Introduction
The healthy cornea is devoid of blood and lymphatic
vessels. However, vascularization of the cornea can occur during a
number of pathological disorders, such as corneal chronic
inflammation, alkali burns and graft rejection (1,2). The
outgrowth of blood and lymphatic vessels from the limbus into the
cornea, which is defined as corneal hemangiogenesis (HG) and
lymphangiogenesis (LG), reduces transparency and visual acuity
(3,4). It is also widely recognized that
corneal HG and LG are strong risk factors for graft failure.
Studies have shown that inhibition of corneal neovascularization
(NV) can promote graft survival in a murine model of corneal
transplantation (5,6). Thus, inhibition of corneal NV is a key
point for not only optimal clarity and vision, but also higher
graft survival rates.
Unlike blood vessels, corneal lymphatic vessels are
not easily visible; therefore, systematic lymphatic research
started much later than the study of blood vessels. With the
advancement of technologies and the discovery of several lymphatic
endothelial-specific markers, such as lymphatic vessel endothelial
hyaluronan receptor-1 (LYVE-1), prospero homeobox protein 1
(Prox-1) and vascular endothelial growth factor receptor-3
(VEGFR-3), significant progress has been made (7–9). A
number of factors involved in LG have been identified to date,
including VEGF-C and VEGF-D (10–12);
however, their underlying mechanisms remain unknown.
Matrix metalloproteinases (MMPs), which are a family
of zinc-dependent enzymes, play key roles in degrading the
extracellular matrix (ECM) associated with metastasis, angiogenesis
and tumor invasion (13,14). MMP-14 (also known as membrane type-1
matrix metalloproteinase), a transmembrane type MMP, has been
reported to possess a broad spectrum of activity against ECM
components such as type I and II collagen, fibronectin,
vitronectin, laminin, fibrin and proteoglycans (15,16). A
previous study suggested that MMP-14 also enhanced cancer cell
migration and invasion by the shedding of cluster of
differentiation (CD)44 and syndecan-1 from the cell surface, which
indicated that MMP-14 participated in tumor invasion and metastasis
(17). MMP-14 may promote
angiogenesis by degrading the ECM (18), and may also regulate corneal HG by
cleaving decorin, an anti-angiogenic factor in corneas with basic
fibroblast growth factor (bFGF)-induced vascularization (19). Furthermore, MMP-14 expression has
been shown to be enhanced in wounded corneal epithelium and stroma
(20). In contrast to HG, LG is
known to also participate in corneal injuries, but the contribution
of MMP-14 to this process remains unclear.
Hence, the present study aimed to determine if
MMP-14 promotes corneal LG, in vivo. The expression of
MMP-14, which is able to induce the outgrowth of lymphatic vessels
after various corneal injuries, was determined by
immunohistochemistry, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blot assays. In addition,
whether corneal LG was increased in a murine model of
suture-induced inflammatory corneal NV was investigated, by the
intrastromal injection of MMP-14 plasmid. Finally, whether VEGF
signaling was involved in the molecular pathway leading to corneal
LG was investigated, through the intrastromal delivery of
MMP-14.
Materials and methods
Plasmid construction
A cDNA encoding the MMP-14 gene was subcloned into
the pCMV vector (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) as previously described (21). Plasmids were purified using a Qiagen
plasmid purification kit (Qiagen, Inc., Santa Clarita, CA, USA)
according to the manufacturer's protocol. The concentration of
plasmid was ~2 µg/µl for naked DNA injection.
Animals and anesthesia
All animal protocols were approved by the local
animal care committee of the First Affiliated Hospital of Harbin
Medical University (Harbin, China), and were in accordance with the
ARVO Statement for the Use of Animals in Ophthalmic and Vision
Research. A total of 153 male C57BL/6 mice (6–8 weeks old; 18–20 g)
were purchased from the Laboratory Animal Center of Harbin Medical
University (Harbin, China). All the mice were raised in constant
room temperature and free for food and water. The room was in 12-h
light/dark cycle. Mice were anesthetized with an intraperitoneal
injection of a combination of ketamine and xylazine (120 and 20
mg/kg bodyweight, respectively). The mice were sacrificed by
CO2 inhalation overdose at the end of the
experiment.
Suture-induced corneal NV and corneal
intrastromal injections
The mouse model of suture-induced inflammatory
corneal NV was established as previously described (22). The central cornea was marked with a
2-mm diameter trephine and three 11–0 nylon sutures (Lingqiao;
Ningbo Medical Needle Co., Ltd., Ningbo, China) were placed in the
intrastromal position. The outer point of suture placement was near
the limbus, and the inner point was near the corneal center
equidistant from the limbus. Sutures were removed after 7 days.
Corneal intrastromal injections were performed on day 3 after
suture placement, as previously described (23). A 0.5-inch 33-gauge needle on a 10-µl
gas tight syringe (Hamilton Robotics, Reno, NV, USA) was introduced
into the corneal stroma, and plasmid (5 µg pCMV-MMP14 or pCMV)
solution was injected into the stroma to separate corneal lamellae
and disperse the plasmid. Thus, MMP-14 and empty vector (pCMV)
groups were established. Normal mice were entirely untreated, and
saline control mice received only standard suture placement, and
treated eyes were then rinsed with sterile physiological saline
(0.9% NaCl, 1 ml, twice daily for 1 week). A total of 35 male
C57BL/6 mice were used, including 8 mice in untreated group, 7 mice
in saline control group, 8 mice in empty vector group and 10 mice
in the MMP-14 group.
Mouse alkali injury model
The alkali burn injury model in the mouse cornea was
used, as described previously (n=9) (24). In brief, a 2-mm diameter filter paper
disc was wetted with 1 mol/l NaOH solution for 20 sec and placed on
the central cornea of the mouse for 30 sec. Injured eyes were
rinsed with sterile physiological saline (0.9% NaCl, 20 ml)
immediately.
Immunohistochemistry
Corneas were cut and fixed in 10% neutral buffered
formalin for 24 h. Paraffin-embedded tissue sections (4 µm) were
deparaffinized, rehydrated, and treated with 0.3% hydrogen peroxide
in methanol for 30 min, to eliminate endogenous peroxidase
activity. The tissue sections were then incubated for 60 min at
room temperature with a rabbit anti-mouse MMP-14 monoclonal
antibody (1:2,000; ab51074; Abcam, Cambridge, UK). After three
washes (3 min each) with phosphate-buffered saline (PBS), a DAB
Detection kit (PV9000; ZSGB-Bio, Beijing, China) was used for
MMP-14 staining, according to the manufacturer's protocol. Images
were acquired with the Leica DM4000B biological microscope equipped
with a Leica DFC 550 digital camera and Leica Application Suite
version 4.2.0 software (Leica Biosystems, GmbH, Heidelberg,
Germany).
RNA isolation and RT-qPCR
Total RNA was extracted from the corneas using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA (400 ng) was
reverse transcribed using the PrimeScript™ RT reagent kit with gDNA
Eraser (Takara Biotechnology Co., Ltd., Dalian, China). qPCR was
performed using SYBR® Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd.) with a LightCycler 480 Real-time PCR
System (Roche Diagnostics, Basel, Switzerland). qPCR was performed
under the following conditions: Initial denaturation step of 95°C
for 30 sec, 40 cycles of 95°C for 5 sec, and of 60°C for 30 sec,
followed by an additional denaturation step of 95°C for 5 sec and
60°C for 60 sec, as a subsequent melt curve analysis to check
amplification specificity. All assays were conducted three times,
and were performed in triplicate. Results were derived from the
comparative threshold cycle method (25,26) and
normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as
an internal control. The primers used for qPCR were as shown in
Table I.
 | Table I.Primers used for quantitative
polymerase chain reaction. |
Table I.
Primers used for quantitative
polymerase chain reaction.
| Gene | Sequence
(5′-3′) |
|---|
| GAPDH | F:
GTATTGGGCGCCTGGTCACC |
|
| R:
CGCTCCTGGAAGATGGTGATGG |
| VEGF-A | F:
ACACGGTGGTGGAAGAAGAG |
|
| R:
CAAGTCTCCTGGGGACAGAA |
| VEGF-C | F:
CTACAGATGTGGGGGTTGCT |
|
| R:
GATTGGCAAAACTGATTGTGAC |
| VEGF-D | F:
GAGGCTGCTGCAACGAAGA |
|
| R:
GCACTTACAACCCGTATGGTT |
| VEGFR-1 | F:
CTGGACTGAGACCAAGCCCAAG |
|
| R:
GCTCAGATTCATCGTCCTGCAC |
| VEGFR-2 | F:
CTGTATGGAGGAAGAGGAAGTG |
|
| R:
GGTTCCTCCAATGGGATATC |
| VEGFR-3 | F:
CTCTGACCTAGTGGAGATCCTG |
|
| R:
CTTCGGTGATATGTAGAGCTGTG |
| MMP-14 | F:
GCTTTACTGCCAGCGTTC |
|
| R:
CCCACTTATGGATGAAGCAAT |
Western blot analysis
The corneas were harvested and lysed in ice-cold
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) with the addition of protease
inhibitors. After separation by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, proteins were
transferred onto nitrocellulose membranes. The membranes were
incubated in blocking solution [2% bovine serum albumin (BSA) in
Tris-buffered saline with Tween-20; Beyotime Institute of
Biotechnology] for 1 h at room temperature, then incubated with a
rabbit anti-mouse MMP-14 monoclonal antibody (1:2,000; ab51074;
Abcam) overnight at 4°C. Each step was followed by extensive
washing with PBS. The membranes were then incubated with
corresponding horseradish peroxidase-conjugated secondary antibody
(1:5,000; A0545; Sigma-Aldrich) for 1 h at room temperature, and
developed using an enhanced chemiluminescence system (RPN2108; GE
Healthcare, Little Chalfont, UK.). β-actin was used as loading
control. The antibody was mouse anti-mouse β-actin monoclonal
antibody (1:1,000; A00702-100; GenScript, Nanjing, China).
Corneal immunofluorescence microscopy
and quantification
The immunofluorescence experiments were performed as
previously described (27). The
excised corneas were rinsed in PBS and fixed in acetone for 30 min.
After washing and blocking with 2% BSA in PBS for 2 h, the corneas
were stained overnight at 4°C with a rabbit anti-mouse LYVE-1
antibody (1:500; Abcam) and a rat anti-mouse CD31 antibody (1:100;
BD Pharmingen, San Diego, CA, USA). On day 2, the tissue was washed
three times with PBS, and was stored at 4°C in the absence of
light. The LYVE-1 antibody (ab14917) was detected with an Alexa
Fluor 647-conjugated goat anti-rabbit IgG antibody (1:200; A-21244;
Invitrogen; Thermo Fisher Scientific, Inc.) and the CD31 antibody
(550274) was detected with an Alexa Fluor 488-conjugated goat
anti-rat IgG antibody (1:200; A-11006; Invitrogen; Thermo Fisher
Scientific, Inc.). The corneas were stained with the Alexa Fluor
antibodies overnight at 4°C. To detect the recruitment of
macrophages into the inflamed corneas, a fluorescein
isothiocyanate-conjugated rat anti-mouse CD11b antibody (1:100;
557396; BD Pharmingen) was used. The excised corneas were rinsed by
PBS and fixed in acetone for 30 min. After washing and blocking
with 2% BSA in PBS, for 2 h, corneas were stained overnight at 4°C,
with a CD11b antibody.
The stained whole mounts were analyzed with a
fluorescence microscope (EVOS f1; AMG; Thermo Fisher Scientific,
Inc.). Each whole mount image was quantified using ImageJ software
(National Institutes of Health, Bethesda, MD, USA), and the version
was 1.24o. A detailed explanation of this method has been described
previously (28). The mean
vascularized area of the control groups was defined as being 100%;
vascularized areas were then related to this value (vessel ratio).
For macrophage analysis, the mean number of macrophages of the
control groups was set as 100%; the numbers of macrophages per
whole mount were then related to this value (cell ratio).
Statistical analysis
Statistical analysis was performed using Student's
t-test with SPSS version 13.0 software (SPSS, Inc., Chicago, IL,
USA). Results were expressed as the mean ± standard error of the
mean, and a value of P<0.05 was considered to indicate a
statistically significant difference. Graphs were drawn using
GraphPad Prism, version 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
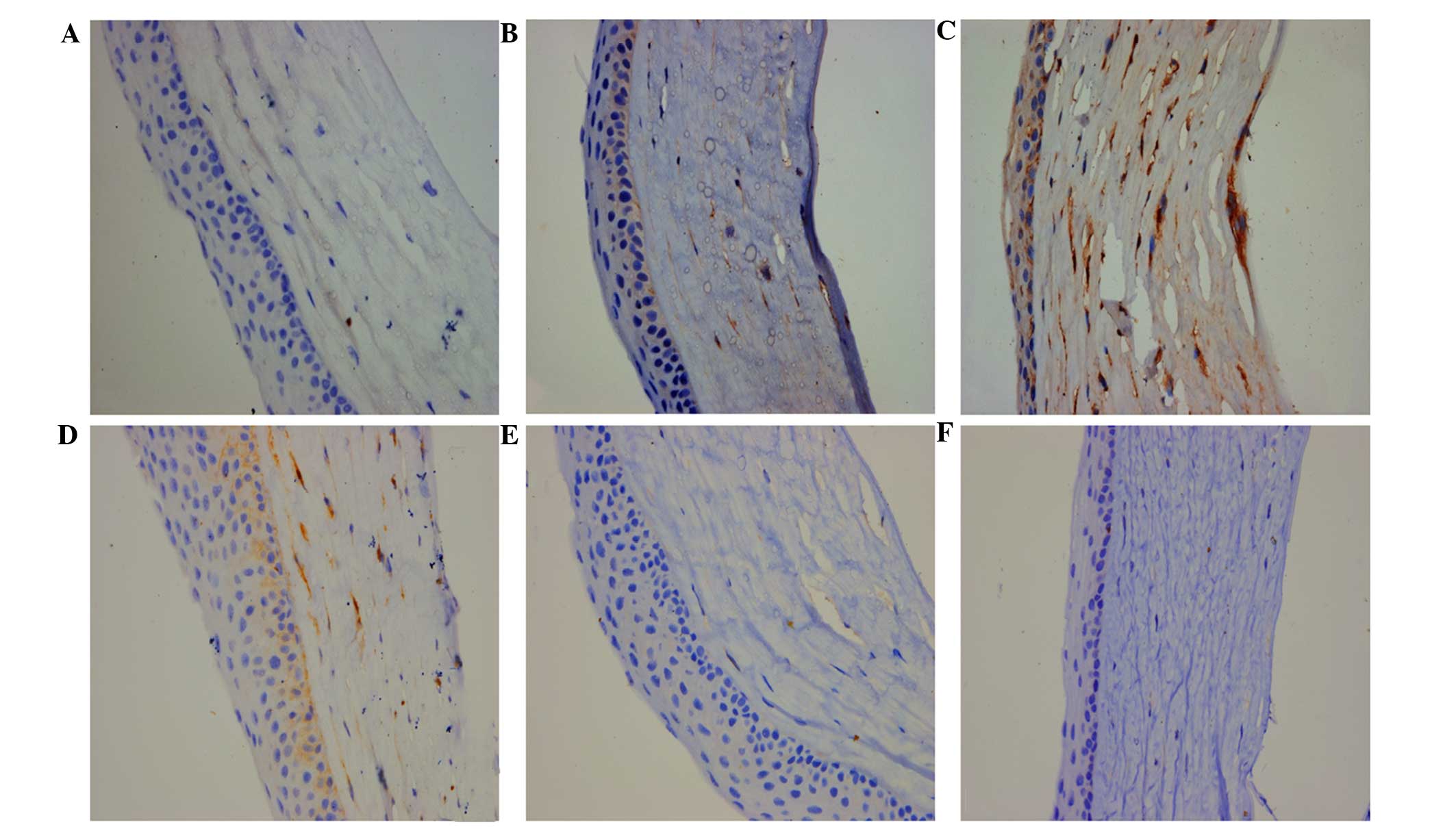
MMP-14 expression in the cornea
Immunohistochemical staining revealed weak
expression of MMP-14 in normal cornea, and high expression levels
of MMP-14 in the cornea on day 3 after standard suture placement or
after alkali injury (Fig. 1A-C). As
shown, these corneal injuries resulted in evident corneal NV.
Moreover, the levels of corneal MMP-14 expression were also
increased on the first day after the intrastromal injection of
MMP-14 plasmid when compared with that of normal cornea, but
increased levels were not detected in the vector-injected cornea.
The data indicated that the intrastromal delivery of MMP-14 plasmid
resulted in increased MMP-14 expression. The levels of MMP-14
expression were almost returned to normal at 3 days after the
injection of MMP-14 DNA (Fig.
1D-F).
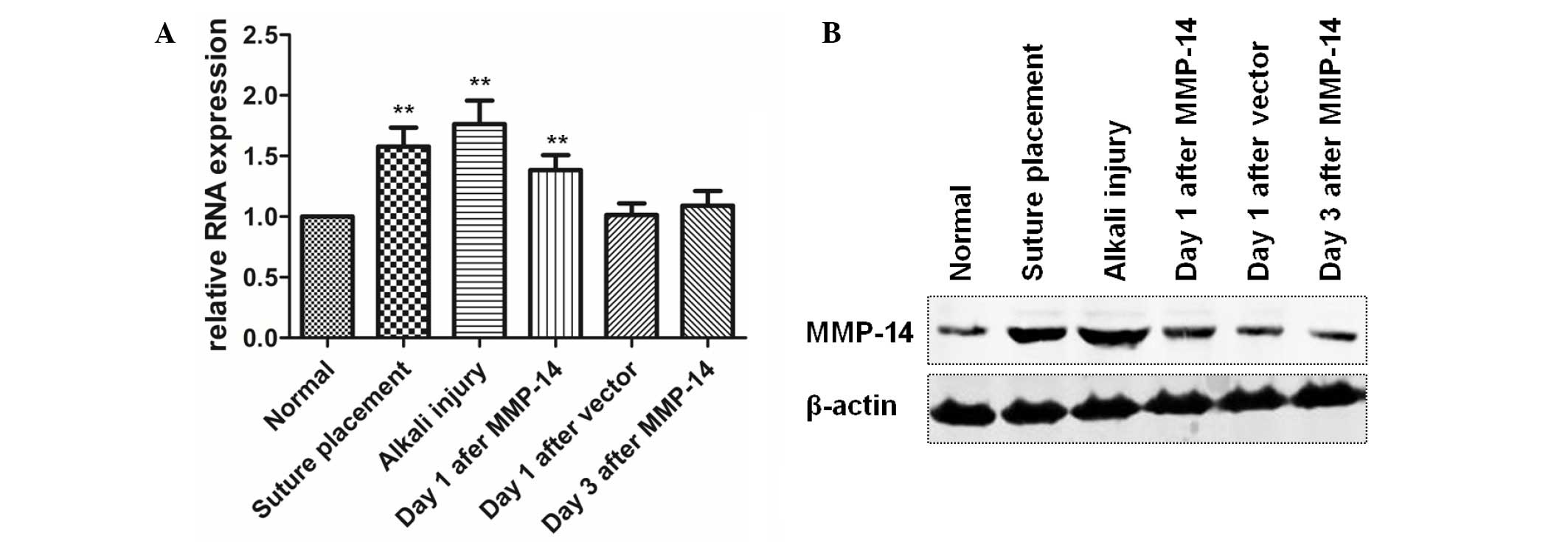
The results of the RT-qPCR assays demonstrated that
MMP-14 expression was significantly upregulated in corneas treated
with an intrastromal injection of MMP-14 plasmid (P=0.007), suture
placement (P=0.003) or alkali injury (P=0.002), whereas MMP-14
expression in corneas treated with empty vector injection (P=0.442)
or in corneas 3 days after MMP-14 vector injection (P=0.239) showed
no significant changes, in comparison with the normal cornea
(Fig. 2A). Furthermore, western blot
assay confirmed the significant upregulation of MMP-14 expression
in the aforementioned corneas (Fig.
2B).
Intrastromal delivery of MMP-14 DNA
promotes corneal LG and HG following suture placement
The standard suture-induced corneal NV assay and
corneal intrastromal injection model were used to investigate the
effect of MMP-14. Mice were randomized to receive intrastromal
injections of either pCMV-MMP-14 or pCMV empty vector on day 3
after suture placement. On day 7, the densities of CD31-positive
blood vessels and LYVE-1-positive lymphatic vessels were detected
by immunohistochemistry as described previously (29). Quantitative immunohistochemical and
morphometric analyses clearly revealed that corneal LG and HG were
induced by standard suture placement, and injection of MMP-14 led
to a significant promotion of LG and HG (Fig. 3A-I). The numbers of lymphatic vessels
in mice treated with MMP-14 were significantly increased (P=0.009),
and the numbers of blood vessels were also markedly increased
(P=0.011), in comparison with those in the mice treated with empty
vector (Fig. 3J and K).
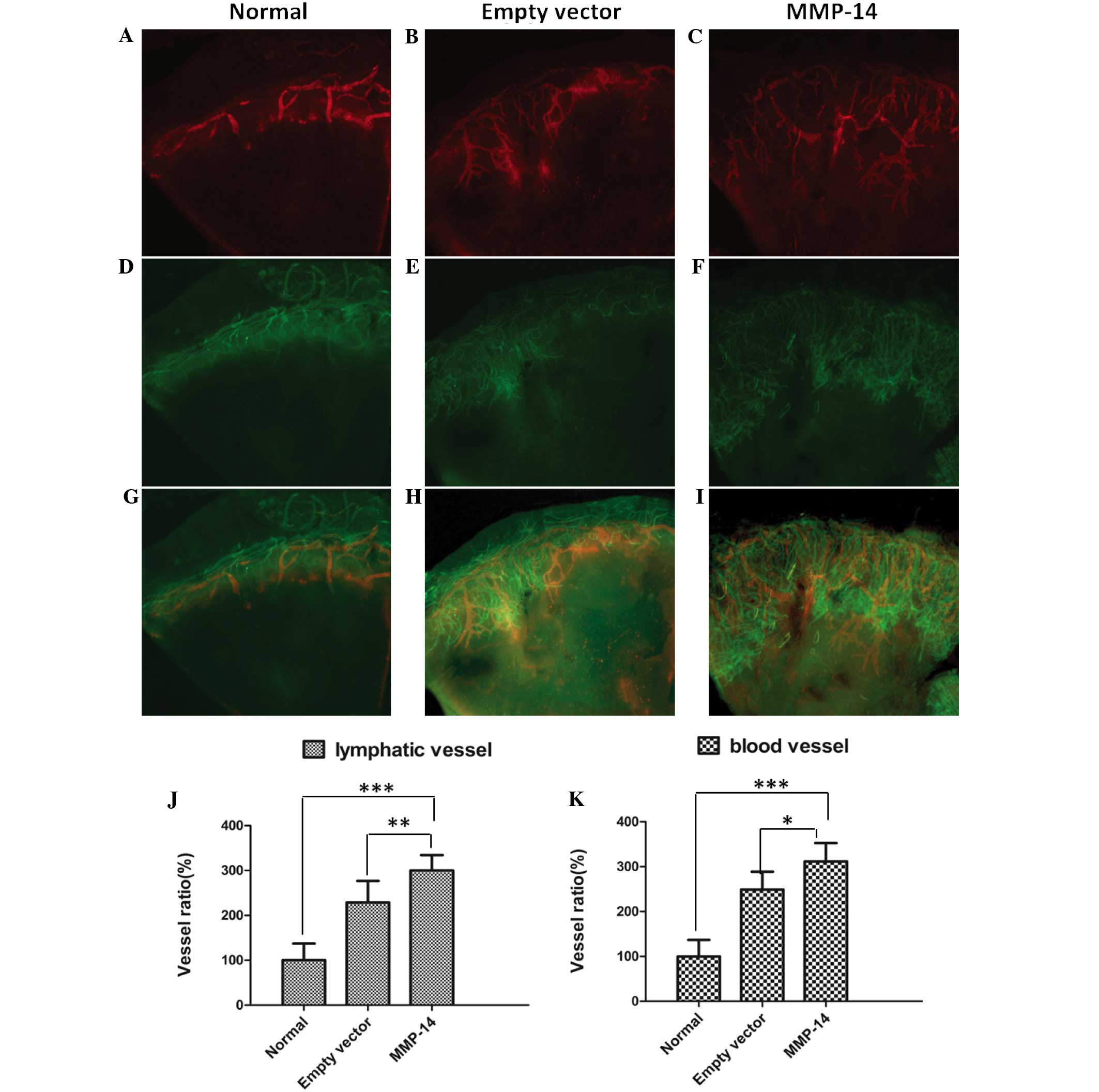 | Figure 3.Intrastromal injection of MMP-14 DNA
significantly promoted suture-induced corneal lymphangiogenesis and
hemangiogenesis. (A-I) Representative images showing corneal whole
mounts in eyes treated with MMP-14 plasmid (C, F, I: n=10 mice) or
empty vector (B, E, H: n=8 mice), compared with the normal eyes (A,
D, G: n=8 mice). Lymphatic vessels are shown in red (Alexa Fluor
647 immunofluorescence staining of LYVE-1) and blood vessels in
green (Alexa Fluor 488 immunofluorescence staining of CD31).
Original magnification, ×40. (J, K) Summarized data showing that
the numbers of lymphatic vessels and blood vessels in eyes treated
with MMP-14 were significantly increased, in comparison with those
in eyes treated with empty vector (*P<0.01 and **P<0.05 vs.
empty vector). MMP, matrix metalloproteinase; LYVE-1, .lymphatic
vessel endothelial hyaluronan receptor-1; CD, cluster of
differentiation. |
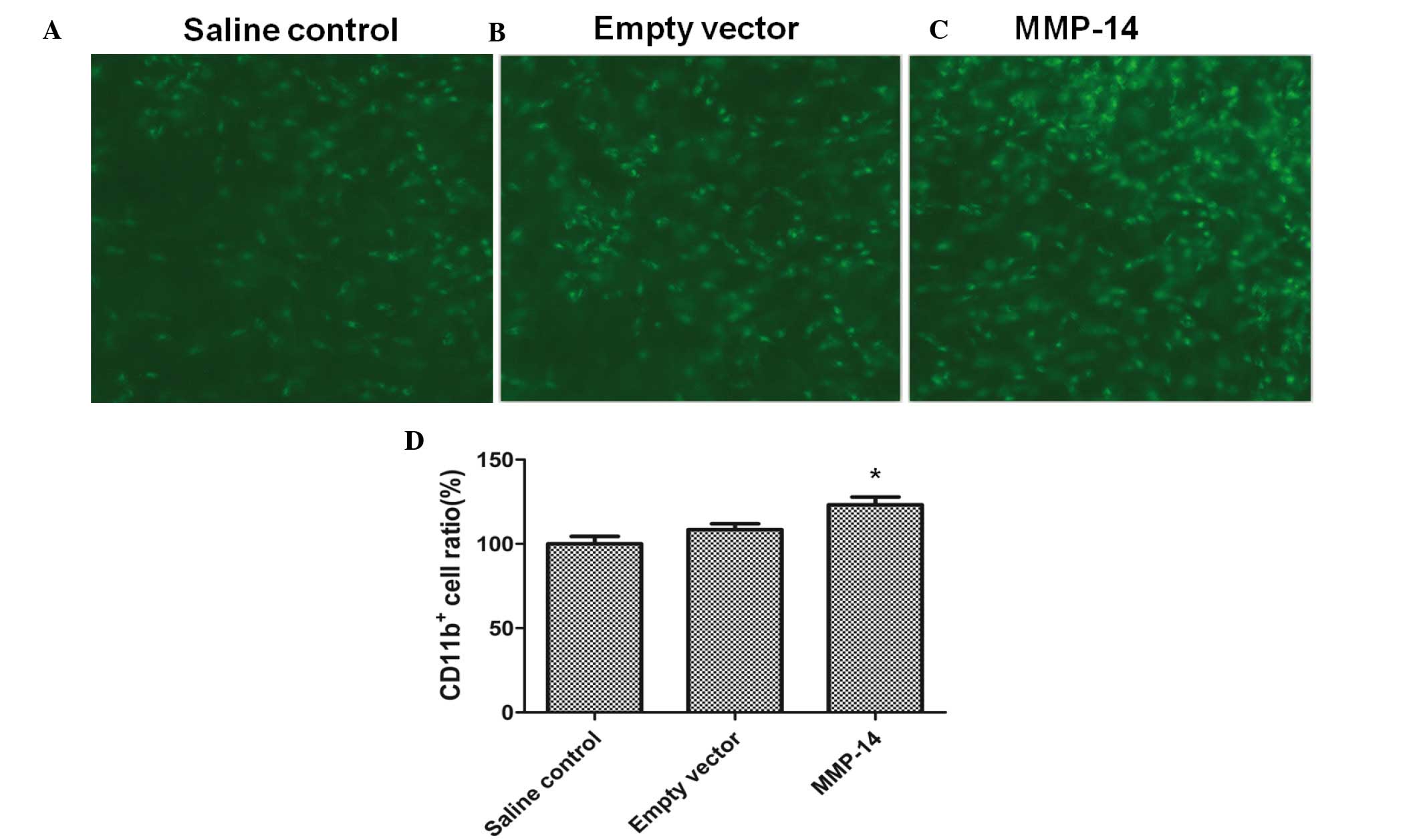
Treatment with MMP-14 promotes the
recruitment of inflammatory macrophages into the cornea
To test whether the lymphangiogenic effect of MMP-14
was also partially caused by an indirect effect on macrophages,
CD11b+ macrophage infiltration into inflamed corneas
(n=7 mice) was evaluated using standard corneal suture placement
and intrastromal injection models. Treatment with MMP-14 resulted
in a significantly increased recruitment of CD11b+
compared with empty vector (P=0.019), as shown in Fig. 4.
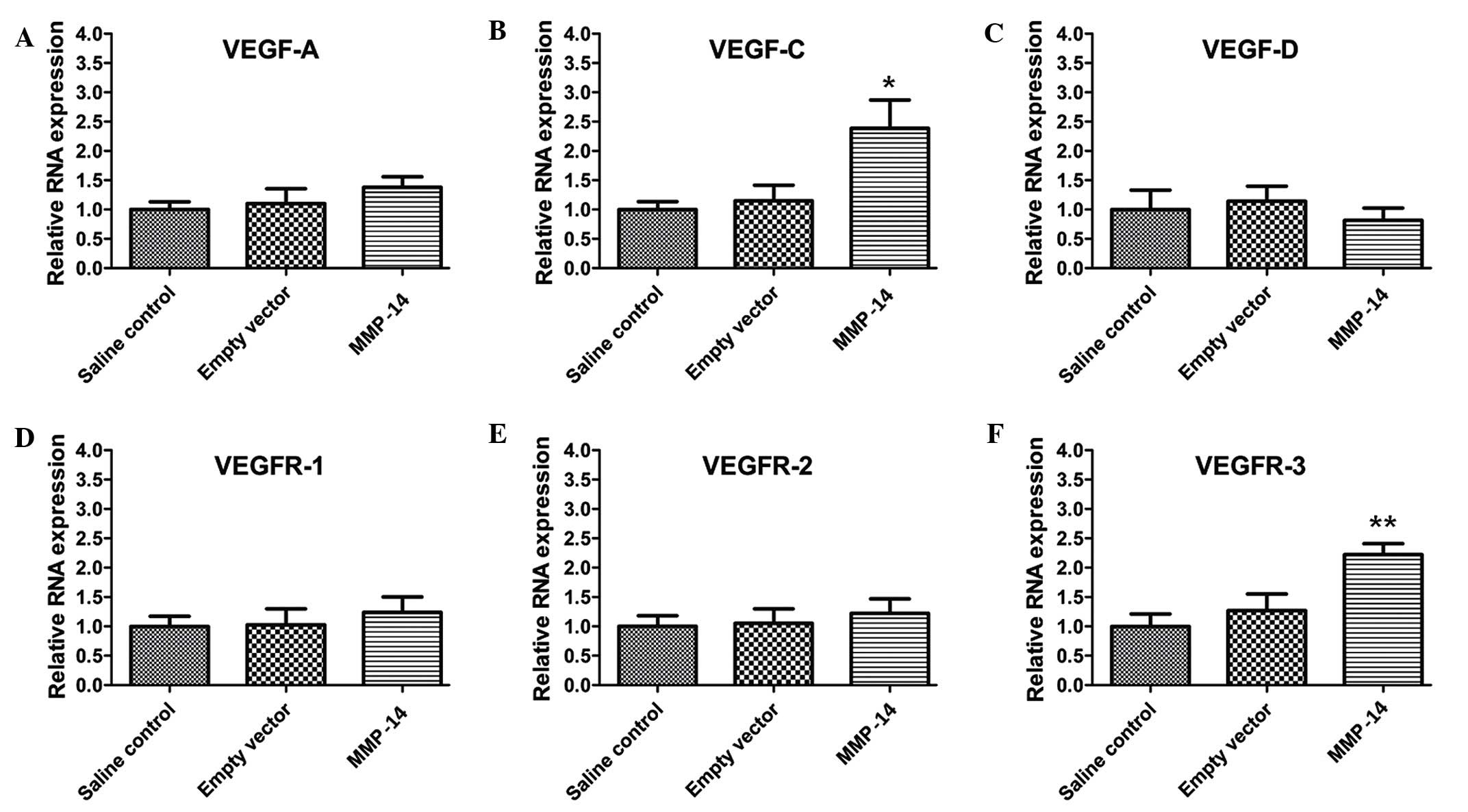
Treatment with MMP-14 upregulates the
expression of VEGF-C and VEGFR-3
HG and LG are driven by the production of
lymphangiogenic growth factors, and the VEGF pathway is central to
these processes (30). To further
investigate the roles of MMP-14 in HG and LG, RT-qPCR was used to
examine the expression of VEGF ligands and receptors. The results
showed that VEGF-C (P=0.028) and VEGFR-3 (P=0.011) expression
levels were significantly upregulated in mice treated with MMP-14,
whereas the expression levels of VEGF-A (P=0.198), VEGF-D
(P=0.183), VEGFR-1 (P=0.296) and VEGFR-2 (P=0.321) showed no
significant changes, in comparison with those in the empty vector
group (Fig. 5).
Discussion
For the first time, to the best of our knowledge,
the present study provides evidence that MMP-14 promotes in
vivo LG in a corneal suture-induced mouse model. Although
previous studies have reported the effects of MMP-14 on several
angiogenesis-related properties, including degradation of ECM and
cleavage of decorin (18), its
contribution during LG has received less attention and so further
investigation is merited.
It has previously been shown that proMMP-2
activation can be blocked by a specific monoclonal antibody against
MMP-14, which resulted in a marked reduction of lymphatic vessel
sprouting (31). However, the impact
of MMP-14 on LG in vivo was not determined in that study. In
the present study, corneal LG and HG were significantly increased
in the suture-induced inflammatory corneal NV model when naked
MMP-14 DNA was added. Thus, it may be concluded that MMP-14 plays
an important role in the development of new lymphatic vessels.
To assess the association between MMP-14 and corneal
NV, MMP-14 expression was investigated under various corneal
conditions using immunohistochemical analysis, RT-qPCR and western
blot analysis. In the present study, corneal intrastromal injection
of MMP-14 plasmid was an effective method of increasing the amount
of the protein, consistent with published reports (32). Additionally, the present study showed
that significantly increased MMP-14 expression existed in the
standard corneal suture model and the alkali burn model. Through
the examination of these models, it was shown that corneal HG and
LG were significantly induced. This was in agreement with previous
results showing that keratocytes and myofibroblasts express MMP-1,
−2 and −9 following corneal injuries (33–35).
Collectively, these results demonstrate that MMP-14 is involved in
corneal NV, at least under certain pathophysiological conditions.
The MMP-14 overexpression in corneal tissues implied that MMP-14
plays an important role in corneal HG and LG.
Macrophages are acknowledged to have a key role in
corneal LG. Previous studies have confirmed that large numbers of
activated CD11b+ macrophages induce LG during corneal
inflammation, by transdifferentiating into lymphatic endothelium
and by releasing lymphangiogenic growth factors (36,37). As
shown in the present study, the numbers of CD11b+
macrophages infiltrating the inflammatory corneas in MMP-14-treated
mice were significantly greater than in vehicle-treated mice. It
may be speculated that the lymphangiogenic effect of MMP-14 might
also be partially caused by an indirect effect on macrophages.
To further investigate the mechanism through which
MMP-14 regulates corneal HG and LG, the associations between MMP-14
and VEGF proteins and receptors were examined. A marked
upregulation of VEGF-C and VEGFR-3 expression levels was detected
in sutured corneas treated with MMP-14, but other members of the
VEGF family exhibited no significant changes. The outgrowth of
lymphatic vessels is primarily triggered by VEGF-C and its receptor
VEGFR-3 (38,39), and the specific inhibition of VEGFR-3
alone is sufficient to block corneal LG (40). In previous studies, corneal LG
induced by fibroblast growth factor-2 or hepatocyte growth factor
could be blocked by VEGFR-3 inhibition (41,42).
These investigations suggest that the VEGFR-3 signaling pathway is
critical for corneal LG.
In addition, the data presented in the present study
indicate that VEGF-C and its receptor VEGFR-3 might induce corneal
HG in addition to LG. Among the VEGFs, VEGF-A is widely studied and
has been found to be responsible for HG by binding to its
receptors, VEGFR-1 and VEGFR-2 (43,44),
while VEGF-C is thought to a dominant factor stimulating LG through
binding to VEGFR-3. However, certain studies have provided evidence
that VEGF-C is also associated with HG (45–47). An
earlier study reported that VEGF-C enhanced microvascular
endothelial cell migration, branching and capillary sprouting in
association with MMP-14 overexpression (48), which is consistent with the findings
of the present study. Several studies have also shown that VEGF-C
and VEGFR-3 are associated with angiogenesis in cancer (49,50). A
possible mechanism by which VEGF-C induces HG has been shown to be
through a RhoA-mediated pathway (51). Furthermore, it may be speculated that
increased expression of VEGF-C are associated with the large
numbers of activated CD11b+ macrophages in the
inflammatory corneas of MMP-14-treated mice. A previous study has
shown that significantly increased numbers of dermal
CD11b+ macrophages expressed higher levels of VEGF-C
(52), which indicates their close
association. It is possible, therefore, that MMP-14 induced corneal
HG and LG by upregulating the expression levels of VEGF-C and
VEGFR-3 in vivo. Additional studies to elucidate the
relationship between MMP-14 and VEGF-C/VEGFR-3 are necessary.
In summary, through the use of a corneal suture
model, the data in the present study demonstrate that MMP-14
promotes corneal HG and LG. The important role of MMP-14 in corneal
LG was closely associated with CD11b+ macrophage
infiltration, and with the VEGF-C/VEGFR-3 signaling pathway. Based
on these findings, MMP-14 inhibition could be a promising new
target for the management of inflammatory corneal disorders and for
maintaining the transparency of the cornea.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (NSFC No. 81300728), and the Scientific
Research Fund of Heilongjiang Provincial Education Department (No.
12521262) and of Heilongjiang Provincial Health Bureau
(2011-031).
References
|
1
|
He Y, Rajantie I, Pajusola K, Jeltsch M,
Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T and
Alitalo K: Vascular endothelial cell growth factor receptor
3-mediated activation of lymphatic endothelium is crucial for tumor
cell entry and spread via lymphatic vessels. Cancer Res.
65:4739–4746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hosseini H and Nejabat M: A potential
therapeutic strategy for inhibition of corneal neovascularization
with new anti-VEGF agents. Med Hypotheses. 68:799–801. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cursiefen C, Chen L, Dana MR and Streilein
JW: Corneal lymphangiogenesis: Evidence, mechanisms and
implications for corneal transplant immunology. Cornea. 22:273–281.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cursiefen C, Maruyama K, Jackson DG,
Streilein JW and Kruse FE: Time course of angiogenesis and
lymphangiogenesis after brief corneal inflammation. Cornea.
25:443–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bachmann BO, Bock F, Wiegand SJ, Maruyama
K, Dana MR, Kruse FE, Luetjen-Drecoll E and Cursiefen C: Promotion
of graft survival by vascular endothelial growth factor a
neutralization after high-risk corneal transplantation. Arch
Ophthalmol. 126:71–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cursiefen C, Cao J, Chen L, Liu Y,
Maruyama K, Jackson D, Kruse FE, Wiegand SJ, Dana MR and Streilein
JW: Inhibition of hemangiogenesis and lymphangiogenesis after
normal-risk corneal transplantation by neutralizing VEGF promotes
graft survival. Invest Ophthalmol Vis Sci. 45:2666–2673. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lymboussaki A, Achen MG, Stacker SA and
Alitalo K: Growth factors regulating lymphatic vessels. Curr Top
Microbiol Immunol. 251:75–82. 2000.PubMed/NCBI
|
|
8
|
Oh SJ, Jeltsch MM, Birkenhäger R, McCarthy
JE, Weich HA, Christ B, Alitalo K and Wilting J: VEGF and VEGF-C:
Specific induction of angiogenesis and lymphangiogenesis in the
differentiated avian chorioallantoic membrane. Dev Biol.
188:96–109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breiteneder-Geleff S, Soleiman A, Kowalski
H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler
E, Alitalo K and Kerjaschki D: Angiosarcomas express mixed
endothelial phenotypes of blood and lymphatic capillaries:
Podoplanin as a specific marker for lymphatic endothelium. Am J
Pathol. 154:385–394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karkkainen MJ, Haiko P, Sainio K, Partanen
J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala
H, et al: Vascular endothelial growth factor C is required for
sprouting of the first lymphatic vessels from embryonic veins. Nat
Immunol. 5:74–80. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel SP and Dana R: Corneal
lymphangiogenesis: Implications in immunity. Semin Ophthalmol.
24:135–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: Multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
d'Ortho MP, Will H, Atkinson S, Butler G,
Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L and Murphy G:
Membrane-type matrix metalloproteinases 1 and 2 exhibit
broad-spectrum proteolytic capacities comparable to many matrix
metalloproteinases. Eur J Biochem. 250:751–757. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koshikawa N, Giannelli G, Cirulli V,
Miyazaki K and Quaranta V: Role of cell surface metalloproteinase
MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol.
148:615–624. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato H, Takino T and Miyamori H: Roles of
membrane-type matrix metalloproteinase-1 in tumor invasion and
metastasis. Cancer Sci. 96:212–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Genis L, Galvez BG, Gonzalo P and Arroyo
AG: MT1-MMP: Universal or particular player in angiogenesis? Cancer
Metastasis Rev. 25:77–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mimura T, Chang JH, Kim TI, Onguchi T,
Kojima T, Sakimoto T and Azar DT: MT1-MMP cleavage of the
antiangiogenic proteoglycan decorin: Role in corneal angiogenesis.
Cornea. 30:(Suppl 1). S45–S49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye HQ, Maeda M, Yu FS and Azar DT:
Differential expression of MT1-MMP (MMP-14) and collagenase III
(MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol
Vis Sci. 41:2894–2899. 2000.PubMed/NCBI
|
|
21
|
Hotary K, Allen E, Punturieri A, Yana I
and Weiss SJ: Regulation of cell invasion and morphogenesis in a
three-dimensional type I collagen matrix by membrane-type matrix
metalloproteinases 1, 2 and 3. J Cell Biol. 149:1309–1323. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cursiefen C, Chen L, Borges LP, Jackson D,
Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ and
Streilein JW: VEGF-A stimulates lymphangiogenesis and
hemangiogenesis in inflammatory neovascularization by macrophage
recruitment. J Clin Invest. 113:1040–1050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stechschulte SU, Joussen AM, von Recum HA,
Poulaki V, Moromizato Y, Yuan J, D'Amato RJ, Kuo C and Adamis AP:
Rapid ocular angiogenic control via naked DNA delivery to cornea.
Invest Ophthalmol Vis Sci. 42:1975–1979. 2001.PubMed/NCBI
|
|
24
|
Cheng HC, Yeh SL, Tsao YP and Kuo PC:
Subconjunctival injection of recombinant AAV-angiostatin
ameliorates alkali burn induced corneal angiogenesis. Mol Vis.
13:2344–2352. 2007.PubMed/NCBI
|
|
25
|
Schefe JH, Lehmann KE, Buschmann IR, Unger
T and Funke-Kaiser H: Quantitative real-time RT-PCR data analysis:
current concepts and the novel “gene expression's CT difference”
formula. J Mol Med. 84:901–910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radonić A, Thulke S, Mackay IM, Landt O,
Siegert W and Nitsche A: Guideline to reference gene selection for
quantitative real-time PCR. Biochem Biophys Res Commun.
313:856–862. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang XL, Sun JF, Wang XY, Du LL and Liu P:
Blocking neuropilin-2 enhances corneal allograft survival by
selectively inhibiting lymphangiogenesis on vascularized beds. Mol
Vis. 16:2354–2361. 2010.PubMed/NCBI
|
|
28
|
Bock F, Onderka J, Hos D, Horn F, Martus P
and Cursiefen C: Improved semiautomatic method for morphometry of
angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye
Res. 87:462–470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Hu X, Tse J, Tilahun F, Qiu M and
Chen L: Spontaneous lymphatic vessel formation and regression in
the murine cornea. Invest Ophthalmol Vis Sci. 52:334–338. 2010.
View Article : Google Scholar
|
|
30
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ingvarsen S, Porse A, Erpicum C, Maertens
L, Jürgensen HJ, Madsen DH, Melander MC, Gårdsvoll H, Høyer-Hansen
G, Noel A, et al: Targeting a single function of the
multifunctional matrix metalloproteinase MT1-MMP: Impact on
lymphangiogenesis. J Biol Chem. 288:10195–10204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onguchi T, Han KY, Chang JH and Azar DT:
Membrane type-1 matrix metalloproteinase potentiates basic
fibroblast growth factor-induced corneal neovascularization. Am J
Pathol. 174:1564–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsubara M, Girard MT, Kublin CL, Cintron
C and Fini ME: Differential roles for two gelatinolytic enzymes of
the matrix metalloproteinase family in the remodelling cornea. Dev
Biol. 147:425–439. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimoda M, Ishizaki M, Saiga T and
Yamanaka N: Expression of matrix metalloproteinases and tissue
inhibitor of metalloproteinase by myofibroblasts-morphological
study on corneal wound healing. Nippon Ganka Gakkai Zasshi.
101:371–379. 1997.(In Japanese). PubMed/NCBI
|
|
35
|
Fini ME, Girard MT and Matsubara M:
Collagenolytic/gelatinolytic enzymes in corneal wound healing. Acta
Ophthalmol Suppl. 26–33. 1992.PubMed/NCBI
|
|
36
|
Maruyama K, Ii M, Cursiefen C, Jackson DG,
Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA,
Stein-Streilein J, et al: Inflammation-induced lymphangiogenesis in
the cornea arises from CD11b-positive macrophages. J Clin Invest.
115:2363–2372. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Watari K, Nakao S, Fotovati A, Basaki Y,
Hosoi F, Bereczky B, Higuchi R, Miyamoto T, Kuwano M and Ono M:
Role of macrophages in inflammatory lymphangiogenesis: Enhanced
production of vascular endothelial growth factor C and D through
NF-kappaB activation. Biochem and Biophys Res Commun. 377:826–831.
2008. View Article : Google Scholar
|
|
38
|
Ji RC: Macrophages are important mediators
of either tumor- or inflammation-induced lymphangiogenesis. Cell
Mol Life Sci. 69:897–914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su JL, Yen CJ, Chen PS, Chuang SE, Hong
CC, Kuo IH, Chen HY, Hung MC and Kuo ML: The role of the
VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 96:541–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bock F, Onderka J, Dietrich T, Bachmann B,
Pytowski B and Cursiefen C: Blockade of VEGFR3-signalling
specifically inhibits lymphangiogenesis in inflammatory corneal
neovascularisation. Graefes Arch Clin Exp Ophthalmol. 246:115–119.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kubo H, Cao R, Brakenhielm E, Mäkinen T,
Cao Y and Alitalo K: Blockade of vascular endothelial growth factor
receptor-3 signaling inhibits fibroblast growth factor-2-induced
lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA.
99:8868–8873. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao R, Björndahl MA, Gallego MI, Chen S,
Religa P, Hansen AJ and Cao Y: Hepatocyte growth factor is a
lymphangiogenic factor with an indirect mechanism of action. Blood.
107:3531–3536. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Goodin S: Development of monoclonal
antibodies for the treatment of colorectal cancer. Am J Health Syst
Pharm. 65:(Suppl 4). S3–S7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang ZS, Xu YF, Huang FF and Ding GF:
Associations of nm23H1, VEGF-C and VEGF-3 receptor in human
prostate cancer. Molecules. 19:6851–6862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cao R, Eriksson A, Kubo H, Alitalo K, Cao
Y and Thyberg J: Comparative evaluation of FGF-2-, VEGF-A- and
VEGF-C-induced angiogenesis, lymphangiogenesis, vascular
fenestrations and permeability. Circ Res. 94:664–670. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Saaristo A, Veikkola T, Enholm B, Hytönen
M, Arola J, Pajusola K, Turunen P, Jeltsch M, Karkkainen MJ,
Kerjaschki D, et al: Adenoviral VEGF-C overexpression induces blood
vessel enlargement, tortuosity and leakiness but no sprouting
angiogenesis in the skin or mucous membranes. FASEB J.
16:1041–1049. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bauer SM, Bauer RJ, Liu ZJ, Chen H,
Goldstein L and Velazquez OC: Vascular endothelial growth factor-C
promotes vasculogenesis, angiogenesis and collagen constriction in
three-dimensional collagen gels. J Vasc Surg. 41:699–707. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Laakkonen P, Waltari M, Holopainen T,
Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR,
Witte L and Alitalo K: Vascular endothelial growth factor receptor
3 is involved in tumor angiogenesis and growth. Cancer Res.
67:593–599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Valtola R, Salven P, Heikkilä P, Taipale
J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, de Waal R and
Alitalo K: VEGFR-3 and its ligand VEGF-C are associated with
angiogenesis in breast cancer. Am J Pathol. 154:1381–1390. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kumar B, Chile SA, Ray KB, Reddy GE,
Addepalli MK, Kumar AS, Ramana V and Rajagopal V: VEGF-C
differentially regulates VEGF-A expression in ocular and cancer
cells; promotes angiogenesis via RhoA mediated pathway.
Angiogenesis. 14:371–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi VY, Bao L and Chan LS:
Inflammation-driven dermal lymphangiogenesis in atopic dermatitis
is associated with CD11b+ macrophage recruitment and
VEGF-C up-regulation in the IL-4-transgenic mouse model.
Microcirculation. 19:567–579. 2012. View Article : Google Scholar : PubMed/NCBI
|