole pretreatment decreased
pathological changes of the kidney after renal I/R
Compared with the sham group (Fig. 2A), the rats in IRI group showed
significant pathological changes, including widespread degeneration
of the tubular architecture, tubular dilation, tubular cell
swelling, cellular vacuolization, tubular cell necrosis and
inflammatory cell infiltration (Fig.
2B). However, pretreatment with osthole resulted in reduced
pathological change in kidney as compared with the IRI group
(Fig. 2C). The histopathological
score of the rats' renal in all groups are presented in Fig. 2D. The scores of IRI group were higher
compared with the sham and osthole groups (P<0.001).
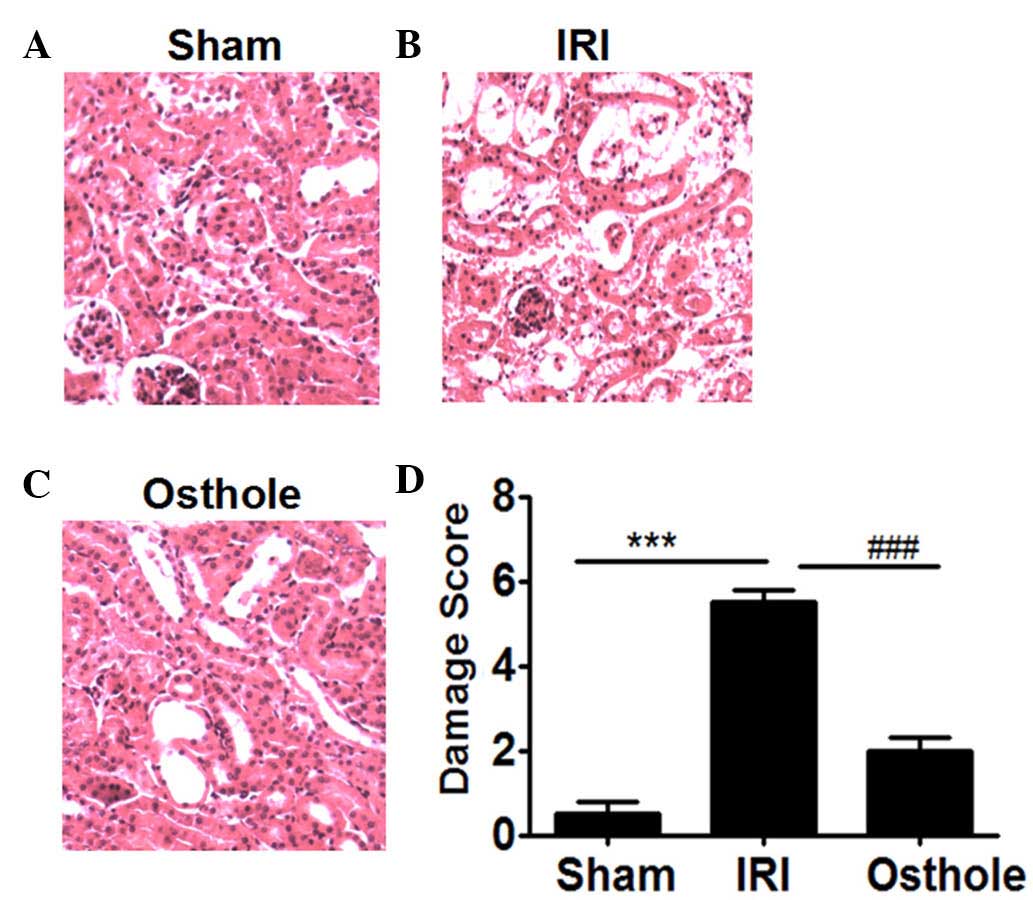 | Figure 2.Hematoxylin and eosin staining for
histopathological changes: Effects of osthole pretreatment on
ischemia/reperfusion (I/R)-induced renal injury. (A) The Sham group
shows no histopathological change 24 h after I/R injury (Sham;
magnification, ×200). (B) The I/R injury (IRI) group shows
widespread degeneration of the tubular architecture, tubular
dilation, tubular cell swelling, cellular vacuolization, tubular
cell necrosis and inflammatory cell infiltration 24 h after IRI
(magnification, ×200). (C) The osthole group shows little
degeneration of the tubular architecture, tubular dilation, tubular
cell swelling, cellular vacuolization, tubular cell necrosis and
inflammatory cell infiltration 24 h after IRI (magnification,
×200). (D) Semi-quantitative assessment of the histological lesions
based on renal histopathological changes. Data are presented as the
mean ± standard error of the mean (n=10). ***P<0.001, vs. Sham;
###P<0.001, vs. osthole. |
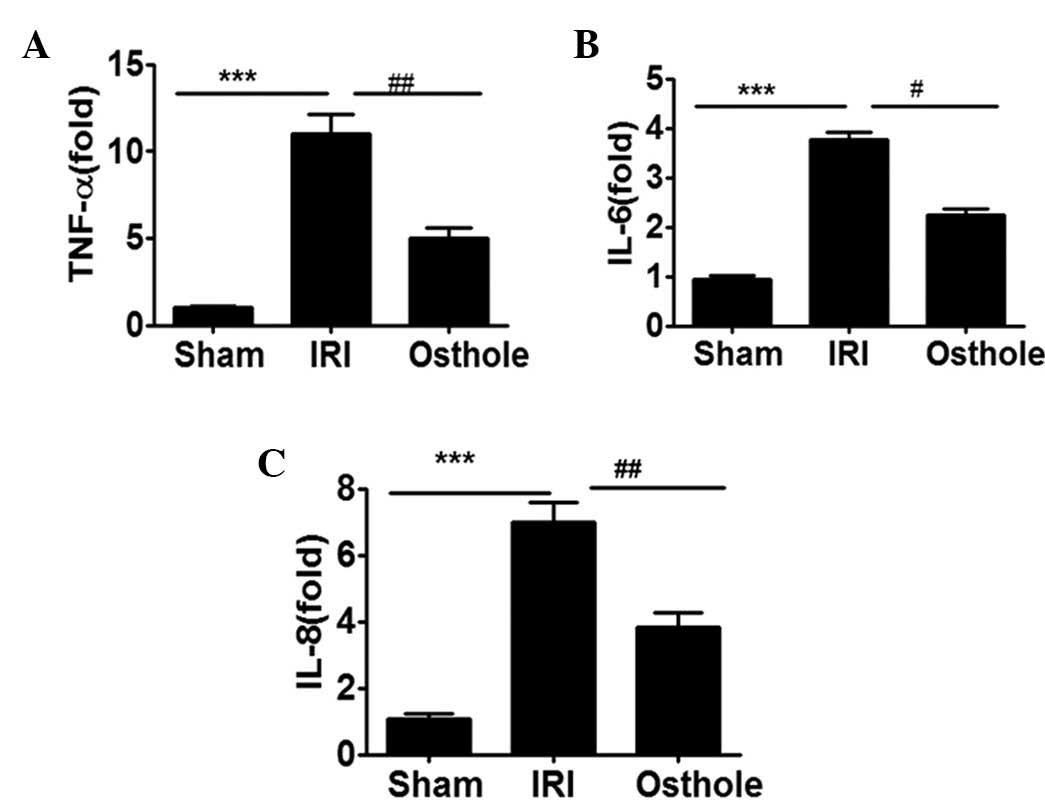
Osthole pretreatment can reduce the
expression of proinflammatory cytokine expression after renal
IRI
To determine whether osthole pretreatment can
interfere with I/R-induced inflammatory response in the kidney,
with respect to production of inflammation-relevant cytokines
TNF-α, IL-6 and IL-8, the renal tissue obtained from each group of
rats were assessed for TNF-α, IL-6 and IL-8 mRNA expression level.
As shown in Fig. 3, the mRNA
expression levels of TNF-α, IL-6 and IL-8 were significantly
upregulated in I/R-damaged kidneys. Notably, the elevated levels of
kidney TNF-α, IL-6 and IL-8 mRNA expression were effectively
reduced by preconditioning with osthole.
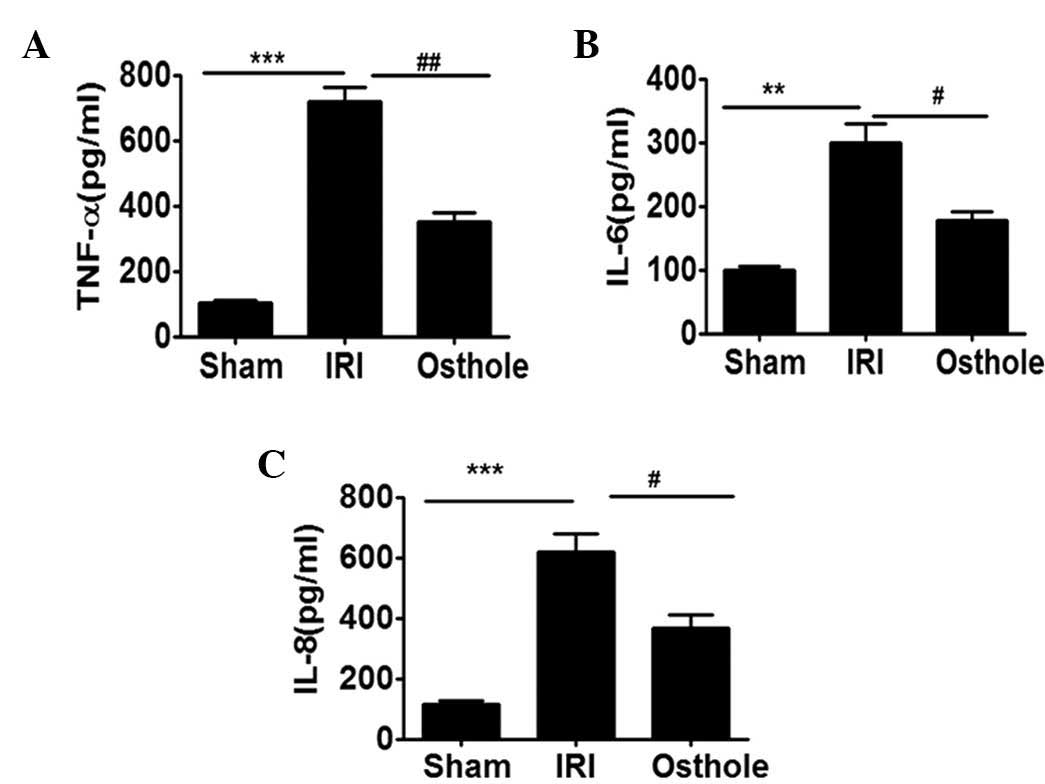
Osthole pretreatment can reduce the
secretion of proinflammatory cytokines after renal IRI
To further demonstrate the effect of osthole
pretreatment in interfering with I/R-induced inflammatory response
in the IRI, the serum obtained from each group of rats were
assessed for TNF-α, IL-6 and IL-8 secretion level. As shown in
Fig. 4, the secretion level of
TNF-α, IL-6 and IL-8 were significantly upregulated in the
I/R-challenged serum. Notably, the elevated levels of serum TNF-α,
IL-6 and IL-8 were effectively reduced by preconditioning with
osthole.
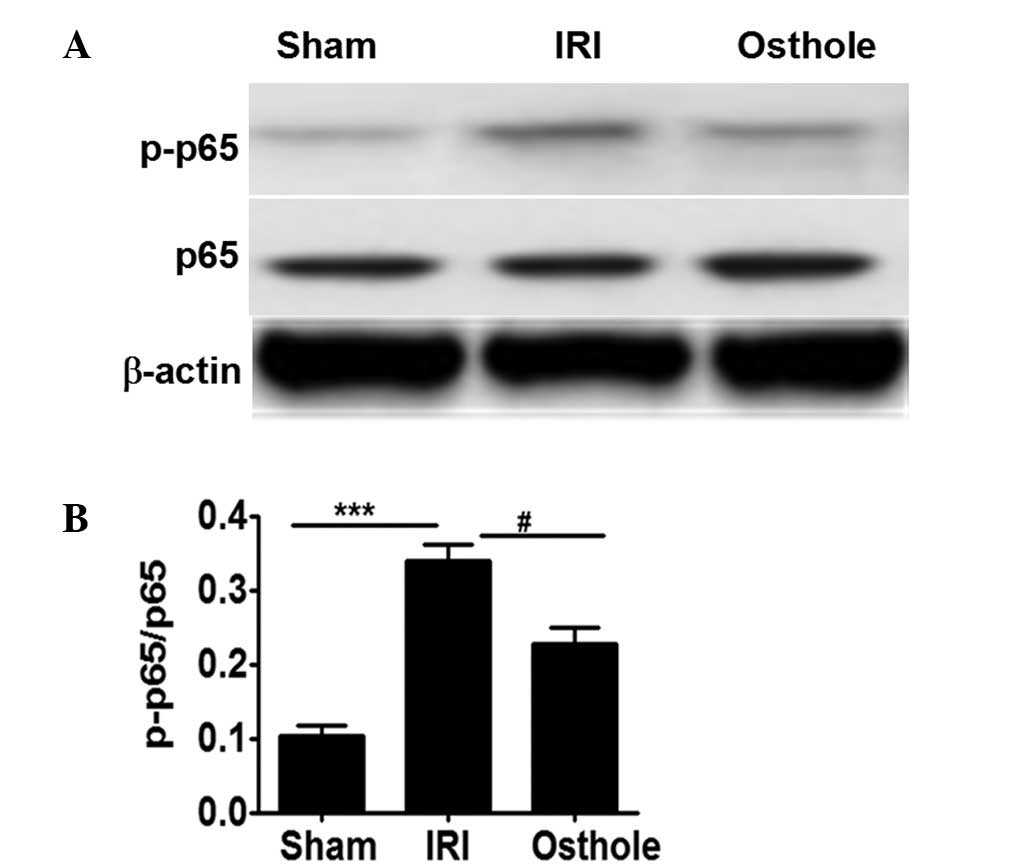
Osthole pretreatment can attenuate
NF-κB activation after renal I/R injury
NF-κB is crucially involved in the inflammatory
response in renal I/R, and its activation is dependent p65
activation (4,5). As shown Fig.
5, neither I/R injury nor osthole pretreatment significantly
affected p65 expression. However, the quantity of activated p65
(p-p65) noted in rats after I/R injury was significantly higher
compared with the sham control rats (Fig. 5A and B). By contrast, pretreatment
with osthole can significantly reduce p65 activation, as manifested
by the lower levels of p-p65 detected in osthole group rats as
compared with that of the IRI group rats. Collectively, the present
results suggest that osthole pretreatment can attenuate
inflammatory response in renal ischemia reperfusion injury by
reducing NF-κB activation (Fig. 5A and
B).
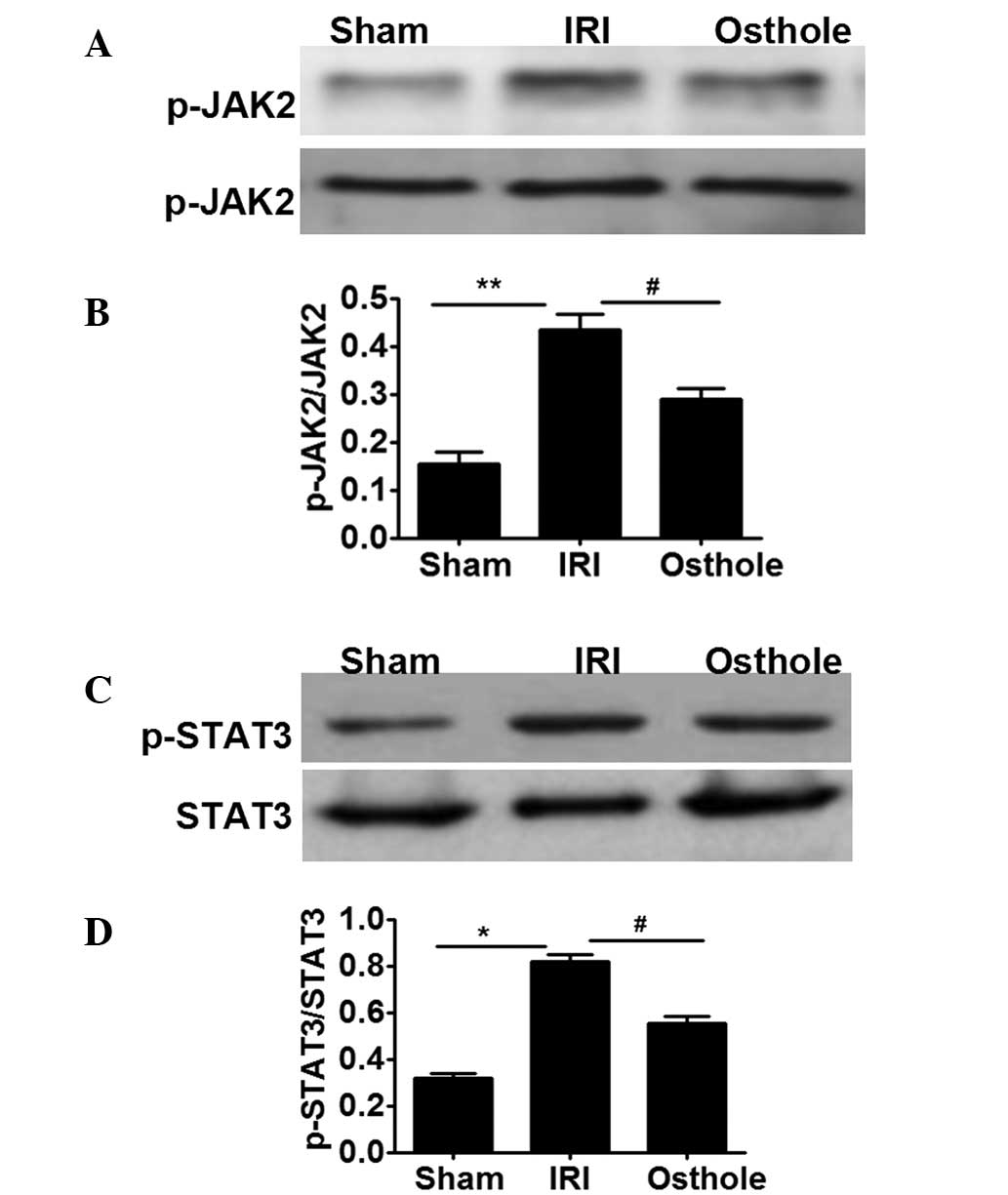
Osthole pretreatment suppressed
JAK2/STAT3 activation after renal I/R
In order to further investigate the mechanism
underlying the osthole-mediated decreasing renal I/R injury, the
activity of JAK2/STAT3 signaling was selectively analyzed in renal
I/R injury. As shown in Fig. 6A and
B, neither I/R insult nor osthole pretreatment have influence
on the expression of JAK2. However, IRI can increase the activation
of JAK2, as indicated by the increased levels of p-JAK2 in the IRI
group compared with the sham group. Furthermore, osthole
pretreatment can decrease the effect of IRI, inducing the
activation of JAK2, as manifested lower levels of p-JAK2 in osthole
group compared with the IRI group (Fig.
6A and B). Since JAK2 activation may provide signals to STAT3,
we next evaluated the expression of STAT3. The results showed that
I/R injury and osthole have no influence on the expression of STAT3
(Fig. 6C and D); however, I/R injury
can increased the expression of p-STAT3, while osthole pretreatment
can decrease the expression of p-STAT3 induced by I/R injury.
Collectively, the present data indicated that osthole pretreatment
can decrease JAK2/STAT3 signaling following renal I/R injury.
Discussion
The acute kidney injury induced by I/R is a clinical
and experimental syndrome characterized by renal dysfunction,
extensive widespread tubular damage, tubular cell necrosis and
inflammatory cell infiltration (4).
Inflammatory responses are believed to play a central role in I/R
injury, in addition to several other factors such as apoptosis,
necrosis and oxidative stress (4,5).
Inflammatory responses exert a range of deleterious effects on
renal tissue, causing a cascade of injury that may lead to organ
failure (4,5).
The present results showed that sham operation did
not alter the renal parameters (serum creatinine, BUN, histological
features and inflammation) as compared with the IRI group rats. By
contrast, renal I/R worsened the renal dysfunction and
histopathological features in rats. In the present study, the
inflammatory response in the kidney during I/R was evaluated by
measuring the expression of proinflammatory cytokine TNF-α, IL-6
and IL-8. In accordance with the change of renal parameters, the
expression of proinflammatory cytokine TNF-α, IL-6 and IL-8 were
significantly increased due to I/R injury (13). Furthermore, histopathological
alterations were evident in the ischemic rat kidney, as well as
alteration of renal function and inflammation. The kidney of the
rats in the IRI group that underwent 45 min ischemia followed by 24
h reperfusion showed a significant pathological difference, as
manifested by widespread degeneration of tubular architecture,
tubular dilation, tubular cell swelling, cellular vacuolization,
tubular cell necrosis and inflammatory cell infiltration.
Therefore, suppressing inflammation is a potential therapeutic
target for reducing renal IRI.
Osthole is a Chinese herbal medicine that has been
shown to have a widespread anti-inflammatory effect and can
decrease cerebral I/R injury (7,8).
However, to date there is a lack of study regarding the precise
function and potential mechanisms of osthole in renal I/R injury.
The present results suggest that osthole protected the rats against
renal I/R injury as manifested by the attenuation of renal
dysfunction, the histopathology alteration and the expression of
the proinflammatory cytokines TNF-α, IL-6 and IL-8 induced by renal
I/R injury.
NF-κB is a ubiquitously acting transcription factor
associated with immune and inflammatory reactions (5). NF-κB has been shown to regulate the
expression of the proinflammatory cytokines TNF-α, IL-6 and IL-8,
which contribute to the further amplification of inflammation
(5,14). Furthermore, NF-κB is crucial to the
propagation of the inflammatory response in the renal I/R injury
and its activation is primarily dependent p65 activation (4,5). In the
present study, I/R insult or osthole influenced the expression of
p65; however, the expression of p-p65 was significantly increased
as compared with the sham group. In addition, osthole pretreatment
can decrease the expression of p-p65 in IRI, which indicated
Osthole pretreatment may significantly reduce NF-κB activation.
The JAK2/STAT3 pathway is a classical signaling
which has been demonstrated to regulate inflammation associated
with renal I/R injury (6).
Therefore, the effect of osthole on JAK2/STAT3 signaling were
investigated in a rat model of I/R. I/R injury and osthole appear
to increase JAK2 activation via phosphorylation, as manifested by
the increased levels of p-JAK2 detected in the IRI group rats
compared with the sham group rats. Furthermore, preconditioning of
rats with osthole can significantly suppress JAK2 activation, as
manifested by the reduced levels of p-JAK2 in osthole group rats
compared with IRI group rats. This result lead us to evaluate STAT3
activity, as JAK2 activation is known to induce STAT3 activation.
Consistent with the aforementioned results, I/R injury and osthole
influence the expression of STAT3. However, I/R injury can increase
the expression of p-STAT3 and osthole pretreatment can reduce the
expression of p-STAT3 induced by I/R injury. These results
indicated that IRI can induce the activation of STAT3, and osthole
pretreatment can attenuate the activation of p-STAT3 induced by I/R
injury. Previous results suggest that blocking JAK2/STAT3
activation can decrease I/R-induced renal injury (6); therefore, in the current report we did
not conduct additional studies to demonstrate that suppressing
JAK2/STAT3 signaling can decrease I/R-induced NF-κB activation in
the kidney. Considering the capacity of osthole preconditioning to
prevent I/R-induced renal injury, it is notable that osthole could
regulate additional pathways other than the JAK2/STAT3 pathway to
suppress NF-κB activation, such as the MAPK kinase cascade
(15). Therefore, further studies
are required to investigate the pathways involved in the
suppression of NF-κB activation by osthole in the context of I/R
injury.
In summary, the present results suggest that
precondition rats with osthole attenuated renal I/R injury, as
manifested by the reduction of pathological and serum changes.
Mechanistic studies demonstrated that osthole preconditioning is
able to decrease NF-κB activation by suppressing the activation of
JAK2/STAT3. Collectively, these data support the conclusion that
osthole may offer an alternative therapy for the prevention of
renal I/R injury in the clinical practice.
Acknowledgements
This study was supported by the Sichuan Provincial
Natural Science Foundation (grant no. 130501).
References
|
1
|
Mangano CM, Diamondstone LS, Ramsay JG,
Aggarwal A, Herskowitz A and Mangano DT: Renal dysfunction after
myocardial revascularization: Risk factors, adverse outcomes and
hospital resource utilization. The multicenter study of
perioperative ischemia research group. Ann Intern Med. 128:194–203.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiffl H, Lang SM and Fischer R: Daily
hemodialysis and the outcome of acute renal failure. N Engl J Med.
346:305–310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chertow GM, Burdick E, Honour M, Bonventre
JV and Bates DW: Acute kidney injury, mortality, length of stay and
costs in hospitalized patients. J Am Soc Nephrol. 16:3365–3370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau A, Wang S, Liu W, Haig A, Zhang ZX and
Jevnikar AM: Glycyrrhizic acid ameliorates HMGB1-mediated cell
death and inflammation after renal ischemia reperfusion injury. Am
J Nephrol. 40:84–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ranganathan PV, Jayakumar C, Mohamed R,
Dong Z and Ramesh G: Netrin-1 regulates the inflammatory response
of neutrophils and macrophages and suppresses ischemic acute kidney
injury by inhibiting COX-2-mediated PGE2 production. Kidney Int.
83:1087–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Si YN, Bao HG, Xu L, Wang XL, Shen Y, Wang
JS and Yang XB: Dexmedetomidine protects against
ischemia/reperfusion injury in rat kidney. Eur Rev Med Pharmacol
Sci. 18:1843–1851. 2014.PubMed/NCBI
|
|
7
|
Liu J, Zhang W, Zhou L, Wang X and Lian Q:
Anti-inflammatory effect and mechanism of osthole in rats. Zhong
Yao Cai. 28:1002–1006. 2005.PubMed/NCBI
|
|
8
|
Li F, Gong Q, Wang L and Shi J: Osthole
attenuates focal inflammatory reaction following permanent middle
cerebral artery occlusion in rats. Biol Pharm Bull. 35:1686–1690.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng Y, Lu M, Ma L, Zhang S, Qiu M and Ma
X: Osthole ameliorates renal ischemia-reperfusion injury by
inhibiting inflammatory response. Urol Int. 91:350–356. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang J, He L, Wang SQ, Ma MJ, Liu HY, Zhu
XH, Zhu P, Wei X and Wang CY: A simplified two-stitch sleeve
technique for arterial anastomosis of cervical heterotopic cardiac
transplantation in mice. Am J Transl Res. 5:521–529.
2013.PubMed/NCBI
|
|
11
|
Zheng X, Feng B, Chen G, Zhang X, Li M,
Sun H, Liu W, Vladau C, Liu R, Jevnikar AM, et al: Preventing renal
ischemia-reperfusion injury using small interfering RNA by
targeting complement 3 gene. Am J Transplant. 6:2099–2108. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S, Lv JW, Yang P, Yu Q, Pang J, Wang
Z, Guo H, Liu S, Hu J, Li J, et al: Loss of dicer exacerbates
cyclophosphamide-induced bladder overactivity by enhancing
purinergic signaling. Am J Pathol. 181:937–946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang S, Chou WP and Pei L: Effects of
propofol on renal ischemia/reperfusion injury in rats. Exp Ther
Med. 6:1177–1183. 2013.PubMed/NCBI
|
|
14
|
Wang X, Xiong M, Zeng Y, Sun X, Gong T and
Zhang Z: Mechanistic studies of a novel mycophenolic
acid-glucosamine conjugate that attenuates renal
ischemia/reperfusion injury in rat. Mol Pharm. 11:3503–3514. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao JL, Li YF and Li RS: A novel mechanism
of NALP3 inducing ischemia reperfusion injury by activating MAPK
pathway in acute renal failure. Med Hypotheses. 80:463–465. 2013.
View Article : Google Scholar : PubMed/NCBI
|