Introduction
Tuberculosis (TB) remains one of the top three
deadly diseases worldwide with 1.4 million deaths reported in 2011
(1). TB pleurisy is the most common
type of extra-pulmonary tuberculosis, accounting for 10–20% of
patients with tuberculous and 10–30% of patients with TB pleurisy
suffer from pleural effusions (PFs) (2). TB-associated PF results from the
infiltration of pleural space by M. tuberculosis (M.
tb) antigens or bacilli, which induce a strong Th1 immune
response (3). Currently,
non-invasive methods including conventional chest X-ray, chest
computed tomography scan, ultrasonography, pleural fluid laboratory
tests, thoracocentesis, pleural biopsy and thoracoscopy are used to
determine the existence and etiology of PFs (4). Pleural fluid M. tb culture
remains the gold standard criterion with a diagnostic detection
rate of <20% (5).
Programmed death ligand 1 (PD-L1), which is also
known as CD274, has been well-characterized as a critical
membrane-bound co-stimulatory molecule that inhibits immune
responses through its receptor, programmed death 1 (PD-1) by
downregulating T cell activation and cytokine secretion (6,7). PD-L1
is constitutively expressed in various immunocytes, including T
cells, B cells, dendritic cells (DCs), macrophages and a wide range
of tumor cell lines and solid tumor tissues, and the expression of
PD-L1 is further upregulated following activation (8). PD-1 is a type I glycoprotein and its
expression can be induced in T cells, B cells, natural killer T
cells and activated monocytes (9).
Various co-stimulatory molecules express PD-1 in two forms, the
soluble (sPD-L1) and membrane-bound (mPD-L1) forms. sPD-L1 has been
demonstrated in various members of the B7 superfamily, including
OX40 and B7-H3 (10,11). sPD-L1 was initially identified in the
blood serum of patients with non-small cell lung cancer, and
increased sPD-L1 expression is associated with lung cancer TNM
staging and the patient's clinical response to treatment (12). Increased levels of serum sPD-L1 have
also been detected in other solid tumors, such as aggressive renal
cell carcinoma, breast cancer and autoimmune diseases, including
systemic lupus erythematosus, rheumatoid arthritis and type 2
diabetes mellitus (13–16). Therefore, sPD-L1 may have important
roles in the regulation of co-stimulatory signals. Th1 cytokines,
including interferon (IFN)-γ, tumor necrosis factor (TNF)-α and
interleukin-2, have also been demonstrated to have critical roles
in granuloma formation and the damage of tubercle bacilli within
macrophages (17). However, whether
sPD-L1 and mPD-L1 are involved in immune regulation and disease
progression of TPE has yet to be elucidated. In the present study,
the immune regulatory role of sPD-L1 and the PD-1/PD-L1 pathway
involved in TPE, and the correlation with Th1 immune response, will
be explored. In addition, the possibility of a potential
immune-checkpoint in the treatment of TPE will be investigated.
Materials and methods
Subjects
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Soochow University
(Suzhou, China). Written informed consents for the collection of
blood and PF samples and subsequent analysis were obtained from all
participants prior to the initiation of the study. Fresh PF and
peripheral blood (PB) samples were harvested from 68 subjects. Of
68 patients with evaluable data, 24 were assigned to the
tuberculous pleural effusion (TPE) group, 30 were assigned to the
malignant pleural effusion (MPE) group and 14 were assigned to the
non-tuberculous non-malignant pleural effusion (n-TB n-M) group.
Within the n-TB n-M group, the 14 participants presented with
systemic lupus erythematosus (SLE; n-1), heart failure (n=3),
pneumonia (n=5) and an unknown medical condition (n=5). Clinical
and microbiological characteristics of subjects were obtained from
the patient records and are reported in Table I.
 | Table I.Basic characteristics of the subjects
enrolled in the present study. |
Table I.
Basic characteristics of the subjects
enrolled in the present study.
| Characteristic | TPE | MPE | n-TB n-M |
|---|
| Number of
subjects | 24 | 30 | 14 |
| Age (years) |
46.42±23.44a | 67.70±8.19 | 63.36±17.27 |
| Gender (M/F) | 20/4 | 13/17 | 12/2 |
| Pleural effusion
side (right/left/bilateral) | 7/17/0 | 16/12/2 | 3/3/8 |
| Presence of fever
admission (yes/no) | 14/10 | 2/28 | 4/10 |
| Smoking
(yes/no) | 8/16 | 7/23 | 4/10 |
| Ethnic group | Chinese | Chinese | Chinese |
Patients were diagnosed with TPE on the basis of: i)
Presence of acid fast bacilli in a pleural fluid specimen, growth
of M. tb from pleural fluid, or demonstration of
granulomatous pleurisy on closed pleural biopsy specimen in the
absence of any evidence of other granulomatous diseases; ii) an
exudative lymphocytic effusion with an adenosine deaminase level of
>40 U/l, a positive purified protein derivative skin test result
and the exclusion of any other potential causes of pleurisy; iii)
or a good response to TB treatment (18). Exudative effusion was classified
according the criteria outlined by Light et al (19). A diagnosis of MPE was established
following demonstration of malignant cells in pleural fluid and/or
pleural biopsy specimen (20). SLE
was diagnosed according to the criteria outlined by the American
College of Rheumatology in 1982 (21). Cardiogenic PF was diagnosed according
to the symptoms and laboratory tests of heart failure.
Para-pneumonic PF was diagnosed following evidence of pulmonary
infections associated with acute febrile illness, pulmonary
infiltrates, purulent sputum and response to antibiotic treatment
(22). Ten PB samples were collected
from healthy clinical trial volunteers. At the time of sample
collection, none of the patients had received anti-TB therapy,
anticancer therapy, corticosteroids, other non-steroid drugs or
invasive procedures directed into the pleural cavity.
Determination of immune cell profiles
in PF and PB
PF monocyte cells (PEMCs) were obtained by
Ficoll-Hypaque density centrifugation at 543 × g for 30 min. PEMCs
(~5×105 cells/test) and PB (50 µl/test) were
surface-stained with fluorescein isothiocyanate (FITC)-labeled
anti-human CD4 (clone SK3) and CD8 (clone OKT8) monoclonal
antibodies (mAbs) and (eBioscience, Inc., San Diego, CA, USA),
FITC-labeled anti-human CD14 mAb (clone 61D3; eBioscience, Inc.),
phycoerythrin-labeled anti-human PD-1 (clone EH12. 2H7) and PD-L1
(clone 29E. 2A3) mAbs (BioLegend, Inc., San Diego, CA, USA) for 30
min at 4°C according to the manufacturer's protocol. A blank was
used for the control. For PEMCs, 500 µl fluorescence-activated cell
sorting (FACS) buffer (Beckman Coulter, Inc., Brea, CA, USA) was
used for flow cytometry acquisition. For the blood samples, 300 µl
erythrocyte lysis buffer (Beckman Coulter, Inc.) was added to the
cell suspensions following antibody staining and incubated for a
further 10 min at 42°C, followed by the addition of 500 µl FACS
buffer for flow cytometry acquisition. Data were acquired on a
2-color flow cytometer (Beckman Coulter cytomics FC500) and
analyzed with Expo32 MultiCOMP software version 1.2 (both Beckman
Coulter, Inc.).
Determination of sPD-L1 and IFN-γ
levels
PF (50 ml) and PB (5 ml) samples were centrifuged at
543 × g for 10 min and the supernatants were stored at −80°C for
subsequent ELISA assays. Expression levels of sPD-L1 in PFs and
sera were determined in duplicate wells using the sPD-L1 ELISA
system, as previously described by our group (9). Expression levels of IFN-γ were analyzed
using a commercial ELISA kit (cat no. EK0373; Boster Biological
Technology, Ltd., Wuhan, China) according to the manufacturer's
protocol.
Determination of PD-L1 on
CD14+ monocytes
PB monocyte cells (PBMCs) were collected from
healthy donors by Ficoll-Hypaque density centrifugation and seeded
into 6-well plate at a density of 3×105 cells/well. TPE
were co-cultured with PBMCs for 48 h at 37°C in an incubated
atmosphere containing 5% CO2. Alterations in the levels
of PD-L1 on CD14+ monocytes were evaluated by flow
cytometry.
Determination of cell
proliferation
PEMCs from TPE were collected and CD14+
monocytes were isolated using human CD14 MicroBeads (Miltenyi
Biotec, GmbH, Bergisch Gladbach, Germany) with positive selection.
Subsequently, purified T cells were obtained using a T cell
negative kit (Stemcell Technologies, Inc., Vancouver, BC, Canada).
CD14+ monocytes and T lymphocytes were seeded at a ratio
of 1:5 into a 96-well plate pre-coated with functional anti-human
CD3 mAb (2 µg/ml; cat no. IM1304; Immunotech S.A.S, Marseille,
France). Anti-PD-L1 mAb (2H11; 1 µg/ml), which was developed by our
own group (9), or the control
antibody mouse anti-human IgG (cat no. 6602872) was added into the
medium. Following co-culturing for 3–5 days, cell proliferation was
detected by a cell counting kit-8 (CCK-8; cat no. CK04).
Statistical analysis
Data were analyzed using GraphPad Prism 5.0 (Graph
Pad Software Inc., San Diego, CA, USA) and SPSS 17.0 (IBM, Chicago,
USA). Data with normal distribution were analyzed with paired and
unpaired t-test, and one-way analysis of variance with Newman-Keuls
test. Data without a normal distribution were analyzed with
Wilcoxon matched pairs test, Mann-Whitney U-test and Kruskal-Wallis
test with Dunn's Multiple Comparison. Spearman correlation analysis
was used to calculate the correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
Immune cell profiles in PF differ from
those in PB
Immune cell profiles of CD4+ and
CD8+ cells, which included the expression of PD-1 on
CD4+ cells and CD8+ cells, and
CD14+ monocytes, which included the expression of PD-L1
on CD14+ monocytes in PF and in PB from 41 subjects,
were analyzed (Tables II and
III). With the exception of
CD8+ cells (which were lower in PF compared with those
in PB), expressions of PD-1 on CD4+ cells, PD-1 on
CD8+ cells, CD14+ monocytes and PD-L1 on
CD14+ monocytes in PF were higher compared with those in
PB, and the p-values were P<0.0001, P<0.0001, P=0.0003 and
P=0.0123, respectively.. The ratio of
CD4+/CD8+ cells was significantly increased
in PF, as compared with PB (P<0.001; Tables II and III). For subjects who were diagnosed with
TPE, with the exception of CD14+ monocytes (P=0.2066),
expression levels of CD4+, CD8+,
CD4+PD-1+ and CD8+PD-1+
cells and CD14+PD-L1+ monocytes in PF were
significantly different from those in PB. In addition, in subjects
who were diagnosed with TPE, expression of CD4+PD-1 and
CD8+PD-1+ cells were higher in PF than PB,
and P-values were P=0.0035 and P=0.0131. Percentage of
CD4+ cells was increased in PF, as compared with PB,
whereas the opposite was true for CD8+ cells.
Furthermore, the expression levels of PD-L1 on CD14+
monocytes in PF were significantly increased, as compared with
those in PB.
 | Table II.Comparison between the expression of
immune subsets in the pleural effusion and peripheral blood of the
same subject. |
Table II.
Comparison between the expression of
immune subsets in the pleural effusion and peripheral blood of the
same subject.
|
| TPE (n=18) | MPE (n=16) | n-TB n-M (n=7) |
|---|
|
|
|
|
|
|---|
| Subset (%) | PF | PB | PF | PB | PF | PB |
|---|
| CD4 | 49.8
(41.6–61.2) | 35.2
(25.8–42.8) | 46.2
(29.6–61.4) | 41.1
(35.6–54.7) | 24.7
(10.6–50.2) | 29.2
(26.5–38.7) |
| CD8 | 18.7
(13.5–23.4) | 28.6
(20.0–32.1) | 5.8 (3.6–12.0) | 24.7
(15.6–34.2) | 10.1
(8.2–18.5) | 27.0
(20.3–28.5) |
| CD4/CD8 ratio | 2.5 (2.1–5.0) | 1.23
(0.85–2.33) | 4.9 (3.1–12.2) | 1.9 (1.2–2.7) | 2.7 (1.1–3.0) | 1.2 (1.0–1.7) |
| CD14 | 30.5
(6.9–63.0) | 22.5
(14.1–25.5) | 59.4
(35.9–80.1) | 18.0
(6.4–28.7) | 62.5
(34.6–84.0) | 14.5
(9.6–28.8) |
| CD4+
PD-1+ | 38.6
(24.6–44.8) | 19.5
(13.9–28.3) | 51.2
(31.0–57.7) | 24.4
(18.2–39.7) | 41.5
(24.3–65.2) | 29.0
(26.3–37.1) |
| CD8+
PD-1+ | 19.3
(10.7–25.9) | 11.8
(8.8–20.9) | 35.2
(15.9–44.4) | 18.7
(9.5–21.3) | 23.9
(14.8–39.5) | 19.5
(11.3–35.8) |
|
CD4+/CD8+
PD-1+ ratio | 2.3 (1.2–2.5) | 1.41
(1.20–2.19) | 1.3 (1.17–2.1) | 1.7 (1.2–2.5) | 1.7 (1.2–1.9) | 1.5 (0.8–2.3) |
| CD14+
PD-L1+ | 92.0
(76.9–97.3) | 44.1
(28.4–63.3) | 19.1
(12.2–33.5) | 14.8
(7.8–38.3) | 42.9
(8.9–76.9) | 61.5
(18.8–67.5) |
 | Table III.Comparison of immune subsets in
pleural effusion and peripheral blood. |
Table III.
Comparison of immune subsets in
pleural effusion and peripheral blood.
|
| PF vs. PB | TB (PF vs. PB) | TPE vs. MPE vs.
n-TB n-M |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Subset (%) | P-value | P-value | P-value | TPE (n=18) | Non-TPE (n=23) | P-value |
|---|
|
CD4+ |
0.0919 |
0.0007 |
0.0183 | 49.8
(41.6–61.2) | 44.3
(22.8–55.9) |
0.0902 |
|
CD8+ |
<0.0001 |
<0.0001 |
0.0001 | 18.7
(13.5–23.4) | 8.2 (3.9–14.3) |
<0.0001 |
|
CD4+/CD8+ ratio |
<0.0001 |
<0.0001 |
0.0054 | 2.5 (2.1–5.0) | 3.1 (2.6–8.8) |
0.0853 |
|
CD14+ |
0.0003 |
0.2066 |
0.0434 | 30.5
(6.9–63.0) | 62.4
(34.9–81.8) |
0.0168 |
| CD4+
PD-1+ |
<0.0001 |
0.0035 |
0.3330 | 38.6
(24.6–44.8) | 47.7
(24.9–57.8) |
0.1619 |
| CD8+
PD-1+ |
<0.0001 |
0.0131 |
0.0571 | 19.3
(10.7–25.9) | 34.7
(14.8–43.0) |
0.0195 |
|
CD4+/CD8+
PD-1+ ratio |
0.7558 |
0.1914 |
0.1494 | 2.3
(1.21–2.45) | 1.4
(1.17–1.90) |
0.0719 |
| CD14+
PD-L1+ |
0.0123 |
<0.0001 |
<0.0001 | 92.0
(76.9–97.3) | 21.2
(11.3–40.0) |
<0.0001 |
For immune subsets in PB, the percentage of
CD4+ cells and the level of PD-L1 on CD14+
monocytes in the MPE group were significantly increased compared
with those in the TPE and n-TB n-M groups (P=0.018 and P=0.0128,
respectively). No significant differences in PB were detected in
the other subsets (data not shown). For immune subsets in PF, the
percentage of CD8+ cells was increased in the TPE group,
as compared with the MPE and the n-TB n-M groups. Furthermore,
increased expression of PD-L1 on CD14+ monocytes was
detected in the TPE group, as compared with the MPE and n-TB n-M
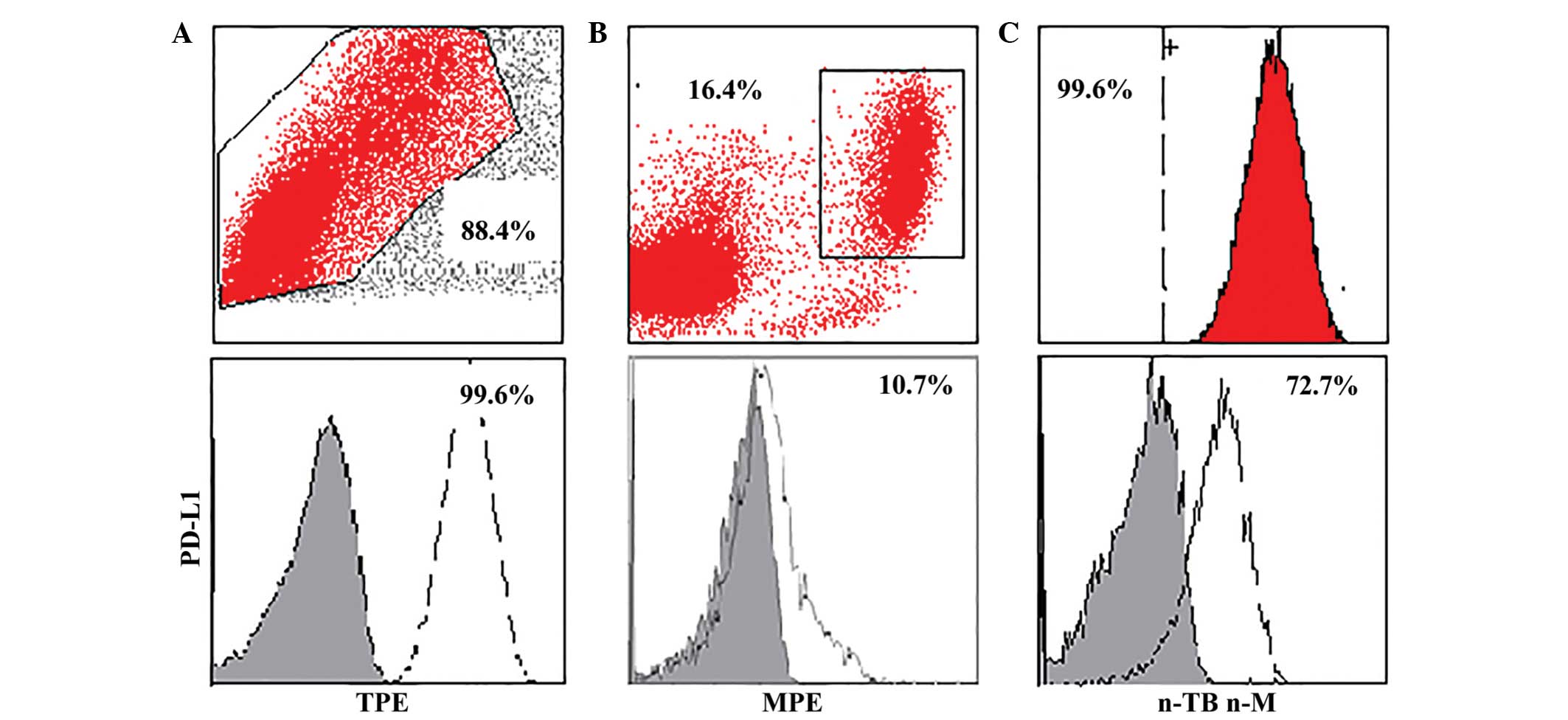
groups (Fig. 1; Tables II and III). Expression of PD-L1 was increased in
the TPE group, as compared with the MPE and n-TB n-M groups at the
mRNA level.
Subjects diagnosed with TPE and non-TPE were
analyzed for potential biomarkers that may distinguish between
them. No differences in the total expression levels of
CD4+, CD8+, CD4+PD-1+
and CD8+PD-1+ cells, and CD14+ and
CD14+PD-L1+ monocytes were detected in PB
(data not shown). For immune subsets in PF, an increased level
CD14+ PD-L1+monocytes was demonstrated in the
TPE group, as compared with the non-TPE group (Table III). The P-values for
CD14+PL-L1+ and CD8+ cells and
CD8+PD-1+ cells were P<0.0001, P<0.0001
and P=0.0195, respectively. Consistent with the result of
CD8+ cells above, the levels of CD8+ cells
were increased in the TPE group, as compared with the MPE and the
n-TB n-M groups; whereas the expression of PD-1 on CD8+
cells was decreased in TPE. Thus, sPD-L1 and mPD-L1 are involved in
the immune regulation and disease progression of TPE.
Expression levels of sPD-L1 and IFN-γ
are elevated in TPE
Expression levels of sPD-L1 were increased in PF, as
compared with PB for the same subject (P=0.0033; Fig. 2A and B). Furthermore, increased
expression levels of sPD-L1 were detected in the TPE group, as
compared with the MPE and n-TB n-M groups (both P<0.05; Fig. 2C); however, no significant
differences were detected between the MPE and n-TB n-M PF groups.
Expression levels of sPD-L1 were increased in the TPE group, as
compared with the non-TPE group (Fig.
2D; Table IV). Increased
expression levels of IFN-γ were detected in the TPE group, as
compared with the MPE and n-TB n-M groups (P<0.05; Fig. 2E); however, no differences were
detected between the MPE and n-TB n-M groups. Expression levels of
IFN-γ in the TPE group were significantly increased, as compared
with the non-TPE group (P<0.0001; Fig. 2F; Table
IV). According to receiver operating curve analysis, levels of
>2.41 ng/ml sPD-L1 in PF were able to discriminate between the
TPE and non-TPE groups, with a specificity (Sp) of 82.9%,
sensitivity (Se) of 82.6% and area under the curve (AUC) of 0.840.
Using an IFN-γ expression level of >31.06 pg/ml as the cut off
value, the TPE group was successfully differentiated from the
non-TPE group (Sp, 95.1%; Se, 87%; AUC, 0.923). Using a value of
>72.75% PD-L1 on CD14+ monocytes as the cut off
value, the TPE group was successfully differentiated from the
non-TPE group (Sp, 92.7%; Se, 78.3%; AUC, 0.898) (Table IV). Furthermore, a combination of
sPD-L1, IFN-γ and PD-L1 on CD14+ monocytes demonstrated
an increased AUC of 0.962 (Sp, 100%; Se, 91.3%), as compared with
the parameters alone, using multivariate analysis. sPD-L1 and PD-L1
on CD14+ monocytes are higher in TPE, which suggests
that they may serve as possible biomarkers of TPE.
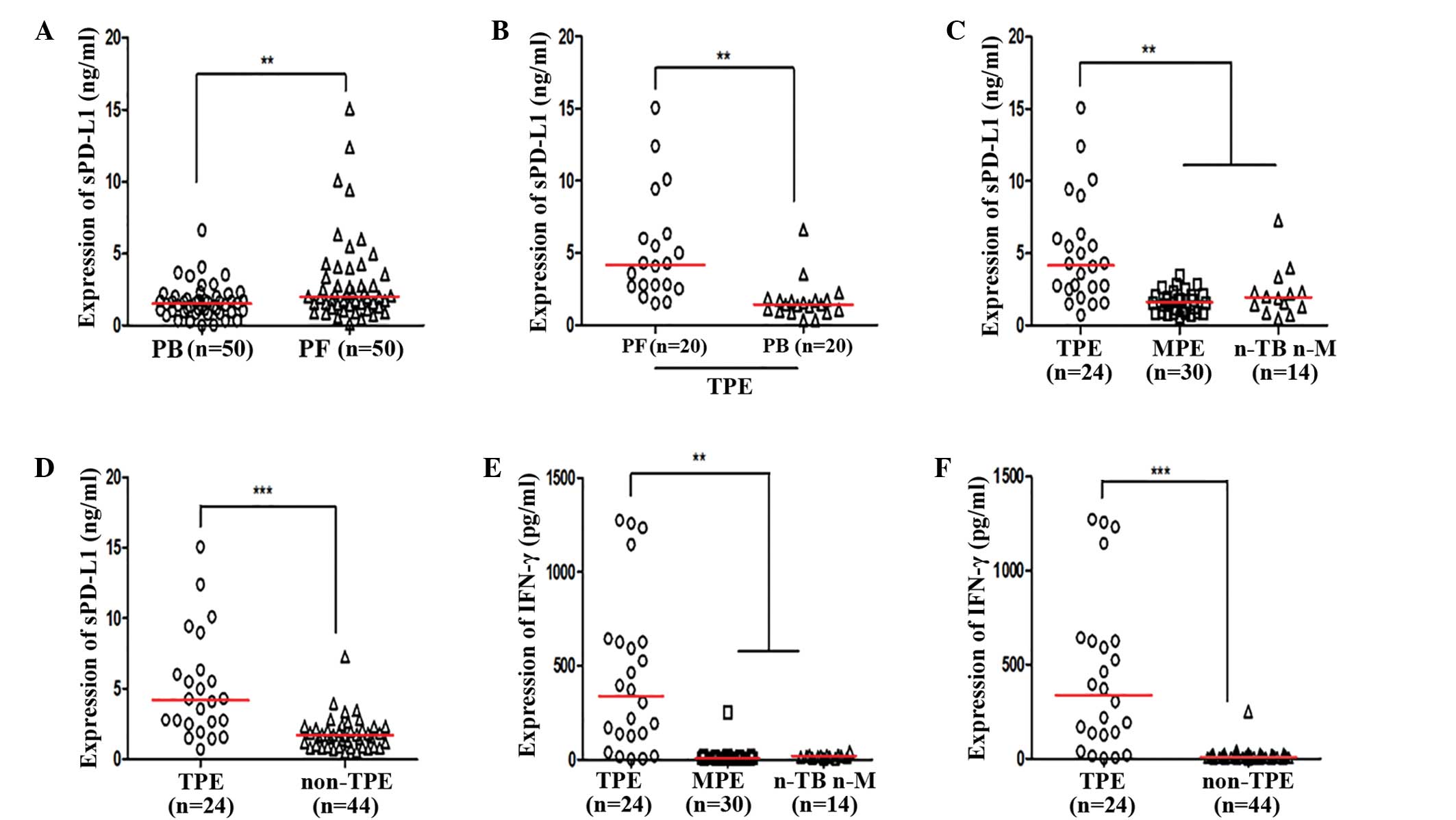 | Figure 2.(A) Expression of sPD-L1 in PB and
PF. (B) Expression of sPD-L1 in PF and PB in patients diagnosed
with TPE. (C) Expression of sPD-L1 in the TPE, MPE and n-TB n-M
groups. (D) Expression of sPD-L1 in patients diagnosed with TPE and
non-TPE. (E) Expression of IFN-γ in the TPE, MPE and n-TB n-M
groups. (F) Expression of IFN-γ in patients diagnosed with TPE and
non-TPE. **P<0.05; ***P<0.0001. sPD-L1, sPD-L1, soluble
programmed death ligand-1; PF, pleural effusion; PB, peripheral
blood; TPE, tuberculous pleural effusion; MPE, malignant pleural
effusion; n-TB n-M, non-TB non-M pleural effusion; IFN,
interferon. |
 | Table IV.Se and Sp of pleural effusion indices
in distinguishing TPE from non-TPE. |
Table IV.
Se and Sp of pleural effusion indices
in distinguishing TPE from non-TPE.
| Parameter | TPE | Non-TPE | P-value | Se (%) | Sp (%) | AUC | Cutoff |
|---|
| sPD-L1 | 4.2 (2.53–6.3) | 1.7
(0.90–2.26) |
<0.0001 | 82.6 | 82.9 |
0.840 | 2.41 ng/ml |
| IFN-γ | 338.6
(128.7–626.9) | 9.4 (6.5–18.6) |
<0.0001 | 87.0 | 95.1 |
0.923 | 31.06 pg/ml |
| CD14+
PD-L1+ | 89.9
(73.5–97.9) | 16.9
(12.6–44.8) |
<0.0001 | 78.3 | 92.7 |
0.898 | 72.75% |
| CD14+
sPD-L1+ IFN-γ+ PD-L1+ | None | None | None | 91.3 | 100.0 |
0.962 | None |
IFN-γ increases the expression levels
of sPD-L1 and PD-L1 on CD14+ monocytes in PBMCs
The results of flow cytometry demonstrated that the
expression levels of PD-L1 on CD14+ monocytes were
improved in the TPE-stimulated group, as compared with the control
(P<0.05; Fig. 3A). In order to
investigate the possible correlation between IFN-γ and mPD-L1 in
PF, all the 68 subjects were studied. The results of this analysis
demonstrated that the expression of PD-L1 on CD14+
monocytes was significantly positively correlated with the level of
IFN-γ, based on Spearman's correlation (P<0.0001; Fig. 3B); however, no significant
differences were detected in the TPE group (r=0.16;
P=0.4552). In addition, Spearman's correlation analysis of sPD-L1
demonstrated that sPD-L1 expression levels were positively
correlated with IFN-γ, (P=0.0004; Fig.
3C) and PD-L1 on CD14+ monocytes (r=0.2984;
P=0.0166). Thus, he immune mechanism of sPD-L1, and the PD-1/PD-L1
pathway involved in TPE are associated with Th1 immune
response.
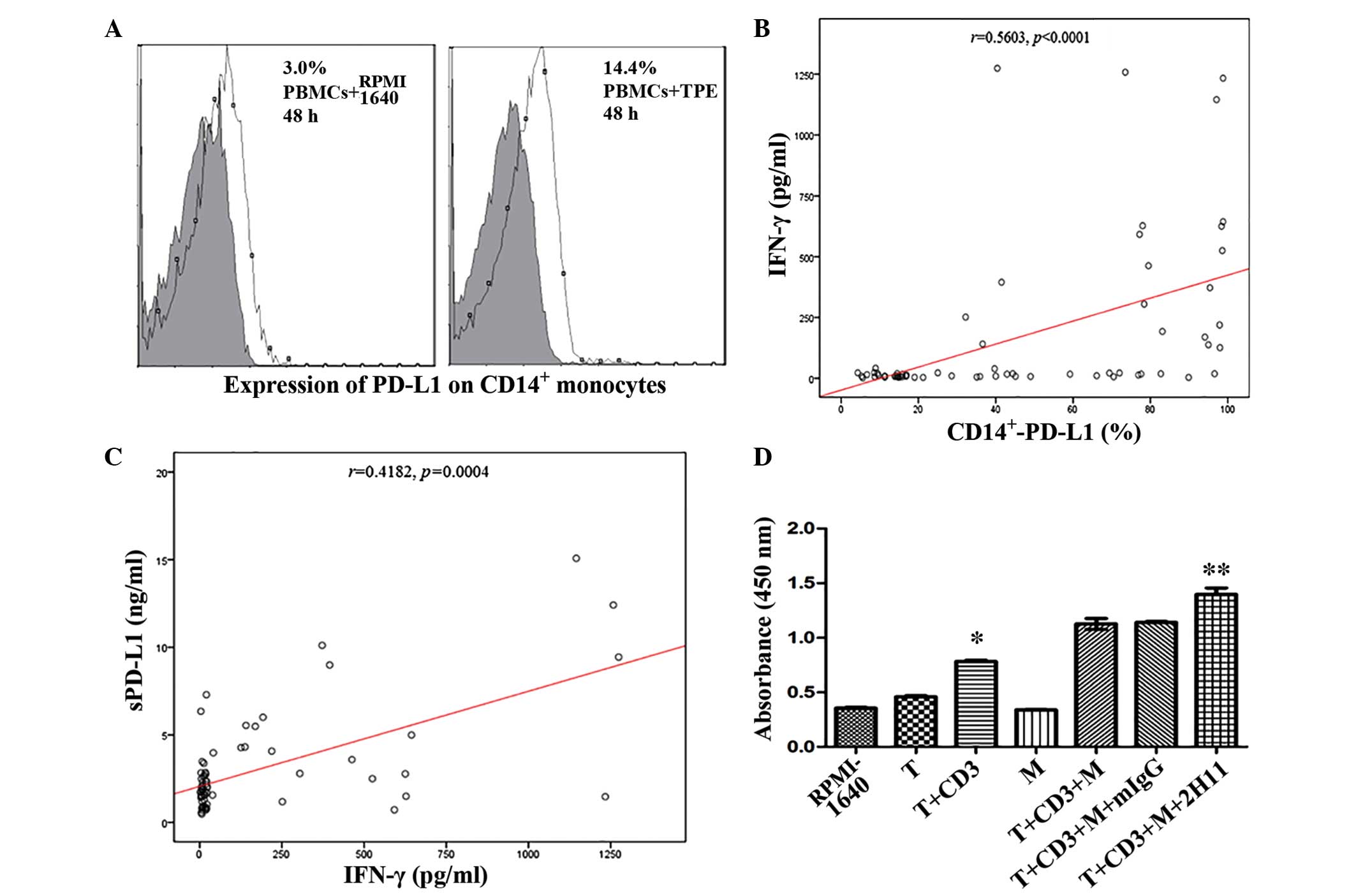 | Figure 3.(A) PBMCs were supplemented with TPE
and co-cultured for 48 h, and the expression levels of PD-L1 on
CD14+ monocytes were subsequently analyzed by flow
cytometry. (B) A significant correlation was detected between IFN-γ
and PD-L1 on CD14+ monocytes in pleural effusion
(P<0.0001) and (C) sPD-L1 and IFN-γ in pleural effusion
(P=0.0004), respectively. (D) Effect of anti-PD-L1 monoclonal
antibodies on the proliferation of T cells following 96-h
co-cultivation with CD14+ monocytes was evaluated with a
cell counting kit-8. *P<0.0001, as compared with the T group;
**P=0.005, as compared with the T+CD3+M group. PBMCs, peripheral
blood monocyte cells; TPE, tuberculous pleural effusion; PD-L1,
programmed death ligand −1; CD, cluster of differentiation; sPD-L1,
soluble PD-L1; IFN, interferon; T, T cells; CD3, agonistic CD3
antibody; M, monocytes that were separated from TPE; mIgG, mouse
anti-human immunoglobulin G control antibody; 2H11, mouse
anti-human PD-L1 antibody. |
Anti-PD-L1 mAb enhances the
proliferation of T lymphocytes in co-cultivation with
CD14+ monocytes
To determine the effect of PD-L1 in TPE, enriched T
cells and purified CD14+ monocytes were co-cultured and
the proliferation of T cells in the co-culture system was evaluated
by CCK-8 kit. The results demonstrated that T cells were activated
in association with anti-CD3 mAb following 4-day stimulation
(P<0.0001; Fig. 3D). Notably,
supplementing the medium with PD-L1 antibody (2H11) partially
reversed this effect (P=0.005; Fig.
3D). Anti-PD-1/PD-L1 pathway is a potential immune-checkpoint
in the treatment of TPE.
Discussion
Tuberculous PF is usually the result of a
hypersensitivity reaction to TB protein with chronic inflammatory
infiltration by immune cells, including monocytes/macrophages and
natural killer cells, in the pleural cavity. Previous studies have
demonstrated that the PD-1/PD-L1 pathway has an important role in
the immune response during infection with M. tb (23–25).
However, the immune mechanism of the PD-1/PD-L1 pathway's
involvement in TB is yet to be fully elucidated. The present study
analyzed the expression levels of mPD-L1 and sPD-L1 in the PB and
PF in patients with PFs, since sPD-L1 acts as an essential cytokine
in lung cancer (12). As
hypothesized, the expression levels of PD-L1 on CD14+
monocytes and sPD-L1 were increased in the TPE group and the Th1
cytokine IFN-γ in PF may enhance this effect. It was also
determined that anti-PD-L1 mAb is able to partially reverse the
attenuated proliferation of T lymphocytes mediated by high levels
of PD-L1 binding to PD-1 in vitro.
An accumulation of lymphocytes, particularly
CD4+ T cells, in TPE has been well documented in
previous studies, demonstrating that patients who were infected
with M. tb may exhibit a strong Th1 immune response to kill
M. tb, particularly in the young (26–28).
Therefore, the PD-1/PD-L1 pathway and increased sPD-L1 levels may
deliver a potent negative co-inhibitory signal to T cells.
Alternatively, this phenomenon may prevent excessive immune injury
and maintain balance in the immune system (29). However, the underlying pathogenesis
remains unclear. Amarnath et al (30) have previously demonstrated that human
regulatory T (Treg) cells promote immune suppression through
dendritic cell modulation via the upregulation of PD-L1. Trinath
et al (31) have also
reported that M. tb may promote the expansion of Treg cells
via the induction of PD-L1 on dendritic cells. In the present
study, high expression levels of membranous and soluble PD-L1 in
TPE indicated that PD-1/PD-L1 and sPD-L1 may have a role in M.
tb infection via Treg cells.
Although both myeloid and T lymphocytes exhibit
mPD-L1 expression, the release of sPD-L1 is a feature of the
myeloid population (32,33). The results of the present study
demonstrated that the expression levels of sPD-L1 in PF were
increased, as compared with PB; this may be due to the high
concentrations of cytokines in the local pleural cavity. High
concentration of IFN-γ were consistently detected in TPE,
supporting the hypothesis that IFN-γ may serve as a diagnostic
biomarker (34). Lee et al
(35) have previously suggested that
IFN-γ may upregulate the expression of PD-L1 via IFN regulatory
factor-1. The results of the present study also indicated that
sPD-L1 may be released with matrix metalloproteinases from the
surface of immune and tumor cells (9). Notably, the expression levels of PD-L1
on CD14+ monocytes increased when the PBMCs were
supplemented with TPE; however, the proliferation rate remained
lower than is achieved with commercial IFN-γ stimulation (16). It is possible that IFN-γ is not the
only cytokine with a role in the TB microenvironment, other
cytokines, such as IFN-α, may also have an effect (26,36). In
a previous study it was reported that IFN-α and IFN-β strongly
inhibited IFN-γ responsiveness and the production of type I
cytokines (15); however, no
differences in IFN-α levels were detected in the TPE, MPE and n-TB
n-M groups in the present study.
Previous studies have determined high levels of
IFN-γ in PF with a cutoff point varying from 60 to 240 pg/ml for
TPE (37–41). The result of the present study
demonstrated a cutoff point of 31.06 pg/ml for IFN-γ. This
difference may be attributed to the different methods, sample
number and ethnicity of participants in the studies. Furthermore,
in the present study it was demonstrated that anti-PD-L1 mAb
enhanced the proliferation of T lymphocytes in co-cultivation with
CD14+ monocytes, which indicated that an immune therapy
method associated with PD-1/PD-L1 pathway in tuberculous PF may be
developed.
In conclusion, the results of the present study
suggested that sPD-L1 and mPD-L1 may be associated with the immune
regulation and disease progression of TPE.sPD-L1. Increased levels
of PD-L1 on CD14+ monocytes were detected in patients
with TPE, indicating that PD-L1 may serve as a potential biomarker
for TPE. The results also suggested that the immune mechanism of
sPD-L1 and the PD-1/PD-L1 pathway in TPE are associated with the
Th1 immune response; therefore, an anti-PD-1/PD-L1 pathway may be a
potential therapeutic strategy for the treatment of TPE.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81272610),
the Science and Technology Support Program of Suzhou, China (grant
no. SS201246), and the Science and Technology Development Program
of Suzhou, China (grant no. SYS201467).
References
|
1
|
WHO global tuberculosis report. 2015,
https://www.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1
|
|
2
|
Porcel JM: Tuberculous pleural effusion.
Lung. 187:263–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valdés L, Pose A, San José E and Vázquez
JM Martínez: Tuberculous pleural effusions. Eur J Intern Med.
14:77–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan FY, Hamza M, Omran AH, Saleh M,
Lingawi M, Alnaqdy A, Rahman MO, Ahmedullah HS, Hamza A, Ani AA, et
al: Diagnostic value of pleural fluid interferon-gamma and
adenosine deaminase in patients with pleural tuberculosis in Qatar.
Int J Gen Med. 6:13–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azimi Q, Rezadoost B, Nadoushan M Jalali
and Davati A: Evaluation of serum cyfra21-1 in patients with
pleural effusion. Iran Red Crescent Med J. 14:613–616.
2012.PubMed/NCBI
|
|
6
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang SC, Greenwald RJ, Latchman YE, Rosas
L, Satoskar A, Freeman GJ and Sharpe AH: PD-L1 and PD-L2 have
distinct roles in regulating host immunity to cutaneous
leishmaniasis. Eur J Immunol. 36:58–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye ZJ, Zhou Q, Yin W, Yuan ML, Yang WB,
Xiang F, Zhang JC, Xin JB, Xiong XZ and Shi HZ: Interleukin
22-producing CD4+ T cells in malignant pleural effusion.
Cancer Lett. 326:23–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L
and Zhang X: Development of a sandwich ELISA for evaluating soluble
PD-L1 (CD274) in human sera of different ages as well as
supernatants of PD-L1+ cell lines. Cytokine. 56:231–238.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu DM, Yan JC, Wang CP, Chen GH, Ding S,
Liu PJ and Du RZ: The clinical implications of increased OX40
ligand expression in patients with acute coronary syndrome. Clin
Chim Acta. 397:22–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang J, Jiang J, Liu C, Zhang G, Gao L,
Chen Y, Zhu R, Wang T, Wang F, Zhang X and Xue Q: Enhancement of
membrane B7-H3 costimulatory molecule but reduction of its soluble
form in multiple sclerosis. J Clin Immunol. 33:118–126. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing YF, Zhang ZL, Shi MH, Ma Y and Chen
YJ: The level of soluble programmed death-1 in peripheral blood of
patients with lung cancer and its clinical implications. Zhonghua
Jie He He Hu Xi Za Zhi. 35:102–106. 2012.(In Chinese). PubMed/NCBI
|
|
13
|
Frigola X, Inman BA, Lohse CM, Krco CJ,
Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H and Kwon
ED: Identification of a soluble form of B7-H1 that retains
immunosuppressive activity and is associated with aggressive renal
cell carcinoma. Clin Cancer Res. 17:1915–1923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muenst S, Soysal SD, Gao F, Obermann EC,
Oertli D and Gillanders WE: The presence of programmed death 1
(PD-1)-positive tumor-infiltrating lymphocytes is associated with
poor prognosis in human breast cancer. Breast Cancer Res Treat.
139:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Paus RA, van Wengen A, Schmidt I,
Visser M, Verdegaal EM, van Dissel JT and van de Vosse E:
Inhibition of the type I immune responses of human monocytes by
IFN-α and IFN-β. Cytokine. 61:645–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi B, Du X, Wang Q, Chen Y and Zhang X:
Increased PD-1 on CD4(+)CD28(−) T cell and soluble PD-1 ligand-1 in
patients with T2DM: Association with atherosclerotic macrovascular
diseases. Metabolism. 62:778–785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Infante-Duarte C and Kamradt T: Th1/Th2
balance in infection. Springer Semin Immunopathol. 21:317–338.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang WB, Liang QL, Ye ZJ, Niu CM, Ma WL,
Xiong XZ, Du RH, Zhou Q, Zhang JC and Shi HZ: Cell origins and
diagnostic accuracy of interleukin 27 in pleural effusions. PLoS
One. 7:e404502012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Light RW, Macgregor MI, Luchsinger PC and
Ball WC Jr: Pleural effusions: The diagnostic separation of
transudates and exudates. Ann Intern Med. 77:507–513. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kastelik JA: Management of malignant
pleural effusion. Lung. 191:165–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan EM, Cohen AS, Fries JF, Masi AT,
McShane DJ, Rothfield NF, Schaller JG, Talal N and Winchester RJ:
The 1982 revised criteria for the classification of systemic lupus
erythematosus. Arthritis Rheum. 25:1271–1277. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niederman MS, Mandell LA, Anzueto A, Bass
JB, Broughton WA, Campbell GD, Dean N, File T, Fine MJ, Gross PA,
et al: Guidelines for the management of adults with
community-acquired pneumonia. Diagnosis, assessment of severity,
antimicrobial therapy, and prevention. Am J Respir Crit Care Med.
163:1730–1754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jurado JO, Alvarez IB, Pasquinelli V,
Martínez GJ, Quiroga MF, Abbate E, Musella RM, Chuluyan HE and
García VE: Programmed death (PD)-1: PD-ligand1/PD-ligand 2 pathway
inhibits T cell effector functions during human tuberculosis. J
Immunol. 181:116–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reiley WW, Shafiani S, Wittmer ST,
Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM and
Woodland DL: Distinct functions of antigen-specific CD4 T cells
during murine Mycobacterium tuberculosis infection. Proc Natl Acad
Sci USA. 107:19408–19413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin W, Tong ZH, Cui A, Zhang JC, Ye ZJ,
Yuan ML, Zhou Q and Shi HZ: PD-1/PD-Ls pathways between CD4(+) T
cells and pleural mesothelial cells in human uberculous pleurisy.
Tuberculosis (Edinb). 94:131–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Porcel JM: Tuberculous pleural effusion.
Lung. 187:263–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia H, Ye ZJ, Zhou Q, You WJ, Cui A, Wang
XJ, Zhai K, Jin XG, Tong ZH and Shi HZ: IL-27 and IL-27-producing
CD4+ T cells in human tuberculous pleural effusion. Tuberculosis
(Edinb). 94:579–588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qama D, Choi WI and Kwon KY: Immune
responses in the lungs of patients with tuberculous pleural
effusion without pulmonary tuberculosis. BMC Immunol. 13:452012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amarnath S, Costanzo CM, Mariotti J,
Ullman JL, Telford WG, Kapoor V, Riley JL, Levine BL, June CH, Fong
T, et al: Regulatory T cells and human myeloid dendritic cells
promote tolerance via programmed death ligand-1. PLoS Biol.
8:e10003022010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trinath J, Maddur MS, Kaveri SV, Balaji KN
and Bayry J: Mycobacterium tuberculosis promotes regulatory T-cell
expansion via induction of programmed death-1 ligand 1 (PD-L1,
CD274) on dendritic cells. J Infect Dis. 205:694–696. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frigola X, Inman BA, Krco CJ, Liu X,
Harrington SM, Bulur PA, Dietz AB, Dong H and Kwon ED: Soluble
B7-H1: Differences in production between dendritic cells and T
cells. Immunol Lett. 142:78–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He XH, Xu LH and Liu Y: Identification of
a novel splice variant of human PD-L1 mRNA encoding an
isoform-lacking Igv-like domain. Acta Pharmacol Sin. 26:462–468.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang J, Shi HZ, Liang QL, Qin SM and Qin
XJ: Diagnostic value of interferon-gamma in tuberculous pleurisy: A
metaanalysis. Chest. 131:1133–1141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI,
Park YM, Oh S, Shin JG, Yao S, Chen L and Choi IH: Interferon
regulatory factor-1 is prerequisite to the constitutive expression
and IFN-gamma-induced up-regulation of B7-H1 (CD274). FEBS Lett.
580:755–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma J, Yang B, Yu S, Zhang Y, Zhang X, Lao
S, Chen X, Li B and Wu C: Tuberculosis antigen-induced expression
of IFN-α in tuberculosis patients inhibits production of IL-1β.
FASEB J. 28:3238–3248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sutherland JS, Garba D, Fombah AE,
Mendy-Gomez A, Mendy FS, Antonio M, Townend J, Ideh RC, Corrah T
and Ota MO: Highly accurate diagnosis of pleural tuberculosis by
immunological analysis of the pleural effusion. PLoS One.
7:e303242012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang T, Lv M, Qian Q, Nie Y, Yu L and Hou
Y: Increased frequencies of T helper type 17 cells in tuberculous
pleural effusion. Tuberculosis (Edinb). 91:231–237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Budak F, Uzaslan EK, Cangür S, Göral G and
Oral HB: Increased pleural soluble Fas Ligand (sFasL) levels in
tuberculosis pleurisy and its relation with T-helper type 1
cytokines. Lung. 186:337–343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu YC, Lee S Shin-Jung, Chen YS, Tu HZ,
Chen BC and Huang TS: Differential diagnosis of tuberculous and
malignant pleurisy using pleural fluid adenosine deaminase and
interferon gamma in Taiwan. J Microbiol Immunol Infect. 44:88–94.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ibrahim L, Salah M, Abd El Rahman A,
Zeidan A and Ragb M: Crucial role of
CD4+CD25+ FOXP3+ T regulatory
cell, interferon-γ and interleukin-16 in malignant and tuberculous
pleural effusions. Immunol Invest. 42:122–136. 2013. View Article : Google Scholar : PubMed/NCBI
|